Introduction
The VX2 tumor cell line originated from Shope
virus-induced human papilloma-derived squamous cell carcinoma and
was established after 72 transplantation passages (1). The VX2 tumor cell line may be used to
establish animal tumor models. The rabbit VX2 tumor is induced in
the skin by Shope virus and is characterized by easy inoculation,
rapid growth, and significant invasiveness. These factors are
similar to the characteristics of human squamous cell carcinoma
(2).
SonoVue, a novel ultrasound reagent, forms
microbubbles after resolution (3).
It contains sulfur hexafluoride and is coated with a stable
phospholipid sheath. The diameter of SonoVue microbubbles is 5 to 7
μm, similar to the size of normal human red blood cells.
This enables them to enter microvessels, including new tumor
vessels (4). Contrast-enhanced
ultrasonography (CEUS) was developed on the basis of the
pharmaceutical and ultrasonic characteristics of SonoVue
microbubbles and may improve the ultrasonic exhibition of
microvasculature.
In the current study, we performed CEUS on VX2
tumors and used a CD34 antibody (a vascular endothelial marker) to
label tumor microvessel endothelial cells. We then compared the
ultrasonic results of tumor microvessel perfusion and pathological
results of tumor angiogenesis. The goal was to investigate the
value of CEUS in diagnosing soft tissue tumors and determine the
correlation between tumor microvessel perfusion and
distribution.
Materials and methods
Animals, main instruments and agents
A total of 17 healthy New Zealand white rabbits,
aged from two to three months and weighing from 2.0 to 2.8 kg, were
obtained from the Experimental Animal Department, China Medical
University (Shenyang, China). The Medical Imaging Institute of
China Medical University provided VX2 tumor tissues, stored at
−198°C. All procedures were conducted with the approval of the
Animal Research Committee at China Medical University.
An Acuson Siemens Sequoia-512 color Doppler
sonographic system (Siemens, Erlangen, Germany), equipped with
coherent pulse sequence (CPS) settings for small organs and 15L8Ws
transducers (frequency range of 7–12 MHz) were used for
conventional and contrast ultrasound scans. A HHW21.600 isothermal
hot water cylinder (Hangzhou Aipu Equipment Co. Ltd., Zhejiang,
China) was used to thaw the VX2 tumor tissue.
The ultrasound contrast agent SonoVue (Braco, Milan,
Italy) was used for the contrast ultrasound scans. A Sumianxin
injection (1 ml/ampul), produced by the military Veterinary
Institute of the Academy of Military Medical Sciences and composed
of amine thiazole xylene, ethyl diamine tetraacetic acid,
hydrochloric acid and dihydroetorphine was used to anesthetize the
rabbits. CD34 monoclonal antibody was used for S-P
immunohistochemical staining.
Preparing the VX2 tumor models in
rabbits
The VX2 tumor tissues were thawed at 36 to 37°C for
15 min. They were then placed in a sterile plate containing normal
saline and were cut into 1-mm3 pieces to prepare the
tumor tissue suspension. One rabbit was injected with a Sumianxin
injection (0.1 ml/kg body weight) for local anesthesia, then
injected with 0.5 ml of VX2 tumor tissue suspension in the vastus
medialis muscle of both hind legs. This procedure prepared the
animal model of the VX2 tumor. The developed VX2 tumor was removed
surgically 21 days after inoculation. The peripheral tumor tissue,
which had grown rapidly, was cut into ∼1-mm3 pieces and
suspended in 5 ml normal saline. The tumor tissue suspension was
then injected into the vastus medialis muscle of both hind legs
(0.5 ml for each side) of the remaining 16 rabbits after injecting
local anesthesia.
Conventional and contrast-enhanced
ultrasonography
Ultrasonography was performed on days 14, 21, 28,
and 35 after tumor inoculation. At each time point, three rabbits
were fixed on an experiment table in the supine position following
intramuscular anesthesia with Sumianxin injection (0.1 ml/kg body
weight). Rabbit fur at the hind legs, inguinal regions and lower
abdomen were removed for ultrasonic observation.
Conventional ultrasonography was performed to
observe the tumors and select the maximum tumor section for
contrast ultrasonic observation. The SonoVue injection was resolved
in normal saline at a ratio of 1:5 ml and agitated for 20 sec for
complete dissolution. Each rabbit underwent bolus injection of 0.3
ml of SonoVue solution and a quick injection of 5 ml of normal
saline via the ear margin vein through a three-way tube. Coherent
pulse sequence and tissue equalization techniques were performed
with a low mechanical index of 0.19, a frame rate of 200 frames/sec
and a trigger time (ΔT) of 125 msec. Dynamic images following
SonoVue injection were recorded until the contrast agent
diminished.
