Introduction
Electroporation occurs when electric pulses with a
field intensity of kV/cm and duration of μsec to msec magnitude act
on cells, altering the transmembrane potential (1,2). Thus,
the lipid bilayer structure is disrupted and small nanopores form
in the cell membrane. This allows micro- and macromolecules to be
transported into and out of the cells (3–7).
Electroporation may be reversible (RE) or irreversible (IRE),
according to whether the membrane recovers or not. RE has been used
for the delivery of drugs or macromolecules into cells, for example
in electrochemotherapy (ECT) (8,9). If
the electric field is strong enough, the pores become permanent and
cause cell death by interfering with cell homeostasis. As IRE is
able to achieve the ablation of desirable tissues without affecting
the surrounding normal tissue it has been considered as a novel
method for cancer treatment (10).
IRE ablates tumors without chemotherapeutics and is
not affected by the heat sink effect. With non-thermal cell death
and a markedly decreased treatment time (usually less than 10 min),
IRE provides a new ablation technique that may be performed in a
well-controlled and focused manner under image monitoring [such as
ultrasonography (US)]. In a previous study (11), researchers reported the correlation
between US and gross section measurements at the same observation
time point (such as 24 h after IRE ablation). However, IRE is not
an acute cell ablation method; tumor cell death occurred sometime
after IRE ablation. Therefore, in the IRE ablation, the correlation
between the ablation zones of US measurements immediately post-IRE
and gross section measurements 24 h after IRE ablation is important
to clinical physicians. The correlation may be used to make a
treatment plan prior to the IRE treatment process and to calculate
the exact ablation extent using a formula immediately following
treatment. In this study, we used an in vivo goat liver
model to evaluate the correlation between ablation areas, as
measured by US, versus gross section examination and aimed to
identify a suitable time point to evaluate the degree of cell
necrosis though histopathological changes of goat livers following
percutaneous IRE treatment.
Materials and methods
Animal care
A total of 24 goats, aged 4–6 months and weighing
25–30 kg, were obtained and maintained by the Division of
Laboratory Animal Medicine of Chongqing Medical University
(Chongqing, China). All animals received appropriate humane care
from properly trained professional staff in compliance with both
the Principals of Laboratory Animal Care and the Guide for the Care
and Use of Laboratory Animals approved by the Animal Care and Use
Committees of Chongqing Medical University.
IRE of goat livers and US
measurement
Goats received a general anesthesia: induction was
performed using an intramuscular injection of diazepam (10–20
mg/100 kg) and xylazine (1.5–2.0 ml/100 kg). The goats were placed
in the supine position following successful anesthesia. The 9–12
ribs which cover the liver tissue had been removed one week prior
to surgery. The right upper quadrant and epigastrium were shaved
and sterilized in the usual fashion. Pre-ablation US was performed
to visualize the normal hepatic anatomy and to locate the desired
area for ablation. Using US guidance, we selected sites in all
hepatic lobes for ablation.
Electroporation was performed with an electric
pulses therapeutic system (manufactured by State Key Laboratory of
Power Transmission Equipment and System Security and New
Technology, Chongqing University) though electrodes at a frequency
of 1 Hz, voltage 2,000 V and pulse duration 100 μsec. The voltage
and wavelength of the electric pulses were monitored throughout the
procedures with a TDS3032B oscilloscope (Tektronix, Beaverton, OR,
USA).
A total of 24 goats underwent ablation of the normal
liver (of those animals, two for sample collection immediately
post-IRE ablation, another two goats received lower voltage
electric fields at frequency of 1 Hz, pulse duration 100 μsec,
voltage 100 V as a control group). Each goat was to be ablated at
two points, mainly at the right lobe of the liver. Two electrodes
with a 2-cm probe distance in the US group were placed into the
liver under US guidance through the animal skin to the target
areas. The ablating area was oblong in shape and the correlation of
long diameters of the ablation zone were measured and analyzed. The
long diameter of the ablation zone was monitored and measured in
real-time with US at the maximum diameter, along the insert course
of the ablation electrode, which was repeated immediately after the
procedure (D1) and again 24 h (D2) after the procedure. A total of
120 pulses were applied through the electrodes.
