Introduction
Epithelial ovarian cancer is the most common cause
of mortality from gynecological malignancy, and most ovarian
carcinomas are of the serous type. Serous carcinomas can be further
subclassified as high- or low-grade, based on histological features
(1). The group termed ‘atypical
proliferative serous tumor (APST)’ behave in a benign fashion and a
second, smaller group designated ‘micropapillary serous carcinoma
(MPSC)’ (also termed ‘noninvasive low-grade serous carcinoma’)
behave in a similar manner to low-grade malignant tumors (2). Moreover, the latter subset has been
found to be closely associated with invasive low-grade serous
carcinoma (LGSC) and the authors proposed that MPSC was the
immediate precursor of LGSC. LGSC is a distinct entity that differs
from high-grade serous carcinoma (HGSC) in several ways, for
example showing specific mutations in genes such as BRAF and KRAS
(2).
Low-grade invasive tumours, which have a better
prognosis, are regarded as ovarian-derived. However, some findings
make a strong argument that the ovarian epithelial inclusions with
a tubal phenotype are likely derived from a Fallopian tube through
an intraovarian endosalpingiosis rather than through Müllerian
metaplasia from the ovarian surface epithelium (3). It is possible that their site of
origin will be re-evaluated in the future. Nevertheless, it is
clear that serous borderline cancers are not precursor lesions for
the majority of high-grade serous ovarian cancer, as they have a
distinct range of mutational events (4).
There is emerging and compelling evidence that a
number of high-grade ovarian serous carcinomas (OSCs) originate
from the epithelium of the distal fimbrial portion of the Fallopian
tube. The Fallopian tube mucosa was thus suggested to be a strong
candidate for the primary source of pelvic (ovarian, tubal or
peritoneal) serous carcinoma. Serous tubal intraepithelial
carcinoma (STIC) has been implicated in the origins of not only
this group, but also serous carcinomas and primary peritoneal
carcinomas. It has been proposed that the earliest neoplastic
change begins in secretory-type cells (5). Further evidence supporting the
proposal that STICs are precursors comes from the identification of
STICs in females without ovarian cancer, as well as the presence of
identical p53 mutations in STICs and concomitant ovarian HGSCs,
indicating a clonal relationship (6).
Most epithelial malignancies arise through a
sequence of genotypic events leading to malignant phenotypes,
regardless of where they occur. Notably, Kindelberger et al
(6) reported that 47% of tumors
classified as OSC coexisted with STIC.
Studies dealing with biomarkers, including markers
of inflammation, micronenvironment, proliferation and invasion, in
normal fimbriae (without STIC) of high- and low-grade OSC are
scarce. In the present study, we detected the expression of 5
markers [E-cadherin, matrix metalloproteinase-2 (MMP-2),
phospho-AKT (pAKT), cyclooxygenase-2 (COX-2), vascular endothelial
growth factor (VEGF) and p53] in the normal-appearing fimbriae with
high- and low-grade OSCs by immunohistochemistry. The aim of this
study was to assess the difference in fimbriae of low- and
high-grade OSC without STIC. We investigated whether the invasion,
proliferation, inflammatory microenvironment, cell adhesion and
angiogenesis of fimbriae of high-grade OSCs without STIC changed
prior to p53 mutation.
Materials and methods
Case selection
Slides of fimbria tissue, all resected between
January 2008 and December 2010 from a total of 52 patients, were
used in this study. All were obtained from the archived files of
the pathology department in the Obstetrics and Gynecology Hospital
of Fudan University (Shanghai, China), following approval from its
institutional review board. Patient consent was received either
from the patient or the patient’s family. The group comprised 28
HGSCc and 24 LGSCs that had previously been categorized as being of
ovarian origin based on conventional criteria (1). All cases were reviewed and classified
independently as low- or high-grade by two gynecological
pathologists using histological criteria described previously
(1). Sectioning and extensively
examining the fimbria (SEE-FIM) was performed and the 2
pathologists reviewed the hematoxylin and eosin-stained sections
from each specimen to exclude fimbria involvement for all the cases
independently.