Image processing and data analysis
Dynamic images were reviewed by two ultrasound
physicians to record the enhancement patterns of the tumors.
Enhanced areas at the margin and inside the VX2 tumors were set as
regions of interest (ROIs) by ACQ software to develop
time-intensity curves of all ROIs and measure arrival time (AT),
time to peak (TTP) and peak intensity (PI) for different areas of
the tumor. Adjacent vessels were avoided during measurement. Three
intratumoral and three marginal ROIs were selected to calculate
average values.
Histopathological examination
Following CEUS, the rabbits were euthanized using
air embolization. VX2 tumors were excised, fixed in a neutral
formalin solution for 24 h and embedded in paraffin. Tumor sections
were prepared for hematoxylin-eosin (HE) staining and S-P
immunohistochemical staining with CD34 monoclonal antibody to label
the tumor microvessels. HE-stained sections and CD34-stained
microvessels (in brown) were observed under a Nikon light
microscope (Japan) by a pathologist. The correlations between the
histopathological findings of the tumor morphology and the
microvessel distribution and CEUS findings were analyzed.
Results
VX2 tumor formation in rabbits
Of the 16 rabbits, four died at 1, 3, 6 and 8 days
after tumor inoculation. A total of 38 tumors developed in the
remaining 12 rabbits; nine had multiple lesions, whereas three had
a single lesion. The tumors grew quickly at 14 days after
inoculation. The size of the tumors ranged from 1.12×1.35 cm to
10.85×7.80 cm.
Conventional ultrasonic findings of VX2
tumors
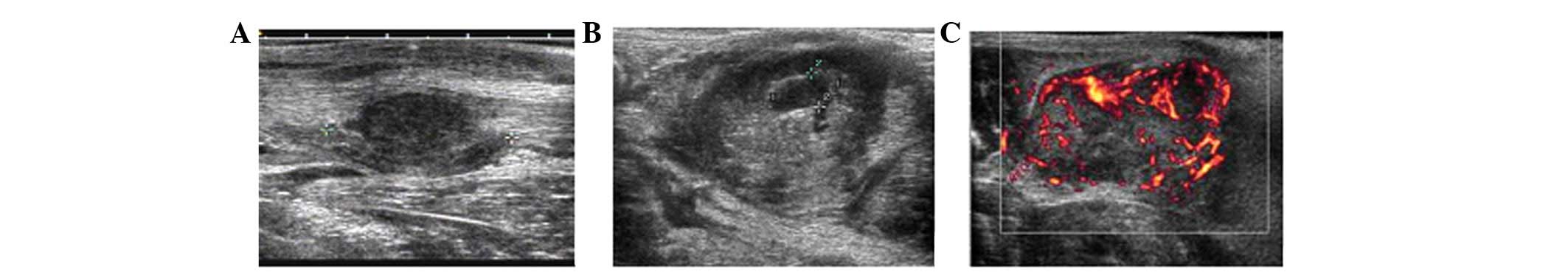
Along with tumor growth, the internal echoes of
tumors on two-dimensional ultrasonography changed gradually. At 14
days after tumor inoculation, homogeneous hypoechoes were observed
in the tumors (Fig. 1A). At 21 days
after tumor inoculation, internal echoes were heterogeneous,
changing from scattered dotted hyperechoes to central dotted
hypoechoes (Fig. 1B). At 35 days
after tumor inoculation, residual separate echoes were observed,
but only in the remaining cysts. The diameter of the minimal tumor
with echo changes was only 2 cm. Color Doppler flow imaging and
color Doppler energy imaging revealed peripheral distribution of
irregular blood branches in VX2 tumors (Fig. 1C).