Tissue collection and gross
measurement
The treated area was observed by ultrasound scanning
in real-time at 0 and 24 h after IRE ablation. Goats were
heparinized with 5,000 units of heparin and then sacrificed with an
overdose of pentobarbital sodium. The livers were harvested and
sectioned at a 2–5 mm thickness along the insert course of the
ablation electrode. At gross section examination, the sections of
the ablation zones were measured and photographed for comparison
with US measurements of the lesions. The largest long diameters of
the gross sections (D3) were measured by electric vernier caliper
after the animals were sacrificed 24 h post-IRE ablation.
Histochemical analysis
Each section was divided into two parts for
subsequent analysis. One part of the treated area was fixed in 4%
paraformaldehyde and embedded in paraffin for hematoxylin and eosin
(HE) stain for histomorphological analysis. The other part of the
treated area was stored in liquid nitrogen for
glucose-6-phosphatase (G-6-P) and succinodehydrogenase (SDH)
staining, to analyze the activity and function of the endoplasmic
reticulum and mitochondria, respectively.
Statistical analysis
Statistical analysis was carried out using the
software SPSS 11.0 (SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard deviation (SD). Analysis of
variance, bivariate correlation analysis and paired t-test were
used for comparing the gross section measurements with US
measurements of the IRE ablation zones. P<0.05 was considered to
indicate a statistically significant result.
Results
Histological analysis result
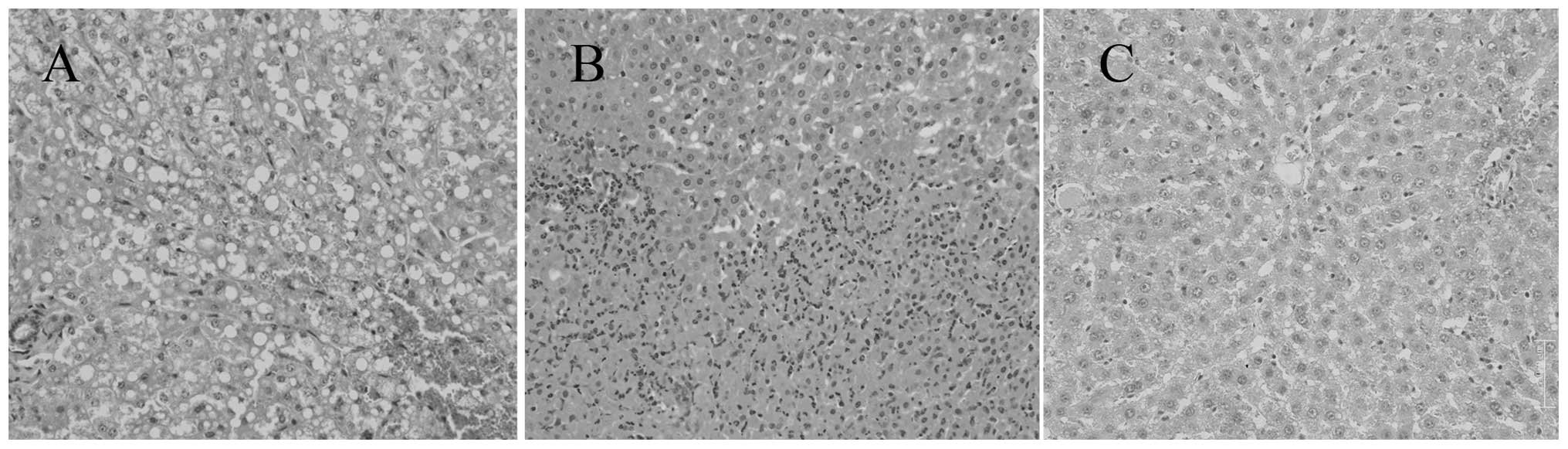
Immediately following IRE ablation, the liver cells
became edemic and liver sinusoids in the ablated areas showed
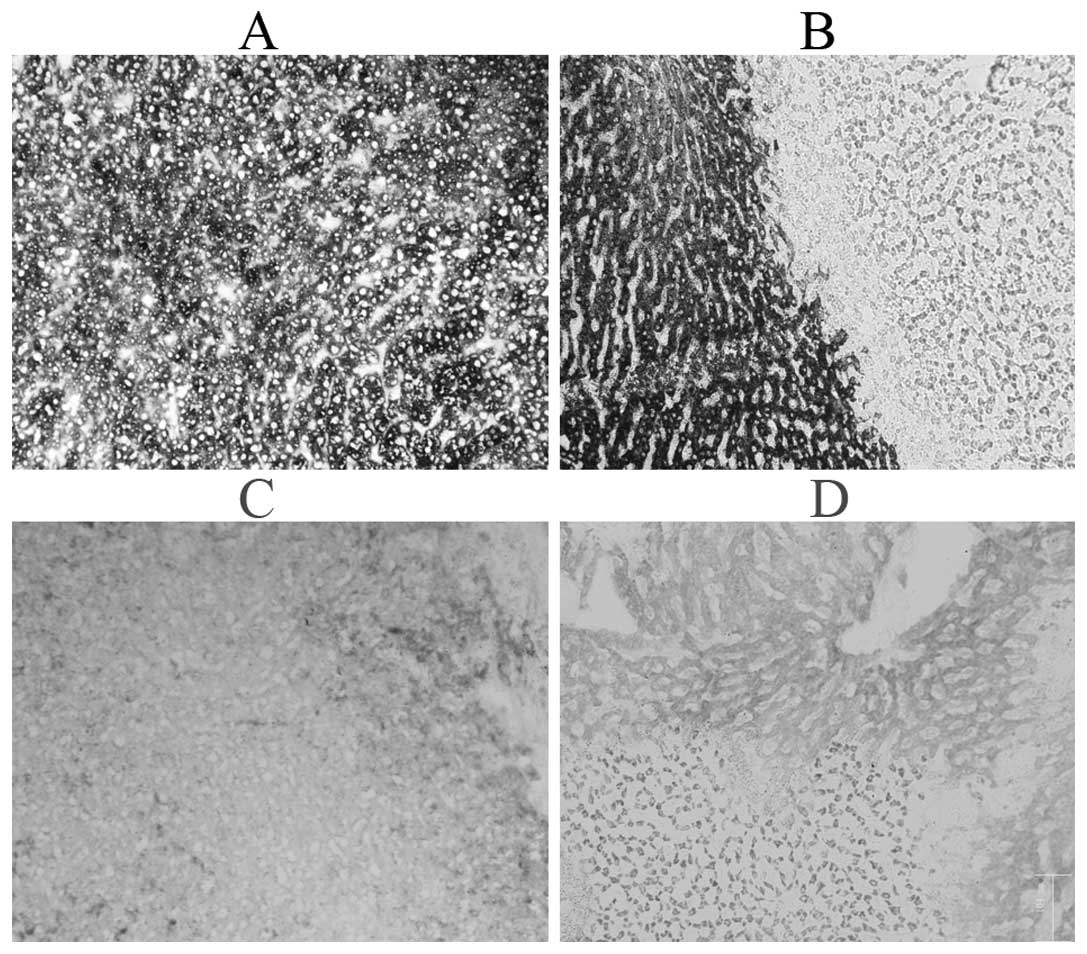
congestion (Fig. 1A). The activity
and function of the endoplasmic reticulum and mitochondria were not
eliminated immediately by the pulsed electric fields (PEFs).
Staining for G-6-P and SDH were positive (Fig. 2A and B). Complete hepatic cell
death, with a sharp demarcation between the ablated and the
non-ablated zones, was well visualized 24 h after the procedure.
The ablation areas were filled with neutrophils and eosinophils
(Fig. 1B). Staining for G-6-P and
SDH were negative (compared with normal control) 24 h post-IRE
(Fig. 2C and D). In the lower
voltage fields group, a few liver cells in the ablation area
appeared slightly swollen and distended, but no necrosis was
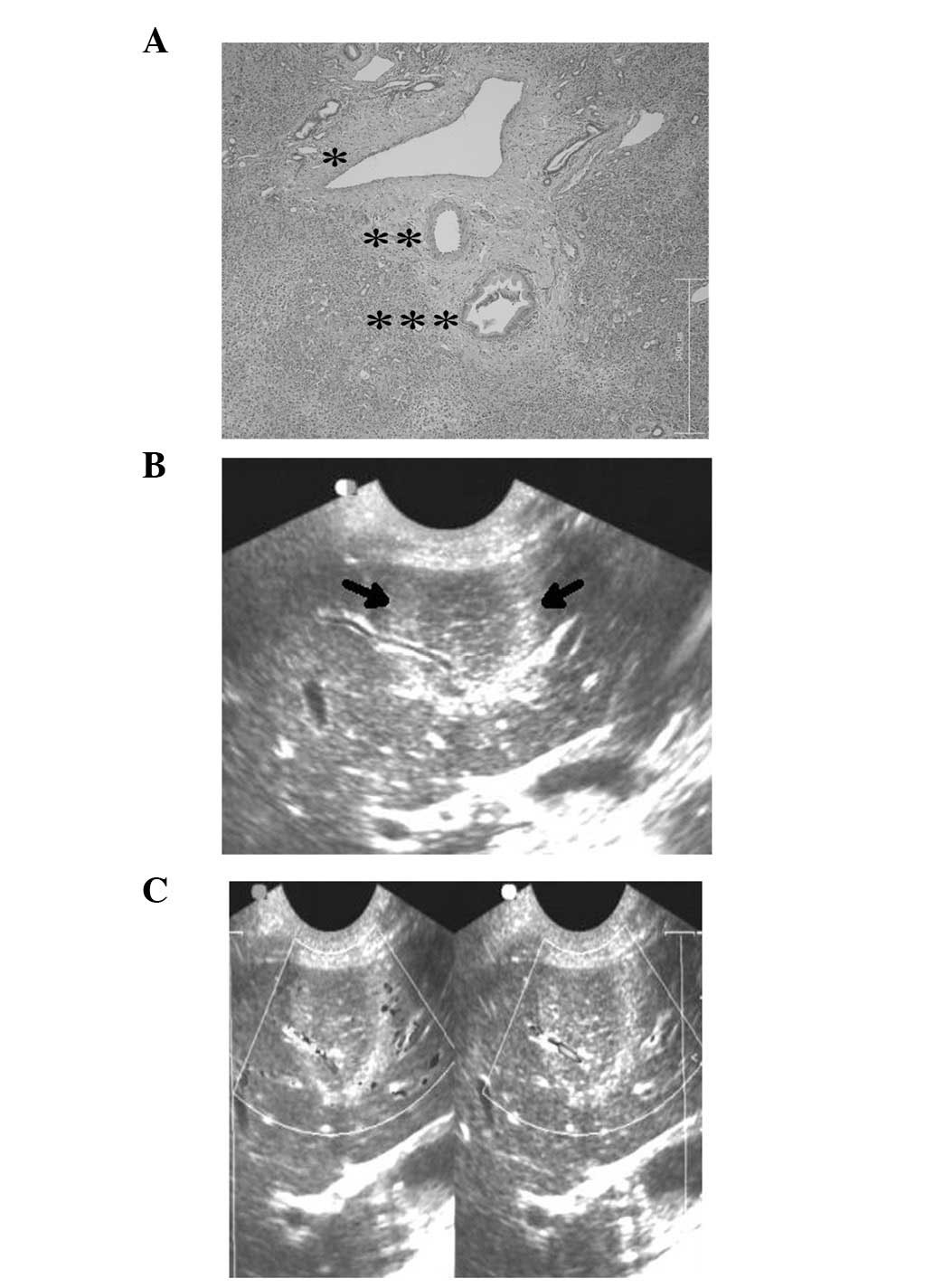
observed (Fig. 1C). Gross section
examination and HE stain revealed the structural integrity of the
blood vessels and bile ducts within the IRE-ablated zone (Fig. 3A).
Gross section and US measurement
US imaging-guidance was used to accurately focus on
the target area. We were able to visualize and measure the ablation
