Introduction
Resveratrol (Res), chemically named
3,4′,5-trihydroxystilbene, is a non-flavone polyphenolic compound
which mainly exists in plants, including grapes, Veratrum
nigrum and giant knot-weed rhizome. It was first extracted from
European Resveratrol (Res). Res, a type of phytoalexin, is
extensively present in nature and is produced by plants under
conditions of fungal infection, ultraviolet radiation or pathology.
Res possesses multiple bioactivities, including antioxidation,
antiinflammation, estrogen-like activity, growth inhibition,
immunoregulation, chemoprevention and antitumor activity (1). Res blocks several processes of
carcinogenesis and has inhibitory effects on the initiation,
promotion and development of tumors (2). However, previous studies have shown
that the inhibitory effect of Res on cancer cells varies between
cancer types and that Res only inhibits the cell growth of certain
types of cancer (3,4). Res has an inhibitory effect on
leukemic osteosarcoma and lung, prostate, colon, pancreatic, liver
and breast cancer (5–10); however, its mechanism of action
remains unknown.
Angiogenesis is one of the prerequisites of the
proliferation and growth of tumor cells. During this process,
vascular endothelial growth factor (VEGF) functions as the most
significant vascular endothelial stimulating factor; VEGF
overexpression is correlated with patient survival rate and the
early relapse, infiltration and lymph node metastasis of tumors
(11–13). Osteosarcoma is a malignant invasive
disease which occurs among young individuals. The molecular
genetics of this medical condition have been elucidated over recent
years. The development of osteosarcoma is associated with
simultaneous changes in multiple genes, for instance, the
cooccurrence of mouse double minute 2 (MDM2) amplification and p53
deactivation promote the initiation and development of osteosarcoma
(14).
In the current study, the effects of Res on
osteosarcoma cell growth and VEGF expression were investigated and
the mechanism of action of Res was further explored.
Materials and methods
Cell culture
The human osteosarcoma cell line U20S was cultured
with 10% fetal bovine serum-containing DMEM in a 5% CO2
incubator at 37°C and then digested with 0.25% trypsin for
subculture.
Methyl thiazolyl tetrazolium (MTT)
assay
Cells in the logarithmic growth phase at a
concentration of 1.5×104 cells/ml (200 μl) were seeded
onto a 96-well plate for 24 h. Res (0, 10, 20 and 40 μmol/l) with a
purity of 99% (Sigma, St. Louis, MO, USA) was added, with four
wells for each group. The cells were cultured for 24, 48 and 72 h,
respectively. The culture medium was removed and 20 μl of MTT (5
g/l) was added to each well for a 4-h culture. The medium was
removed and dimethyl sulfoxide was added to dissolve the crystals.
The absorbance of the solution at 570 nm (A570 nm) was read and the
cell inhibition ratio was calculated as follows: cell inhibition
ratio = [(A570 nm value of the control group - A570 nm value of the
experimental group)/A570 nm value of the control group] x 100%.
Real-time polymerase chain reaction
(RT-PCR)
Based on GenBank (AF022375.1), the primer sequences
of human VEGF were designed using the Primer 5.0 software: 5′- CA
AGTG GTCCCAG G CTG CAC-3′ (upstream) and
5′-CGCGAGTGTGTGTTTTTGCAGG-3′ (downstream). GAPDH was taken as the
internal standard and the primer sequences were as follows:
5′-AAAGTGGATATTGTTGCCATC-3′ (upstream) and
5′-CAAATGAGCCCCAGCCTTCTCC-3′ (downstream). Syntheses were performed
by Sangon Biotech Co., Ltd. (Shanghai, China). The amplification
fragment length of GAPDH was 198 bp. The concentration of the VEGF
cDNA original templates was standardized according to that of the
GAPDH cDNA original templates and cDNA-free wells were used for
negative controls.
Total RNA was extracted with TRIzol reagent. A260
and A280 values were read on the ultraviolet spectrophotometer, RNA
was qualified using agarose gel electrophoresis and the rest of the
RNA was stored at −80°C. cDNA was reverse transcribed in line with
the manufacturer’s instructions (Shanghai Biology Engineering,
Shanghai, China) and the products were kept at −20°C. The reaction
system, with a volume of 30 μl, contained 1 μl of the
reverse-transcribed products, 1 μl of VEGF primers, 1 μl of GAPDH
primers, 3 μl of 10X bufer, 2 μl of 2.5 mmol/l dNTP and 2 units of
Taq polymerase. The amplification conditions consisted of a
pre-denaturation step at 94°C for 3 min, 35 cycles of 94°C for 30
sec, 55°C for 30 sec and 72°C for 1 min and a final extension step
at 72°C for 5 min. In the interest of quantitation, GAPDH and VEGF
were amplified at the same time. The amplified products were
observed following 1.5% agarose gel electrophoresis and ethidium
bromide (EB) staining. Total A value (average A value x band area)
was taken as the quantity of mRNA and VEGF mRNA expression and was
then calculated based on the A value ratio between VEGF and
GAPDH.