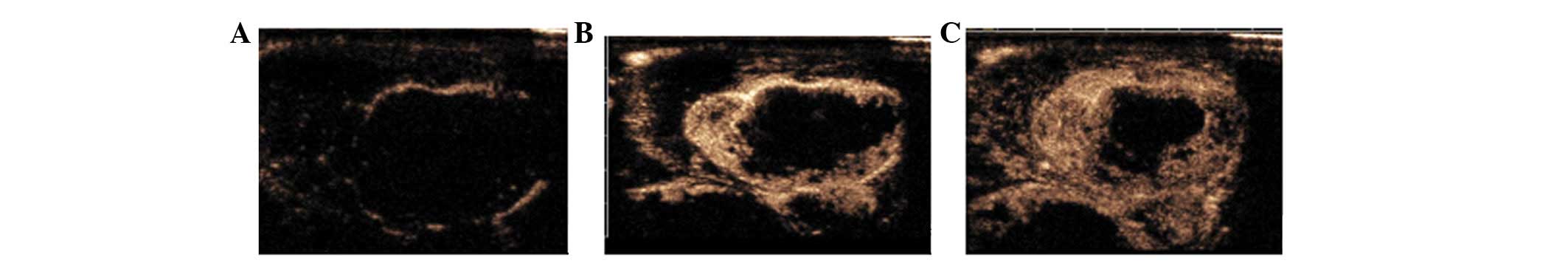
Contrast-enhanced ultrasonography
CEUS showed marked peripheral enhancement of the VX2
tumors at the early arterial phase and transient increased
enhancement, then quick washout of contrast agents. All tumors
showed rim enhancement at ∼6 sec after the contrast agent was
injected (Fig. 2A) and a little
intratumoral enhancement at 12 to 18 sec (Fig. 2B and C). Tumor contours were clear
as the surrounding muscles showed no enhancement. The central areas
of the tumors with hypoechoes on conventional ultrasonography
showed irregular weak enhancement, whereas the anechoic necrotic
areas showed no enhancement.
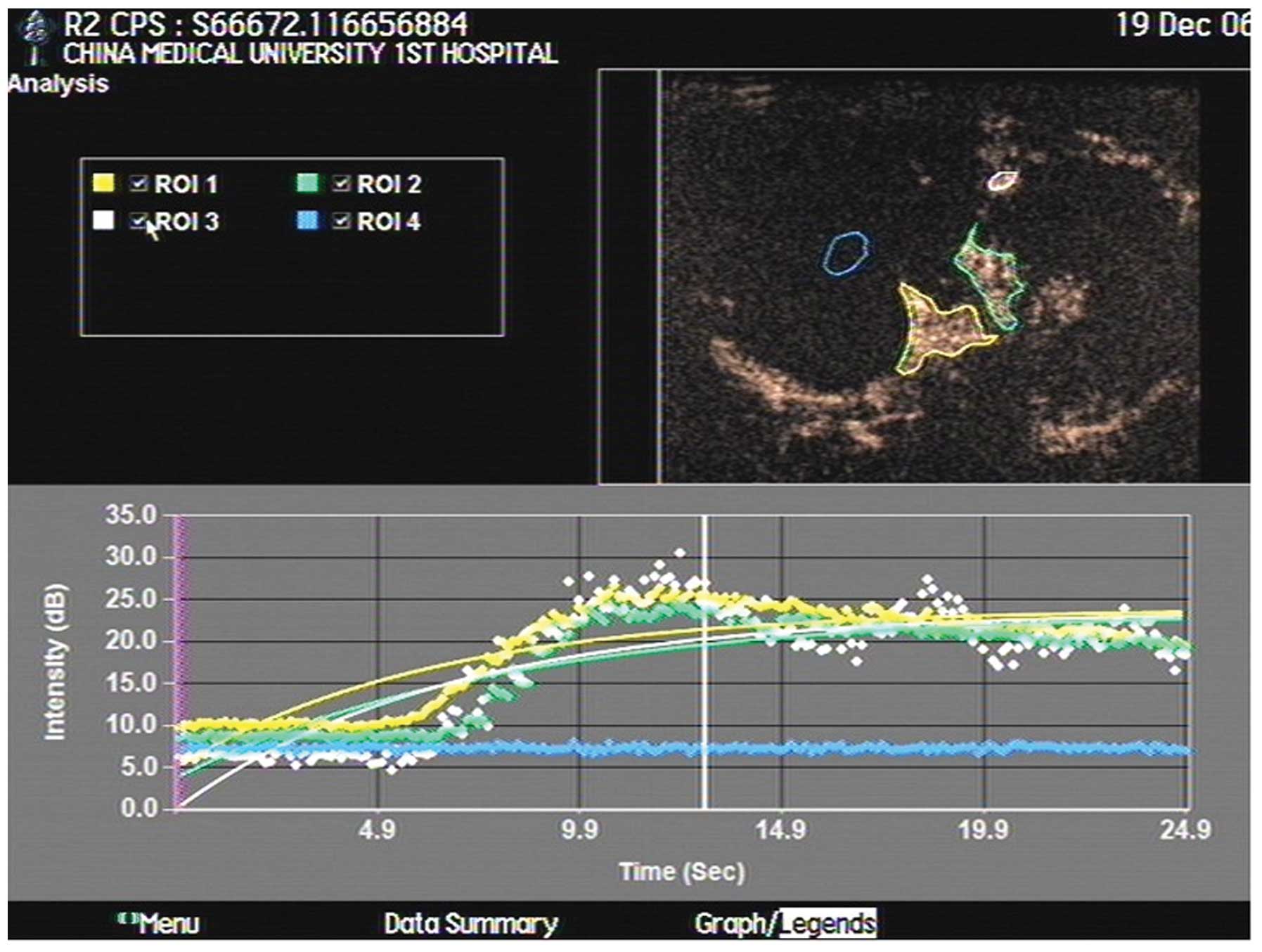
CEUS time-intensity curves of VX2 tumors showed a
subdued uplift with a blunt peak, indicating slow enhancement, and
a subdued decrease lasting ∼40 sec, on average, indicating slow
washout of the contrast agent (Fig.
3). As analyzed by the ACQ software, BI (base intensity) was
8.61±5.89 db, A (slope of the ascending curve) was 9.7±0.3 db, AT
was 6.91±1.01 sec, TTP was 11.3±3.5 sec and PI was 9.85±8.39
db.
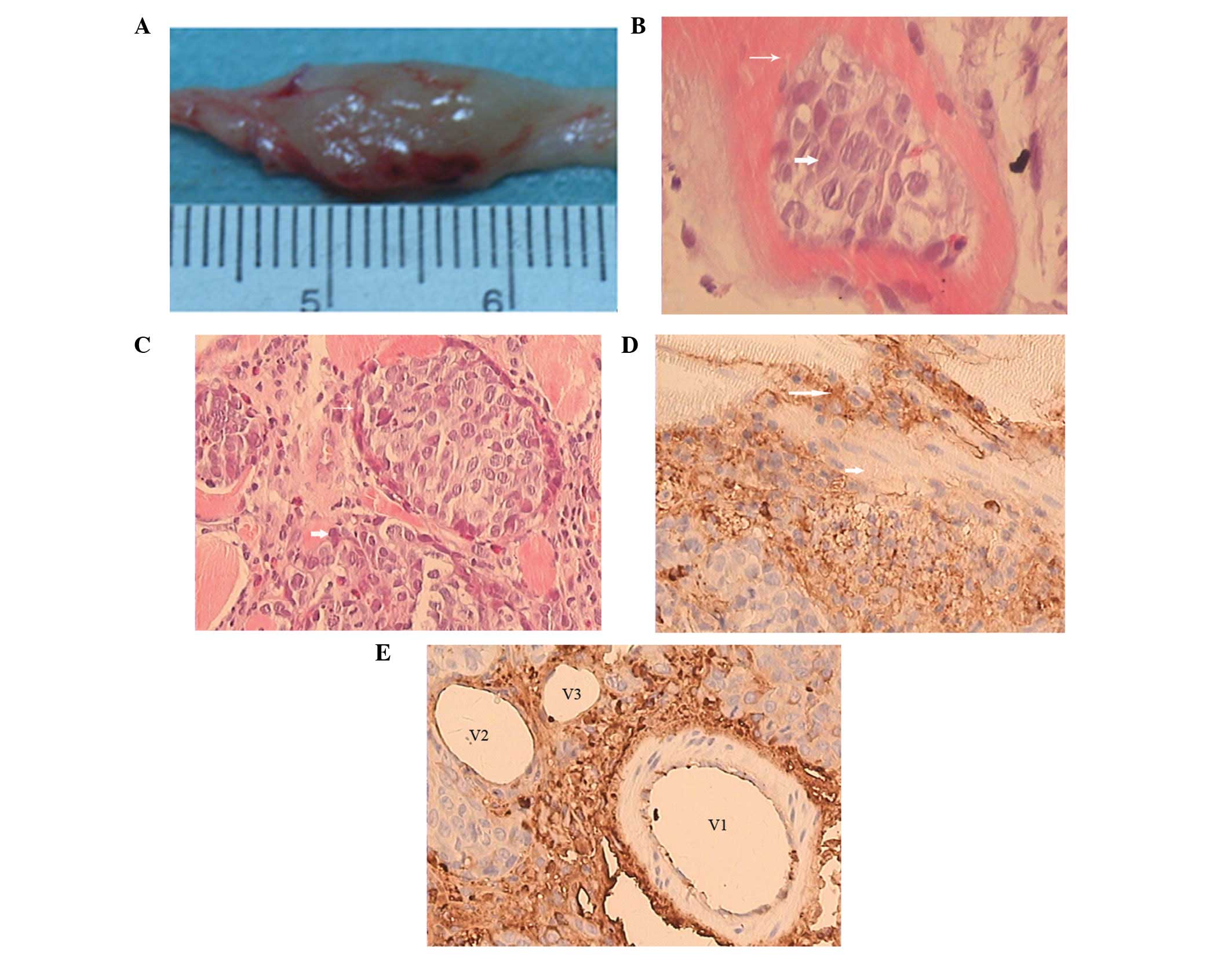
Pathological findings
The surface of the tumors had a high tension, with
vessel dilation and a pseudocapsule. The tumors were white and
cystic with necrosis in the central area and unclear boundaries
with the surrounding normal muscles (Fig. 4A).
On microscopic examination, the VX2 tumor cells that
exhibited large nuclei with pathological mitosis were nested or
scattered in striated muscles (Fig.