areas of IRE under direct real-time US. Among the three sets of
data (D1, D2 and D3), the largest long diameters measured by
intraprocedural US immediately following the IRE ablation procedure
(D1, 39.58±2.13 mm) was the largest diameter, followed by the
measurement by US 24 h after the procedure (D2, 37.07±3.51 mm). The
two sets were significantly different (P<0.05). The gross
section measurement (D3, 36.44±2.04 mm), which was measured 24 h
after imaging-guided IRE ablation, was the smallest, but there was
no significant difference between D2 and D3 (P>0.05; Table I). D1 showed a good linear
correlation with D3 (r=0.949). The regression equation between
these two sets of data was [Y(D3)=0.906X(D1)+0.058,
R2=0.9013].
 | Table IComparison of the measurement under
ultrasound and of the gross specimen (mean ± SD). |
Table I
Comparison of the measurement under
ultrasound and of the gross specimen (mean ± SD).
| Maximum ablation zone
dimensions | Measurement (mm) |
|---|
| US measurement 0 h
post-imaging-guided IRE ablation | 39.58±2.13 (D1) |
| US measurement 24 h
post-imaging-guided IRE ablation | 37.07±3.51 (D2) |
| Gross section
measurement 24 h post-imaging-guided IRE ablation | 36.44±2.04 (D3) |
The two-dimensional ultrasound imaging revealed that
blood vessels were intact and that there was color flow angiography
in the blood vessels (Fig. 3B and
C).