Western blot analysis
Cells in each group were collected, sonicated
slightly for 10 sec and centrifuged for total protein samples.
Concentrations were determined using a Bio-Rad DC protein assay kit
(Shanghai Biology Engineering). The protein sample (50 g) was
fractionated by 12% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane. The membrane was treated with skimmed milk for
2 h and incubated with rabbit antibody against human MDM2 (1:200
dilution) at 37°C for 2 h. Following washing in TBST for 30 min,
the membrane was incubated with goat anti-rabbit antibody (1:1,000
dilution) at 37°C for 1 h. Following washing in TBST for 30 min,
the membrane was colored and images were captured.
Statistical analysis
Data are presented as mean ± SD and were analyzed
using the SPSS 12.0 software. P<0.05 was considered to indicate
a statistically significant difference.
Results
Inhibitory effect of Res on U20S
The MTT assays revealed that Res had an inhibitory
effect on U20S at each concentration; the inhibitory effect was
strengthened with the increase of the Res concentration as well as
with the prolongation of action time, showing time- and
dose-dependent characteristics (Table
I).
 | Table I.The inhibitory effect of Res on U20S
cells. |
Table I.
The inhibitory effect of Res on U20S
cells.
| 24 h
| 48 h
| 72 h
|
|---|
| Res dose
(μmol/l) | OD570 (mean ±
SD) | IR (%) | OD570 (mean ±
SD) | IR (%) | OD570 (mean ±
SD) | IR (%) |
|---|
| 0 | 1.2011±0.3112 | - | 1.4407±0.2312 | - | 1.6389±0.2234 | - |
| 10 |
1.0232±0.1503a | 3.20 |
0.7890±0.2312a | 6.71 | 0.6784±0.4321 | 13.52 |
| 20 |
0.9756±1.2724a | 23.32 | 0.6745±1.51b | 43.21 |
0.5462±0.3424a | 54.62 |
| 40 | 0.7567±1.34a | 46.72 | 0.5432±1.48b | 65.08 |
0.4321±1.2613b | 87.71 |
RT-PCR
The purity of total RNA was assayed using the
ultraviolet spectrophotometer. The results revealed that all the
A260/A280 ratios fell between 1.9 and 2.0, satisfying the
requirements for the experiment.
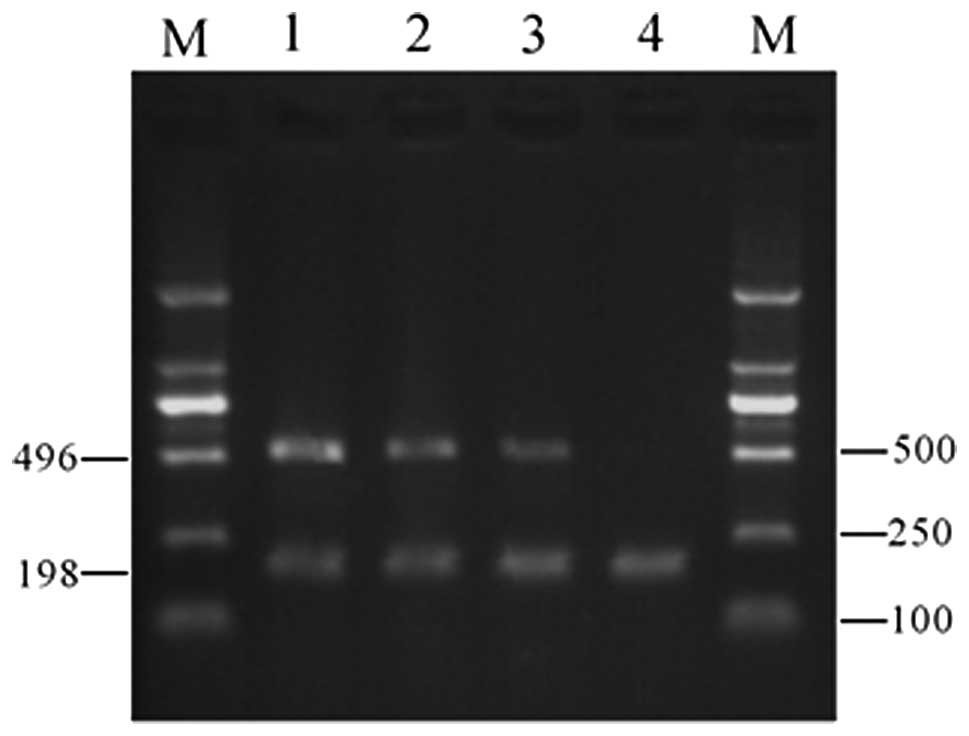
Gel electrophoresis revealed that VEGF expression in
the 10 μmol/l Res group was significantly lower than that in the
control group; in the 40-μmol/l Res group, VEGF expression was not
detected (Fig. 1). These results
indicate that Res inhibits VEGF mRNA expression within a certain
concentration range.