4B). Abundant immature capillaries and fibrosis tissues were
distributed in the tumor nests (Fig.
4C). Degenerating tumor cells with no nuclei were observed in
necrotic areas at 28 days after tumor inoculation. CD34-positive
vascular endothelial cells (in brown) were detected in peripheral
areas of the VX2 tumors and interstitial tissues (Fig. 4D and E).
Correlation between pathological and CEUS
findings
The scope of the tumor enhancement on CEUS imaging
was consistent with the distribution of CD34-positive vessels. All
VX2 tumors showed the same enhancement pattern; that is, quick
perfusion and transient enhancement at the arterial phase, with no
capillary or venous phase. The enhancement first appeared in the
peripheral regions of the tumors, then quickly showed in internal
reticular vessels. No complete intratumoral enhancement was
observed despite the tumor size and the presence of necrosis.
Immunohistochemical staining revealed that the CD34-positive cells
were scattered or clustered mainly in the muscles adjacent to
vessels and peripheral tumor tissues, but could not form normal
vessel lumens. No CD34-positive cells were observed in necrotic
areas.
Discussion
The invasion and metastasis routes of VX2 tumors are
similar to those ofhuman head and neck squamous cell and
hepatocellular carcinomas (5,6).
Animal models of VX2 tumors, therefore, have commonly been used to
study these cancers (7,8). When planted to the bottom of culture
bottles, VX2 tumor cells grew in a single layer with partially
overlapping areas. Multinuclear giant cells were also observed.