Marker selection and
immunohistochemistry
Immunohistochemical (IHC) staining for all 6 markers
(E-cadherin, cell adhesion; MMP-2, invasion; pAKT, proliferation;
COX-2, inflammatory microenvironment; VEGF, angiogenesis; and p53)
was performed following standard IHC procedures. Details of
antibodies used and staining conditions are provided in Table I.
 | Table IAntibodies. |
Table I
Antibodies.
| Marker name | Specific | Supplier | Dilution | Catalog no. |
|---|
| pAKT | Rabbit | CST | 1:1,000 | 4060 |
| E-cadherin | Rabbit | CST | 1:1,000 | 3195 |
| COX-2 | Rabbit | CST | 1:1,000 | 4842 |
| MMP-2 | Goat | R&D | 1:200 | AF902 |
| VEGF | Goat | R&D | 1:200 | AB-293-NA |
| p53 | Goat | Dako | 1:200 | M 7001 |
The rabbit monoclonal antibodies against E-cadherin,
pAKT and COX-2 were purchased from Cell Signaling Technology
(Denvers, MA, USA) and the goat polyclonal antibodies against
MMP-2, VEGF and p53 were purchased from R&D Systems
(Minneapolis, MN, USA) and Dako (Carpinteria, CA, USA), all of
which were used as primary antibodies. The dilutions are listed in
Table I. Formalin-fixed,
paraffin-embedded specimens were sliced into 5-μm sections,
placed on glass slides and routine deparaffinization and
rehydration procedures were performed. For antigen retrieval, the
slides were heated at 98˚C in an EDTA buffer (pH 9.0) for a total
of 45 min and cooled naturally to the room temperature. The cooled
slides were rinsed with distilled water and transferred to
phosphate-buffered saline (PBS). After the slides were rinsed in
PBS, the endogenous peroxidase activity was quenched by incubating
the sections for 15 min with 0.3% H2O2 in
absolute methanol. The slides were subsequently dehydrated in PBS
and incubated with blocking serum (10% nonimmune goat serum) for 30
min at room temperature. After slides were rinsed, they were
incubated with biotinylated secondary antibody detection reagent at
room tempetature for 30 min. Following incubation with the
antibodies, the sections were washed with PBS 3 times and incubated
with an avidinbiotinylated horseradish peroxidase macromolecular
complex for 10 min, according to the manufacturer’s instructions.
The bound antibody complexes were stained for 3-5 min or until
appropriate for microscopic examination with diaminobenzidine and
then counterstained with hematoxylin (for 30 sec) and mounted.
Briefly, images were captured with the microscope (Olympus BX51,
Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP70,
Olympus).
All IHC stainings were performed independently by
the same pathologists with experience of this technique.
Evaluation and scoring of
immunohistochemically analyzed tissue sections
All immunostained tissue sections were evaluated and
scored by a board-certified pathologist with 10 years’ experience
of IHC techniques both in research and diagnostic pathology who was
blinded to any clinical or pathological information about the
sections. For each case, 1,000 cells were assessed in 3 or 4
different fields at a magnification of x400. Expression scores were
assigned semiquantitatively according to the percentage of cells
stained (1, <25%; 2, 25%–75%; 3, >75%) and the intensity of
the staining (1, weak; 2, moderate; 3, strong). The two scores were
then multiplied. When <25% of the cells were stained, the
intensity of the stain was weak; when the product of the 2 scores
was ≤3, the expression was categorized as negative. When ≥25% of
the cells were stained, the intensity of the stain was moderate or
strong; when the product of the 2 scores was ≥4, the expression was
categorized as positive.
Statistical analysis
Fisher’s exact test was used to compare the
expression of each protein in fimbrial epithelial cells of high-
and low-grade OSCs. P<0.05 was considered to indicate a
statistically significant result.