Discussion
IRE is a new tumor ablation technique. PEFs with an
electric field intensity of at least 500 to 1,000 V/cm and
permanent duration (100 μsec) permanently permeabilize the cell
membrane, causing the formation of innumerable permanent nanopores
in the cell membrane and leading to cell death. IRE has certain
unique advantages, such as ablating tissue non-thermally,
shortening the ablation time and safety. There is a sharp
demarcation between the ablated and non-ablated zones. IRE provides
a novel and unique ablation method for cancer treatment.
In this study, we found that IRE ablation of tissues
is not an acute effect compared with radiofrequency ablation,
microwave ablation, cryoablation and high-intensity focused
ultrasound. We selected histochemical stain for G-6-P and SDH to
detect the cell viability and HE stain for histological
observation. Enzymohistochemical staining for G-6-P and SDH were
used in this study. Researchers usually use G-6-P to detect the
activity and function of the endoplasmic reticulum and SDH to
detect the activity and function of the mitochondria in liver
tissues. When we observed the samples under a microscope
immediately post-IRE, it was found that the structure and
enzymohistochemical viability of the hepatic cells remained intact.
The activity and function of the endoplasmic reticulum and
mitochondria were not eliminated immediately by the PEF. Staining
for G-6-P and SDH were positive. The complete destruction of
hepatic tissue structure and hepatic cell viability was observed
through HE, G-6-P and SDH stains in the experiment group 24 h
post-IRE ablation. There was a sharp demarcation between the
ablated and the non-ablated zones. However, no necrosis was
observed in the control group. Therefore, in the present study, the
effective focus area of IRE was observed and measured at 24 h
post-IRE ablation.
In imaging-guided IRE application in the clinic, the
accurate focusing on the target area and image monitoring during
the process were crucial. Placing the electrodes accurately ensured
that the high intensive electric energy ablated the target area
effectively and did not injure the surrounding normal tissues.
Real-time monitoring by the US may aid the assessment of the extent
of the ablation area and observation of tissue response to the
electric field energy. Among the three sets of data (D1, D2 and
D3), the long diameter measured by intraprocedural US immediately
after the IRE ablation procedure (D1) was the largest, followed by
the data measured by US 24 h after the procedure (D2). The two sets
of data were significantly different (P<0.05). The levels of
intracellular water molecules increased following the opening of
the transmembrane pores by the high voltage of electroporation
immediately after IRE ablation. The ablating area became edemic,
which was likely caused by increased water content in the area of
ablation as a result of the disruption of cellular homeostasis.
However, the extent of the ablation area at 24 h decreased due to
hepatic cell necrosis and dehydration. The US measurement of D2, 24
h post-IRE ablation, is more accurate for calculating the necrotic
area.
Another important finding of this study was the
accuracy of imaging in guidance and monitoring the extent of the
ablation area. We compared the maximum diameters of US-guided IRE
ablation areas 24 h post-IRE ablation measured by US (D2) with its
gross section measurement (D3). There was no significant difference
between the two sets of data (P>0.05). The result showed that
imaging-guided IRE ablation measured the target area
accurately.
In a previous study (11), researchers reported the correlation
between US and gross section measurements at the same time point
(such as 24 h after IRE ablation). However, in the development of
IRE ablation clinical application, it may be more important to
calculate the exact necrotic extent of the ablation areas during
the imaging-guided IRE ablation therapy procedure. This determines
whether the ablating dosage (voltage and pulses) and electric field
intensity are sufficient to completely ablate the lesion of the
tumor tissue. Therefore, investigating the correlation between the
maximum diameters of the IRE lesions measured by intraprocedural US
immediately after the IRE ablation procedure (D1) and gross section
measurement 24 h after IRE ablation (D3) when the animals were
scarified was necessary. The results may be used to make a
treatment plan prior to the IRE treatment process and to calculate
the exact ablation extent using a formula during the IRE process.
The statistical results indicated that D1 showed a good linear
correlation with D3 (r=0.949). The regression equation between
these two sets of data was [Y(D3)=0.906X(D1)+0.058,
R2=0.9013]. Therefore, physicians may be able to assess
the exact extent of necrosis using the regression equation during
the imaging-guided IRE ablation treatment procedure, and decide
whether complementary ablation energy should be added to the lesion
areas. Intraprocedural US may not only be used for IRE ablation
guidance, but also for measuring and regulating the ablation
extent. However, this study had some limitations. The experimental
data were not sufficient and the study was a primary investigation
of the regression equation between D1 and D3.