Cells proliferated quickly with 58 to 62 hypotriploid chromosomes.
The success rates of inoculation in both athymic nude mice and
homogeneous rabbits were 100% (9).
VX2 tumors over-express multiple matrix metalloproteins during the
invasion process (10). The methods
used, therefore, represent a good model of tumor metastasis and
invasion.
In this study, we implanted VX2 tumors in the hind
leg muscles of rabbits. Pathological examination confirmed the
intramuscular distribution of tumor cells, indicating that VX2
tumor-bearing animal models were established successfully. Tumors
grew quickly in the first three weeks and showed necrosis and cysts
at advanced stages, which were consistent with VX2 tumor
characteristics (11,12). Due to the similarities between VX2
tumor and human carcinomas, determining the acoustic
characteristics of VX2 tumors may contribute to the diagnosis of
human tumors.
Tumor growth relies on tumor feeding arteries.
Forming tumor vessels is a complex process, which currently cannot
be assessed directly or effectively. Tumor vessel endothelial cells
may be labeled using immunohistochemistry. For example, the CD34
antibody is expressed in the cytoplasm of active vessel endothelial
cells (13). Assessing the
distribution and density of CD34-positive vessels aids the
determination of tumor angiogenesis. Tumor specimens, however,
cannot be used to evaluate the activity of new tumor vessels. In
this study, we used CEUS, a non-invasive imaging method, to
demonstrate tumor microvessel perfusion and assess tumor
angiogenesis in vivo.