Results
The immunohistochemical analysis results are
summarized in Table II. Three of
the 6 markers (pAKT, E-cadherin and COX-2) showed significant
differences between the fimbriae of low- and high-grade OSCs,
whereas the remaining 3 markers (MMP-2, VEGF and p53) had similar
expression levels in both low- and high-grade OSCs.
 | Table IIImmunohistochemical staining results
for antibodies. |
Table II
Immunohistochemical staining results
for antibodies.
| Positive staining,
n (%)
| |
|---|
| Biomarker | High-grade
tumors | Low-grade
tumors | P-value |
|---|
| pAKT | 17/28 (61) | 2/24 (8) | 0.005 |
| MMP-2 | 6/28 (21) | 3/24 (13) | 0.78 |
| E-cadherin | 6/28 (21) | 20/24 (83) | 0.003 |
| VEGF | 7/28 (25) | 5/24 (21) | 0.86 |
| COX-2 | 20/28 (71) | 5/24 (21) | 0.007 |
| p53 | 4/28 (14) | 2/24 (8) | 0.82 |
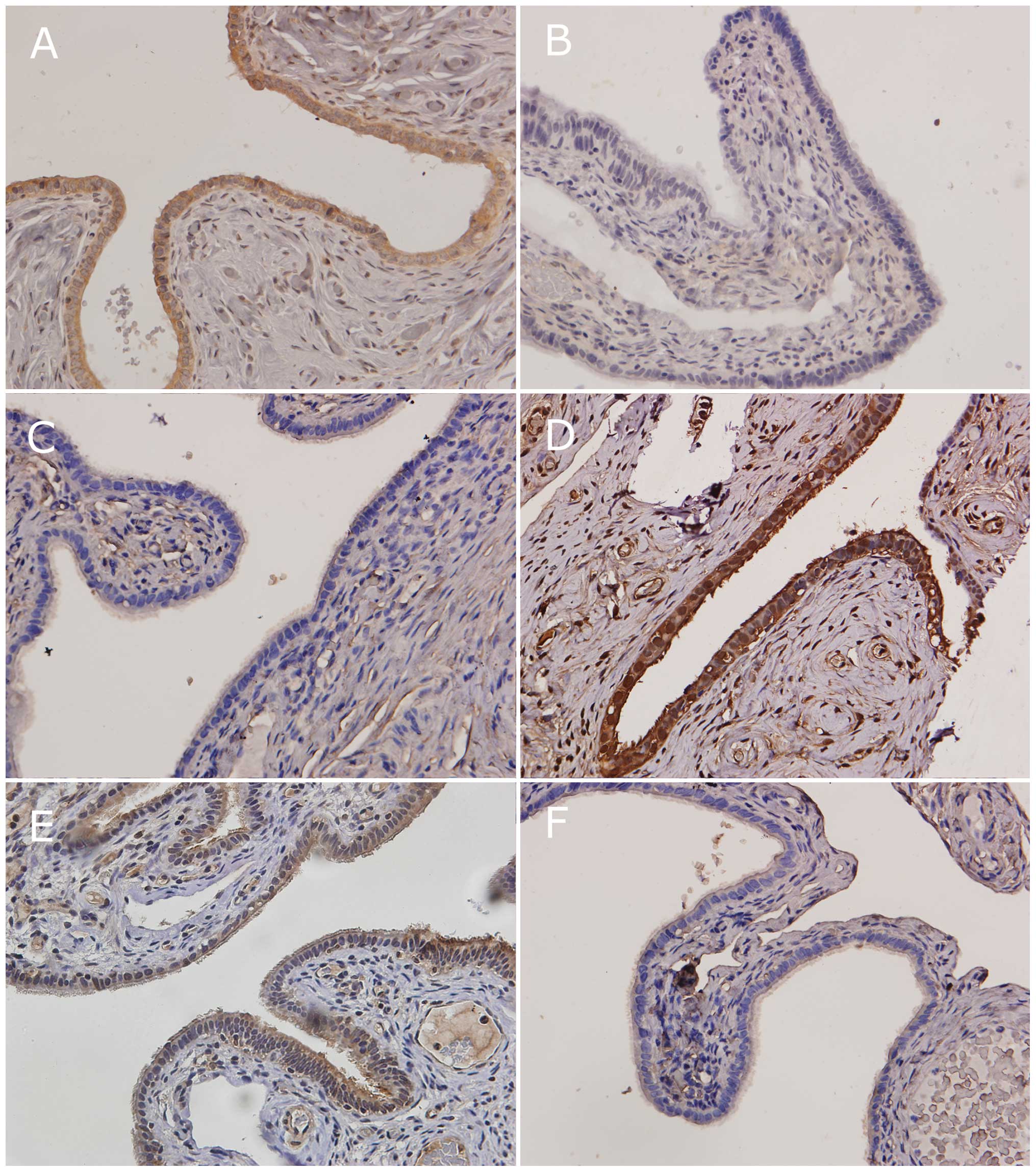
The immunostaining levels of pAKT and COX-2 were
significantly higher in the fimbriae of high-grade OSCs than those
of low-grade OSCs. E-cadherin expression was significantly lower in
the fimbriae of high-grade OSCs than those of low-grade OSCs
(Fig. 1).
