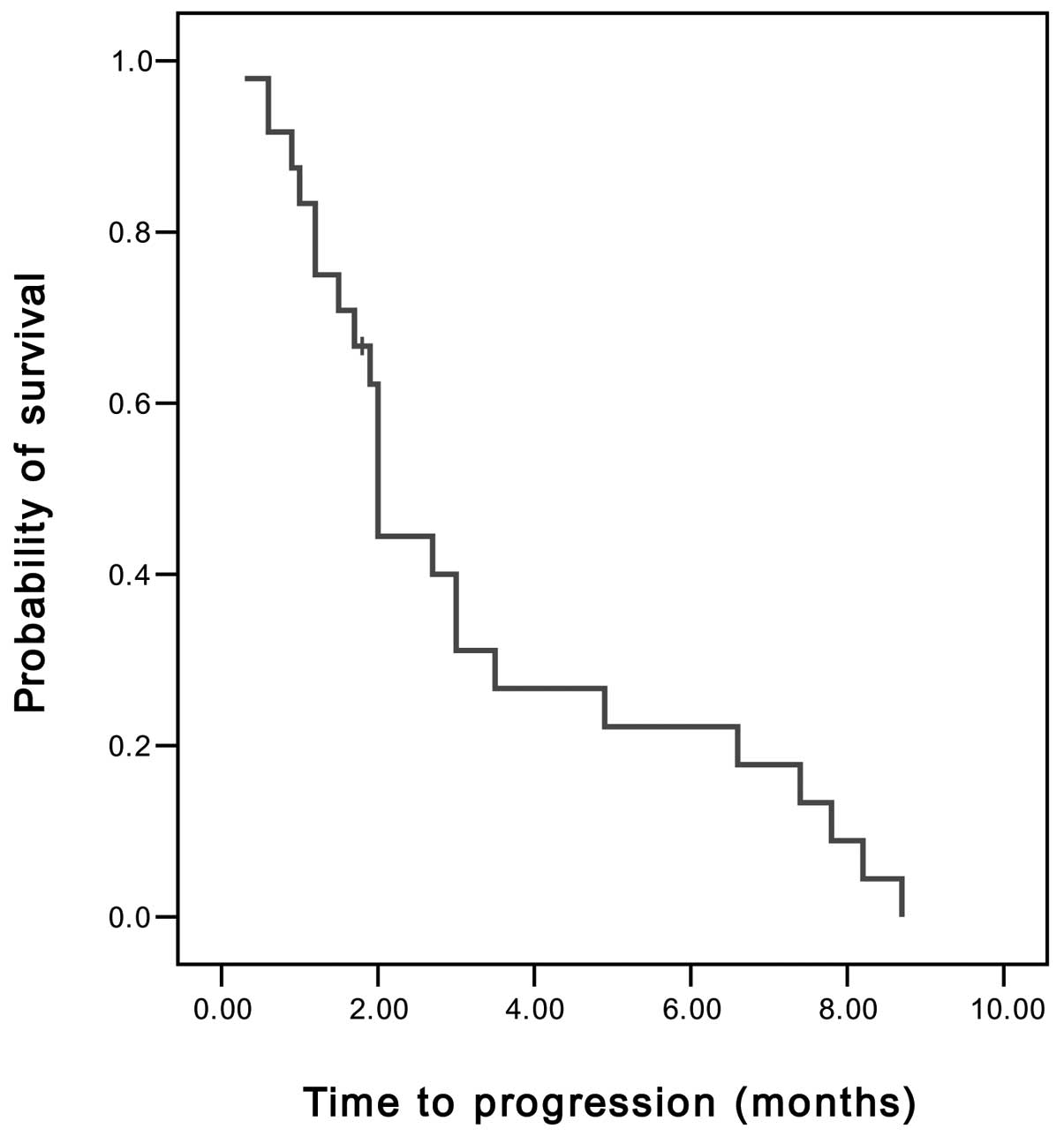
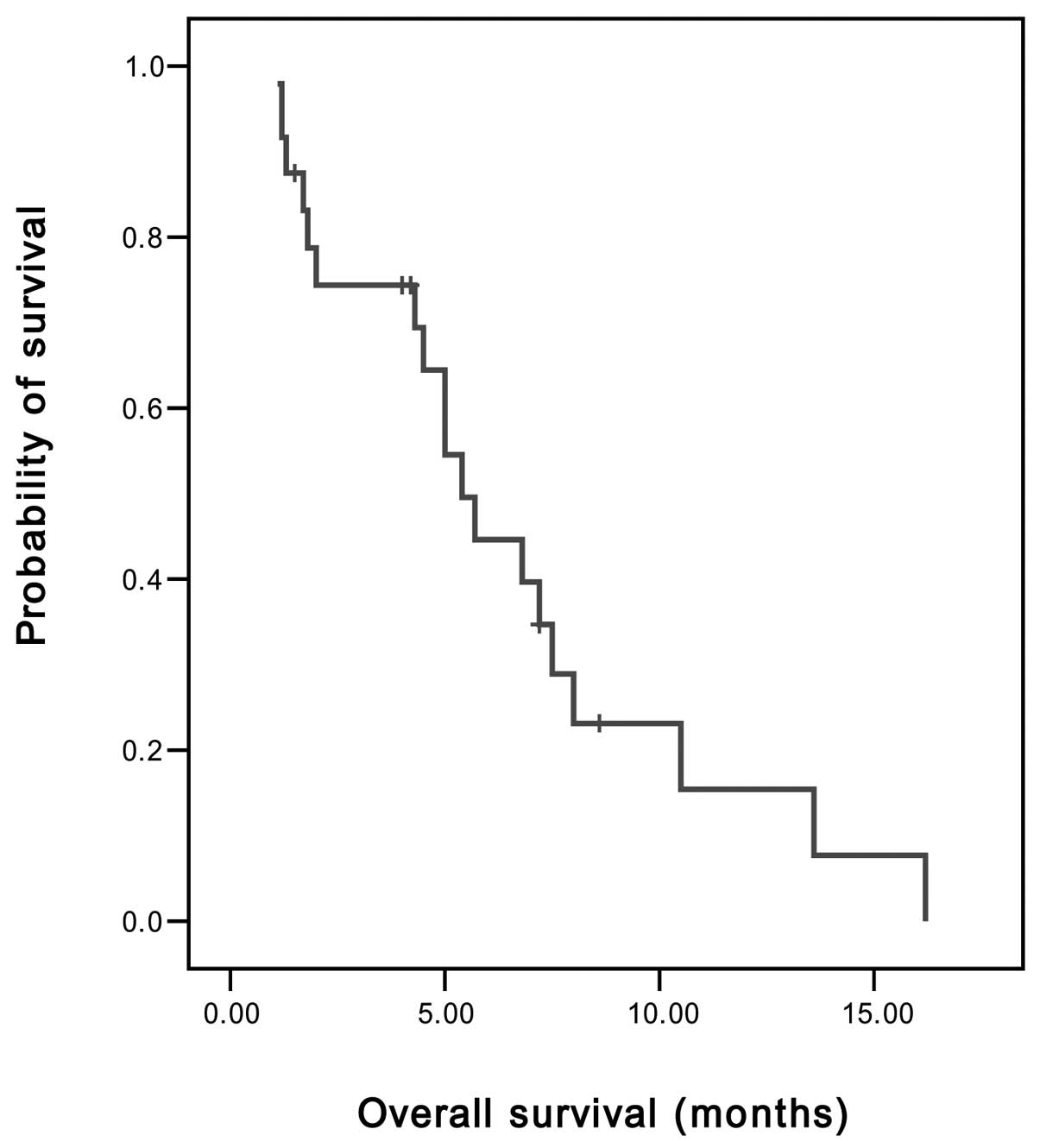
Spandidos Publications style
Kim
JH, Kim HS, Han AR, Moh IH, Chung DC, Choi DR, Jang HJ, Kim JB, Yang DH, Lee SI, Lee SI, et al: Irinotecan, leucovorin and 5‑fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer. Oncol Lett 4: 751-754, 2012.
APA
Kim
, J.H., Kim, H.S., Han, A.R., Moh, I.H., Chung, D.C., Choi, D.R. ... Zang, D.Y. (2012). Irinotecan, leucovorin and 5‑fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer. Oncology Letters, 4, 751-754. https://doi.org/10.3892/ol.2012.782
MLA
Kim
, J. H., Kim, H. S., Han, A. R., Moh, I. H., Chung, D. C., Choi, D. R., Jang, H. J., Kim, J. B., Yang, D. H., Lee, S. I., Zang, D. Y."Irinotecan, leucovorin and 5‑fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer". Oncology Letters 4.4 (2012): 751-754.
Chicago
Kim
, J. H., Kim, H. S., Han, A. R., Moh, I. H., Chung, D. C., Choi, D. R., Jang, H. J., Kim, J. B., Yang, D. H., Lee, S. I., Zang, D. Y."Irinotecan, leucovorin and 5‑fluorouracil (modified FOLFIRI) as salvage chemotherapy for frail or elderly patients with advanced gastric cancer". Oncology Letters 4, no. 4 (2012): 751-754. https://doi.org/10.3892/ol.2012.782