Using color Doppler flow and energy imaging to
assess the distribution of tumor vessels is based on color and the
energy signals of the tumor vessels. As it is limited by spatial
resolution, it is prone to disturbance and cannot fully detect
flows in microvessels. The lipid membrane structure, good in
vivo stability of SonoVue, a second generation contrast agent,
and the combination of acoustic features and second harmonic
imaging improve the accuracy of CEUS. Contrast-enhanced gray-scale
ultrasonography with a low mechanic index, a real-time imaging
method with high spatial resolution, shows enhancements similar to
that of computed tomography (CT) (14).
In this study, we assessed tumor vessel perfusion on
CEUS and analyzed its correlation with tumor morphology and
CD34-positive tumor vessel distribution. CEUS images of VX2 tumors
demonstrated marked enhancements at the tumor margins. Pathological
examination also revealed that CD34-positive regions increased and
accumulated in the muscles close to normal vessels, suggesting that
new tumor supply vessels are formed from the sites of existing
vessels and provide nutrition to the tumor cells. Recent studies
have found that tumor cells cannot live without sufficient oxygen
and nutrition or protection from toxic molecules. Oxygen
disseminates to capillaries from a distance of 150 to 200 μm
to tumor cells (15). To continue
growing, tumor cells need continuous nutrition supplied by new
vessels. New vessels are formed by either budding or non-budding.
Budding develops new capillaries from existing vessels; in
non-budding, tumor cells proliferate under the mediation of
existing vascular endothelial cells, divide extensively and fuse to
form new vessels.
Using CEUS, we found that all VX2 tumors exhibited
the same enhancement pattern: quick perfusion and transient
enhancement at the arterial phase, with no capillary or venous
phase. Enhancement was first shown in the peripheral regions of
tumors, then quickly showed in internal reticular vessels. No
complete intratumoral enhancement was observed despite the tumor
size and the presence of necrosis. Other researchers have
transplanted VX2 tumor cells to the liver or kidneys in rabbits and
also found tumor periphery-dominant enhancement on CEUS (16). We labeled tumor vessel endothelial
cells by immunohistochemistry and found increased new vessels in
the peripheral areas of tumors. Their basement membranes, however,
were incomplete and did not form normal vessel lumens, presenting
as scattered or clustered CD34-positive areas. In addition, these
CD34-positive areas around arteries were more abundant than those
found around veins, suggesting that the transient enhancement of
VX2 tumors at arterial phase is related to new arteries formed in
tumors.
Pathological examination confirmed the unenhanced
tumor tissues as coagulation necrosis with cell apoptosis and
revealed no CD34-positive new tumor vessels. This local necrosis
differs from the liquefaction necrosis shown on conventional
ultrasonography. Local necrosis presents homogenous solid
hypoechoes, whereas liquefaction necrosis presents liquid or dotted
echoes. This difference is more marked when assessing tumor vessel
perfusion on CEUS. Pathological examination revealed that
hemorrhagic necrotic regions existed in tumor tissues, but their
acoustic impedance difference might not be large enough to be
reflected using ultrasound. This minimal difference may be detected
only on CEUS; therefore, the CEUS findings accurately reflect tumor
vessel perfusion and indicate that new vessels in tumors are
forming and distributing.
When analyzing the CEUS findings of solid tumors
with a single blood supply using the ACQ software, we should pay
attention to the length of the entire tumor enhancement phase
(reflecting the distribution of arteries or veins and the maturity
of vessels) and to the enhancement pattern and intensity of
peripheral tumor vessels (relating to forming and distributing new
vessels). For the tumor areas with weak or no enhancement, the CEUS
findings should be analyzed, taking into account the pathological
findings. Not all solid areas present homogenous enhancement; not
all unenhanced areas are necrotic areas. The characteristics of the
contrast agent determine whether CEUS reflects only the
distribution of tumor microvessels. Clinical studies have shown
that the distribution and quantity of tumor vessels determine the
tumor enhancement pattern. Illustrating tumor blood supply aids the
differentiation of benign and malignant solid tumors.
Whether the transplanted VX2 tumors mimic the
angiogenesis of original or metastatic human tumors fully needs to
be investigated. Room for improvement exists in selecting the
maximal section, the influence of the contrast agent dose, time
recording, the stability of operators and the selection of
ROIs.