The results suggested important biological
differences in the behavior of the fimbria in high- and low-grade
OSCs and indicate that the proliferation, cell adhesion and
inflammatory microenvironment of fimbriae of high-grade OSCs
without STIC had changed prior to p53 mutation.
Discussion
According to Vaughan et al, ovarian cancer is
many diseases (7). In the past few
years, on the basis of a series of morphological and molecular
genetic studies, a dualistic model has been proposed that
categorizes various types of ovarian cancer into two groups, types
I and II. The high-grade OSCs, which are the most common and most
lethal of all ovarian epithelial neoplasms, may arise in the distal
part of the Fallopian tubes (fimbria, secretory-type cells) and are
only secondarily deposited on the ovarian surface. They commonly
show genetic instability and p53 mutations (8).
However, a PubMed search revealed no earlier studies
dealing with normal fimbriae of high- and low-grade OSCs and
evaluating which characteristics had altered prior to p53 mutation.
In this study, we evaluated the expression of 6 proteins in 28
cases of high-grade OSC and in 26 cases of low-grade OSC. In
high-grade OSCs, there was a trend toward increased expression of
pAKT and COX-2 in fimbriae without involvement of cancer. Higher
E-cadherin positivity was observed in fimbriae of low-grade OSCs
than in fimbriae of high-grade OSCs. This indicates that the
proliferation, cell adhesion and inflammatory microenvironment of
the fimbriae of high-grade OSCs without STIC had changed prior to
p53 mutation.
An abundance of basic investigative studies has
established the pAKT pathway as a major driver of cancerous
behavior, inolved in proliferation, dysregulation of the cell
cycle, apoptosis, metabolism, protein synthesis, senescence and
other aspects of cell function. In addition, pAKT
immunohistochemistry may be useful in the diagnosis and
characterization of precancerous and intraepithelial lesions
(9). We observed an increased AKT
expression in 61% of high-grade OSCs, as compared with 8% of
low-grade OSCs by immunohistochemistry.
E-cadherin is an adhesion molecule that may be
involved in the metastasis of ovarian cancer (10). It has been suggested that E-cadherin
acts as a tumor suppressor; furthermore, the transfection of tumor
cells with E-cadherin cDNA prevents invasive growth (11). Thus, reduced cytoplasmic positivity
of E-cadherin in high-grade OSC in this study is consistent with
the poor outcome of patients with this disease. In our study, its
expression was higher in the fimbriae of low-grade OSC than those
of high-grade OSC. It is therefore possible that transformed
fimbrial epithelial cells of high-grade OSC lose adhesion early in
their progression and may slough off and migrate to the ovarian
surface or directly to the peritoneum, with minimal ovarian deep
involvement.