Western blot analysis
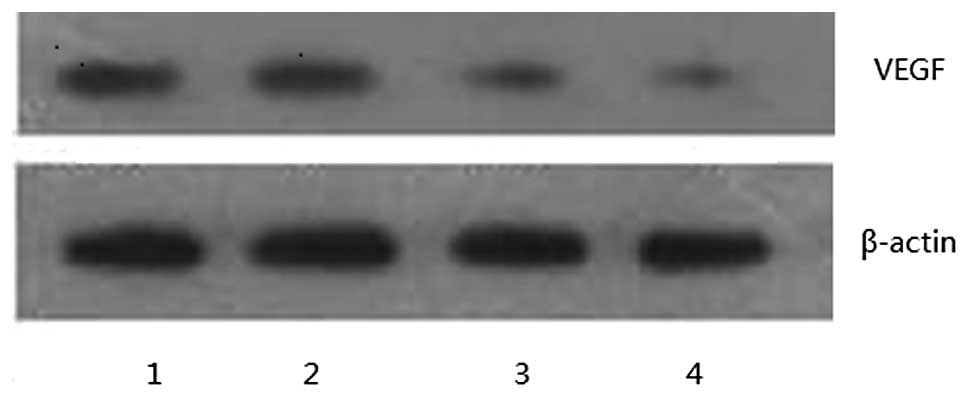
Western blot analyses demonstrated that VEGF
expression in the 10- and 20-μmol/l Res groups was significantly
lower than that in the control group and no VEGF expression was
detected in the 40 μmol/l Res group (Fig. 2). These results were consistent with
the RT-PCR results, indicating that Res has an inhibitory effect on
VEGF protein expression in U20S.
Discussion
Res is a non-flavone polyphenolic compound which is
extensively present in plants and is abundant in red wines. In
recent years, Res has attracted much attention from researchers and
has become a hotspot in the prevention of tumors. Res inhibits
tumor cell proliferation by reducing the number of G-phase cells
and blocking S-phase cells (15).
Res shows a bidirectional effect on the cell proliferation of
prostate, breast, pancreatic and esophageal cancer, namely, a low
concentration of Res promotes cell growth while a high
concentration inhibits the growth (15–18).
Osteosarcoma is a primary malignant bone tumor with
a strong invasive power, which occurs most commonly among young
individuals. This disease is characterized by organ metastasis at
an early stage and has a high relapse rate following surgery.
Therefore, the study of the mechanisms of osteosarcoma invasion and
metastasis, as well as other related factors, have become hot
topics in the field of osteology. The proliferation, invasion and
metastasis of tumor cells, as well as tumor relapse, are correlated
with the interactions among multiple factors, in which angiogenesis
is a prerequisite. VEGF and basic fibrolast growth factor perform
significant roles in angiogenesis (19,20).
In the present study, the MTT assays revealed that
Res has an inhibitory effect on osteosarcoma cell proliferation and
that this effect is strengthened with the increase of Res
concentration as well as with the prolongation of Res action time,
showing time- and dose-dependent characteristics. The RT-PCR and
western blot analysis performed in this study further confirmed
that Res inhibits VEGF expression within a certain concentration
range in U20S cells. These results suggest that Res exerts its
anti-osteosarcoma function by inhibiting VEGF expression in tumor
cells.