In conclusion, CEUS combined with administering the
contrast agent SonoVue may be used to assess angiogenesis and blood
perfusion in VX2 tumors in rabbits. Investigating tumor
angiogenesis with CD34 immunohistochemical staining is the
pathological basis for CEUS on VX2 tumors. The enhancement pattern
of VX2 tumors is consistent with tumor vessel distribution.
References
|
1.
|
E GeorgesF BreitburdN JibardG OrthTwo
Shope papillomavirus-associated VX2 carcinoma cell lines with
different levels of keratinocyte differentiation and
transplantabilityJ Virol552462501985
|
|
2.
|
C LiW WangH DingB HuangJ CaoF MaoZ JiValue
of contrast-enhanced sonography in the diagnosis of peripheral
intrahepatic cholangiocarcinomaJ Clin
Ultrasound39447453201110.1002/jcu.2079721626512
|
|
3.
|
E DomenechD Berná-Serna JdeL PoloM ReusD
Berná-Mestre JdeM CanterasEffect of SonoVue on the synovial
membrane in rabbit kneesJ Ultrasound Med3012411246201121876095
|
|
4.
|
S TaoZ QinW HaoL YongquanY LanhuiY
LeiUsefulness of gray-scale contrast-enhanced ultrasonography
(SonoVue®) in diagnosing hepatic alveolar
echinococcosisUltrasound Med
Biol3710241028201110.1016/j.ultrasmedbio.2011.04.01421640477
|
|
5.
|
KC LeeWK MoonJW ChungAssessment of lymph
node metastases by contrast-enhanced MR imaging in a head and neck
cancer modelKorean J
Radiol8914200710.3348/kjr.2007.8.1.917277558
|
|
6.
|
A SonodaN NittaA Nitta-SekoTime-course
studies of implanted rabbit VX2 liver tumors to identify the
appropriate time for starting hepatic arterial embolization in
animal modelsOncology809296201110.1159/00032876321677452
|
|
7.
|
AA DünneR MandicA RamaswamyLymphogenic
metastatic spread of auricular VX2 carcinoma in New Zealand white
rabbitsAnticancer Res22327332792002
|
|
8.
|
AA DünneA SchmidtC KuropkatA RamaswamyS
SchulzJA WernerThe auricular VX2 carcinoma--an animal model for
sentinel node conceptIn Vivo17457461200314598609
|
|
9.
|
XF LiuLR RenGY SuEstablishment and
characterization of a rabbit tumor cell line VX2Zhonghua Bing Li
Xue Za Zhi346616632005(In Chinese)
|
|
10.
|
R MandicAA DünneN EikelkampExpression of
MMP-3, MMP-13, TMP-2 and TMP-3 in the VX2 carcinoma of the New
Zealand white rabbitAnticancer Res2232813284200212530076
|
|
11.
|
J LiB DongX YuC LiUltrasonographic
portography with low mechanical index gray-scale imaging in hepatic
VX2 tumorUltrasound Med
Biol32641647200610.1016/j.ultrasmedbio.2006.01.00816677923
|
|
12.
|
H MaruyamaS MatsutaniH SaishoN KamiyamaY
MineT HirataM SasamataSonographic shift of hypervascular liver
tumor on blood pool harmonic images with definity: time-related
changes of contrast-enhanced appearance in rabbit VX2 tumor under
extra-low acoustic powerEur J
Radiol566065200510.1016/j.ejrad.2005.04.004
|
|
13.
|
DL SimmonsAB SatterthwaiteDG TenenB
SeedMolecular cloning of a cDNA encoding CD34, a sialomucin of
human hematopoietic stem cellsJ Immunol14826727119921370171
|
|
14.
|
N XingZL CaiSH ZhaoL YangBX XuFL WangThe
use of CT perfusion to determine microvessel density in lung
cancer: Comparison with FDG-PET and pathologyChinese J Cancer
Res23118122201110.1007/s11670-011-0118-z23483098
|
|
15.
|
IJ FidlerLM EllisNeoplastic angiogenesis -
not all blood vessels are created equalN Engl J
Med351215216200410.1056/NEJMp04808015254281
|
|
16.
|
S ElagozR EgilmezA KoyuncuA
MuslehiddinogluS AriciThe intratumoral microvessel density and
expression of bFGF and nm23-H1 in colorectal cancerPathol Oncol
Res122127200610.1007/BF0289342716554912
|