COX-2 overexpression has been reported in most
gynecological neoplasms, including breast, cervix, endometrial and
epithelial ovarian cancers. COX-2 expression promotes tumor cell
proliferation, reduces apoptosis and induces angiogenesis (12). With regard to the inflammatory
process, the cell proliferation rate has a well-known association
with prognosis in ovarian carcinoma (13). The different expression levels of
Cox-2 in low- and high-grade OSCs in our study possibly indicates
the different inflammatory microenvironments.
The mechanism by which MMP-2 facilitates early
adhesion and invasion involves cleavage of multiple extracellular
matrix (ECM) proteins into smaller fragments that serve as better
attachment sites. In addition, the inhibition of MMP-2, but not
MMP-9, has been reported to significantly reduce ovarian cancer
metastasis (14). A previous study
detected active MMP-2 enzyme (62 kDa) only in ovarian cancer (66%)
and corresponding metastases (93%), but never in benign or low
potential malignancy tumors (15).
We found that the expression level of MMP-2 was similar between the
fimbriae of low- and high-grade OSCs, suggesting that the
invasiveness of the fimbrial epithelial cells had not emerged.
VEGF expression in ovarian cancer has been evaluated
in several studies. In early stage ovarian cancers, increased VEGF
expression has been shown to correlate with worse disease-free
survival (DFS) and poor overall survival (OS) (16). In addition, a higher serum level of
VEGF associated with ovarian cancer have been considered as an
independent risk factor and a prognostic parameter for ascites,
more metastasis, advanced-stage disease and reduced survival
(17,18). We have confirmed that the expression
of VEGF had similar expression levels in low- and high-grade OSCs,
maybe due to the alteration of fimbrial epithelial cells being an
early event in the pathogenesis of high-grade OSCs and angiogenesis
having not yet started.
p53 is a useful biomarker for detecting not only the
early precursor lesions of high-grade OSCs, but also the later
stages of this disease (19). Lee
et al hypothesized that p53 signatures represent the elusive
OSC precursor. In addition, short stretches of normal appearing
Fallopian tube epithelium that strongly express p53, and in which
p53 mutations have been identified in some cases, have been termed
‘p53 signatures’ (5). Although
these lesions may represent early events in serous carcinogenesis,
it is not clear, at this time, whether p53 signatures are precursor
lesions or if they are benign ‘reactive’ changes that overexpress
p53 and have no biological relevance to neoplasia. It has been
shown that the expression of p53 mutations was similar between the
fimbriae of normal appearance of low- and high-grade OSCs in our
study. It is possible that p53 mutations were preceded by other
neoplastic changes of cells, maybe after the increase of pAKT and
COX-2 and the decrease of E-cadherin; this is an area that requires
further investigation.
The present study has limitations, including its
retrospective design, which is prone to selection bias, and a small
sample size. In addition, the technique of immunohistochemistry
does not always reflect the structure and functionality of the
protein.
In conclusion, our results showed that the
immunostaining of pAKT and COX-2 were significantly higher in the
fimbriae of normal appearance of high-grade OSCs than those of
low-grade OSCs and that the immunostaining of E-cadherin was
significantly higher in the fimbriae of low-grade OSCs than those
of high-grade OSCs. The remaining 3 markers (MMP-2, VEGF and p53)
had similar expression levels in low- and high-grade OSCs. The
relative importance of the Fallopian tube compared with the ovarian
surface epithelium in the genesis of high-grade serous ovarian
cancers is still being debated. However, our results suggest marked
biological differences in the behavior of the fimbriae in high- and
low-grade OSCs and indicate that the proliferation, cell adhesion
and inflammatory microenvironment of fimbriae of high-grade OSCs
without STIC had changed prior to p53 mutation.