With advantages of real-time monitoring,
practicability, non-thermal effect and well-controlled ablating
range, percutaneous IRE provides a novel and unique method in
ablating living cells with lipid bilayer membranes. The activity
and function of endoplasmic reticulum and mitochondria in hepatic
cells were lost at the same time. Previous studies found that
(12,13) the IRE ablation technique is not
affected by the heat sink effect of blood flow, which ensured
effective transmission and accumulation of electric field energy.
In our study, we found that the IRE ablation method destroyed
capillaries and cholangioles, but retained vital structures such as
the hepatic arteries, hepatic veins, bile ducts and perivascular
elastic and collagen fiber structure in the ablation zone (2,14-16).
Gross section examination and HE stain showed the structural
integrity of blood vessels and bile ducts in the IRE-ablated zone.
The two-dimensional ultrasound imaging also showed that blood
vessels were intact and that there was color flow angiography in
the blood vessels. Thus, IRE produced a more complete and safer
tumor ablation effect within the lesion area and did not damage the
integrity and basic function of blood vessels in the target
area.
Acknowledgements
This study was supported by the Key Program of
National Natural Science Foundation of China (NSFC 50637020).
References
|
1
|
Rubinsky B: Irreversible electroporation
in medicine. Technol Cancer Res Treat. 6:255–260. 2007. View Article : Google Scholar
|
|
2
|
Davalos RV, Mir IL and Rubinsky B: Tissue
ablation with irreversible electroporation. Ann Biomed Eng.
33:223–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weaver JC: Electroporation: A dramatic,
nonthermal electric field phenomenon. Proceedings of the First
World Congress for Electricity and Magnetism in Biology and
Medicine. Academic Press; Lake Buena Vista, Florida: 1992
|
|
4
|
Weaver JC and Chizmadzhev YA: Theory of
electroporation: a review. Bioelectrochem Bioenerg. 41:135–160.
1996. View Article : Google Scholar
|
|
5
|
Weaver JC: Electroporation of cells and
tissues. IEEE Trans Plasma Sci IEEE Plasma Sci Soc. 28:24–33. 2000.
View Article : Google Scholar
|
|
6
|
Neumann E and Rosenheck K: Permeability
changes induced by electric impulses in vesicular membranes. J
Membr Biol. 10:279–290. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crowley JM: Electrical breakdown of
biomolecular lipid membranes as an electromechanical instability.
Biophys J. 13:711–724. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mir LM: Nucleic acids
electrotransfer-based gene therapy (electrogenetherapy): Past,
current and future. Mol Biotechnol. 43:167–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neumann E, Schaefer-Ridder M and Wang Y:
Gene transfer into mouse lyoma cells by electroporation in high
electric fields. EMBO J. 1:841–845. 1982.PubMed/NCBI
|
|
10
|
Sersa G, Miklavcic D, Cemazar M, Rudolf Z,
Pucihar G and Snoj M: Electrochemotherapy in treatment of tumours.
Eur J Surg Oncol. 34:232–240. 2008. View Article : Google Scholar
|
|
11
|
Lee EW, Chen C, Prieto VE, Dry SM, Loh CT
and Kee ST: Advanced hepatic ablation technique for creating
complete cell death: irreversible electroporation. Radiology.
255:426–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daniels C and Rubinsky B: Electrical field
and temperature model of nonthermal irreversible electroporation in
heterogeneous tissues. J Biomech Eng. 131:0710062009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maor E, Ivorra A and Rubinsky B: Non
thermal irreversible electroporation: novel technology for vascular
smooth muscle cells ablation. PLoS One. 4:e47572009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rubinsky B, Onik G and Mikus P:
Irreversible electroporation: a new ablation modality - clinical
implications. Technol Cancer Res Treat. 6:37–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maor E, Ivorra A, Leor J and Rubinsky B:
The effect of irreversible electroporation on blood vessels.
Technol Cancer Res Treat. 6:307–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee EW, Loh CT and Kee ST: Imaging guided
percutaneous irreversible electroporation: ultrasound and
immunohistological correlation. Technol Cancer Res Treat.
6:287–294. 2007. View Article : Google Scholar : PubMed/NCBI
|