References
|
1.
|
BB AggarwalA BhardwjRS AggarwalNP SeeramS
ShishodiaY TakadaRole of resveratrol in prevention and therapy of
cancer: preclinical and clinical studiesAnticancer
Res2427832840200415517885
|
|
2.
|
JK KunduKS ChunSO KimYJ SurhResveratrol
inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse
skin: MAPKs and AP-1 as potential molecular
targetsBiofactors2l3339200410.1002/biof.55221010815630167
|
|
3.
|
MH AzizR KumarN AhmadCancer
chemoprevention by resveratrol in vitro and in vivo studies and the
underlying mechanisms (review)Int J Oncol231728200312792772
|
|
4.
|
H JiangU ZhangJ KuoResveratrol-induced
apoptotic death in human U251 glioma cellsMol Cancer
Ther4554561200510.1158/1535-7163.MCT-04-005615827328
|
|
5.
|
TT WangNW SchoeneEK KimYS KimPleiotropic
effects of the sirtuin inhibitor sirtinol involves
concentration-dependent modulation of multiple nuclear
receptor-mediated pathways in androgen-responsive prostate cancer
cell LNCaPMol CarcinogApr112012(Epub ahead of
print)10.1002/mc.21906
|
|
6.
|
ME JuanI AlfarasJM PlanasColorectal cancer
chemoprevention by trans-resveratrolPharmacol
Res65584591201210.1016/j.phrs.2012.03.01022465196
|
|
7.
|
P LiuX WangC HuT HuInhibition of
proliferation and induction of apoptosis by trimethoxyl stilbene
(TMS) in a lung cancer cell lineAsian Pac J Cancer
Prev1222632269201122296367
|
|
8.
|
JH ZhouHY ChengZQ YuResveratrol induces
apoptosis in pancreatic cancer cellsChin Med J
(Engl)12416951699201121740780
|
|
9.
|
HB YuHF ZhangX ZhangResveratrol inhibits
VEGF expression of human hepatocellular carcinoma cells through a
NF-kappa B-mediated
mechanismHepatogastroenterology5712411246201021410066
|
|
10.
|
Y LiCM BäckesjöLA HaldosénU
LindgrenResveratrol inhibits proliferation and promotes apoptosis
of osteosarcoma cellsEur J
Pharmacol6091318200910.1016/j.ejphar.2009.03.00419285066
|
|
11.
|
HF DvorakLF BrownM DetmarAM DvorakVascular
permeability factor/vascular endothelial growth factor,
microvascular hyperpermeability, and angiogenesisAm J
Pathol1461029103919957538264
|
|
12.
|
G RanieriR PatrunoE RuggieriS MontemurroP
ValerioD RibattiVascular endothelial growth factor (VEGF) as a
target of bevacizumab in cancer: from the biology to the clinicCurr
Med Chem1318451857200610.2174/09298670677758505916842197
|
|
13.
|
LC BakerJK BoultS Walker-SamuelThe
HIF-pathway inhibitor NSC-134754 induces metabolic changes and
anti-tumour activity while maintaining vascular functionBr J
Cancer10616381647201210.1038/bjc.2012.13122498643
|
|
14.
|
P RieskeJK BartkowiakAM SzadowskaB
OlborskiB Harezga-BalM Debiec-RychterA comparative study of
P53/MDM2 genes alterations and P53/MDM2 proteins immunoreactivity
in soft-tissue sarcomasJ Exp Clin Cancer
Res18403416199910606188
|
|
15.
|
AL KimY ZhuH ZhuResveratrol inhibits
proliferation of human epidermoid carcinoma A431 cells by
modulating MEK1 and Ap-1 signalling pathwaysExp
Dermatol15538546200610.1111/j.1600-0625.2006.00445.x16761963
|
|
16.
|
N KuwajerwalaE CifuentesS
GautamResveratrol induces prostate cancer cell entry into s phase
and inhibits DNA synthesisCancer Res6224882492200211980638
|
|
17.
|
S VyasY AsmeremDD De LeónResveratrol
regulates insulin-like growth factor-II in breast cancer
cellsEndocrinology14642244233200510.1210/en.2004-134416037384
|
|
18.
|
L GolkarXZ DingMB UjikiResveratrol
inhibits pancreatic cancer cell proliferation through
transcriptional induction of macrophage inhibitory cytokine-1J Surg
Res138163169200710.1016/j.jss.2006.05.037
|
|
19.
|
J Gora-TyborJZ BlonskiT RobakCirculating
vascular endothelial growth factor (VEGF) and its soluble receptors
in patients with chronic lymphocytic leukemiaEur Cytokine
Netw164146200515809205
|
|
20.
|
DJ HicklinLM EllisRole of the vascular
endothelial growth factor pathway in tumor growth and angiogenesisJ
Clin Oncol2310111027200510.1200/JCO.2005.06.08115585754
|