References
|
1.
|
A MalpicaMT DeaversK LuGrading ovarian
serous carcinoma using a two-tier systemAm J Surg
Pathol28496504200410.1097/00000478-200404000-0000915087669
|
|
2.
|
RT BurksME ShermanRJ KurmanMicropapillary
serous carcinoma of the ovary. A distinctive low-grade carcinoma
related to serous borderline tumorsAm J Surg
Pathol2013191330199610.1097/00000478-199611000-000038898836
|
|
3.
|
RJ KurmanJD SeidmanIM ShihSerous
borderline tumours of the
ovaryHistopathology47310315200510.1111/j.1365-2559.2005.02186.x16115232
|
|
4.
|
DD BowtellThe genesis and evolution of
high-grade serous ovarian cancerNat Rev
Cancer10803808201010.1038/nrc294620944665
|
|
5.
|
Y LeeA MironR DrapkinA candidate precursor
to serous carcinoma that originates in the distal fallopian tubeJ
Pathol2112635200710.1002/path.209117117391
|
|
6.
|
DW KindelbergerY LeeA MironIntraepithelial
carcinoma of the fimbria and pelvic serous carcinoma: Evidence for
a causal relationshipAm J Surg
Pathol31161169200710.1097/01.pas.0000213335.40358.4717255760
|
|
7.
|
S VaughanJI CowardRC Bast JrRethinking
ovarian cancer: recommendations for improving outcomesNat Rev
Cancer10719725201110.1038/nrc314421941283
|
|
8.
|
RJ KurmanIe-M ShihMolecular pathogenesis
and extraovarian origin of epithelial ovarian cancer - shifting the
paradigmHum
Pathol42918931201110.1016/j.humpath.2011.03.00321683865
|
|
9.
|
V ShtilbansM WuDE BursteinCurrent overview
of the role of Akt in cancer studies via applied
immunohistochemistryAnn Diagn
Pathol12153160200810.1016/j.anndiagpath.2007.12.00118325479
|
|
10.
|
T FujiokaY TakebayashiT KihanaExpression
of E-cadherin and beta-catenin in primary and peritoneal metastatic
ovarian carcinomaOncol Rep8249255200111182035
|
|
11.
|
K VleminckxL Vakaet JrM MareelGenetic
manipulation of E-cadherin expression by epithelial tumor cells
reveals an invasion suppressor
roleCell66107119199110.1016/0092-8674(91)90143-M2070412
|
|
12.
|
A MunkarahR Ali-FehmiCOX-2: a protein with
an active role in gynecological cancersCurr Opin Obstet
Gynecol174953200510.1097/00001703-200502000-0000915711411
|
|
13.
|
J KaernM AghmeshehJM NeslandPrognostic
factors in ovarian carcinoma stage III patients. Can biomarkers
improve the prediction of short- and long-term survivors?Int J
Gynecol
Cancer1510141022200510.1111/j.1525-1438.2005.00185.x16343177
|
|
14.
|
HA KennyE LengyelMMP-2 functions as an
early response protein in ovarian cancer metastasisCell
Cycle8683688200910.4161/cc.8.5.770319221481
|
|
15.
|
B SchmalfeldtD PrechtelK HärtkingIncreased
expression of matrix metalloproteinases (MMP)-2, MMP-9, and the
urokinase-type plasminogen activator is associated with progression
from benign to advanced ovarian cancerClin Cancer
Res723962404200111489818
|
|
16.
|
P PaleyK StaskusK GebhardVascular
endothelial growth factor expression in early stage ovarian
carcinomaCancer8098106199710.1002/(SICI)1097-0142(19970701)80:1%3C98::AID-CNCR13%3E3.0.CO;2-A9210714
|
|
17.
|
BC CooperJM RitchieCL
BroghammerPreoperative serum vascular endothelial growth factor
levels: significance in ovarian cancerClin Cancer
Res831933197200212374688
|
|
18.
|
L HeflerR ZeillingerC GrimmPreoperative
serum vascular endothelial growth factor as a prognostic parameter
in ovarian cancerGynecol
Oncol103512517200610.1016/j.ygyno.2006.03.05816750560
|
|
19.
|
IeM ShihRJ KurmanOvarian tumorigenesis: a
proposed model based on morphological and molecular genetic
analysisAm J
Pathol16415111518200410.1016/S0002-9440(10)63708-X15111296
|