Introduction
Adenoid cystic carcinoma (ACC) in the minor salivary
glands, including the larynx, nasal cavity, paranasal sinus,
external auditory canal and trachea, is uncommon (1–4). ACC
is noted for its slow growth and indolent course and, as with ACC
in the major salivary glands, patients with ACC of these sites
often experience recurrence and distant metastases many years after
diagnosis and definitive treatment (5). The patterns of disease include
perineural and vascular invasion. The most significant modalities
for the primary treatment of local and locoregional disease are
surgery and/or irradiation. However, the long-term outcome of these
treatments is unfavorable (6).
Several studies have identified clinicopathological factors in ACC
with an unfavorable effect on survival, including old age, tumor
location, advanced stage, solid histological subtype, high grade,
tumor-node-metastasis (TNM) staging, major nerve involvement, the
presence of perineural invasion, a positive surgical margin and
lymph node metastasis (7,8). However, the real prognostic and
recurrence factors are still unknown. Thus, further studies are
needed to identify the pathogenesis, prognostic factors and new
diagnostic and therapeutic methods for ACC, particularly at the
molecular level.
Like other malignant tumors, ACC cells exhibit
increased glucose uptake and utilization compared with their
nonmalignant counterparts. This phenomenon has been demonstrated by
positron emission tomography (PET) using
18F-2-fluoro-2-deoxy-D-glucose (18F-FDG)
(9). Many mechanisms of
18F-FDG uptake have been proposed for accelerated
glucose use in growing tumors and in transformed and malignant
cells. These include passive diffusion, Na+-dependent
glucose transport and facilitative glucose transporters (GLUTs)
(10), with GLUTs considered to be
the most significant mechanism for enhancing glucose influx into
cells. GLUTs are membrane proteins that facilitate the transport of
glucose across cellular membranes. Thirteen members of the
facilitative glucose transporter family are now recognized (GLUT-1
to -12 and HMIT; gene name SLC2A) (11). The human genes encoding these
proteins are named GLUT-l to -5 and GLUT-7 to -13; GLUT-6 and -14
are now known to be pseudogenes. Of the 14 isoforms, GLUT-1 appears
to be the most ubiquitously distributed (10,12,13).
Increased GLUT-1 levels and glucose uptake correlate with increased
cellular growth and proliferation (12,13).
This isoform is overexpressed in many human cancer cells, and its
appearance is correlated with aggressive biological behavior
(10,12). Thus, control of GLUT-1 trafficking
and activity are also key elements in regulating glucose uptake.
Similarly to the insulin-responsive glucose transporter GLUT-4,
GLUT-1 cell-surface localization is controlled by extrinsic
signals. Among the signaling pathways initiated in cell activation,
the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)
pathway has been shown to promote both GLUT-1 cell-surface
trafficking and activity (14,15).
PI3K is a heterodimeric enzyme important for growth
and proliferation and Akt is a downstream serine-threonine kinase
that transmits survival signals from growth factors (15). The PI3K/Akt pathway is frequently
overactive in various tumors and triggers a cascade of responses,
from cell growth and proliferation to cell survival and motility,
which drives tumor progression (15–17).
The PI3K/Akt pathway has been shown to be involved in translocating
the GLUT-1 glucose transporter from the cytosol to the plasma
membrane in endocrine organs such as the pancreas (15).
In the present study, we assessed the expression of
GLUT-1, PI3K and p-Akt protein in ACCs using immunohistochemistry.
Additionally, we examined the possible correlations between GLUT-1,
PI3K and p-Akt protein expression and clinicopathological
parameters in this cohort of patients.
Patients and methods
Patients
Forty-two patients with ACC from the Departments of
Otolaryngology and Oral and Maxillofacial Surgery, the First
Affiliated Hospital, College of Medicine, Zhejiang University,
China were evaluated between January 1993 and February 2010. The
institutional review board approved the present study, and written
informed consent was obtained from the patients before inclusion.
The clinicopathological findings (including age, gender, site, TNM
stage, pathological type, recurrence, metastasis and follow-up)
were analyzed (Table I).
 | Table I.Clinicopathological findings of 42
adenoid cystic carcinomas. |
Table I.
Clinicopathological findings of 42
adenoid cystic carcinomas.
| | Recurrence
|
|---|
| Clinical
characteristics | N | N | χ2 | P-value |
|---|
| Gender | | | | |
| Female | 29 | 11 | 0.25 | >0.05 |
| Male | 13 | 6 | | |
| Age (years) | | | | |
| <60 | 15 | 9 | 3.69 | >0.05 |
| ≥60 | 27 | 8 | | |
| Site | | | | |
| Major salivary
gland | 16 | 6 | 0.21 | >0.05 |
| Minor salivary
gland | 22 | 9 | | |
| Other | 4 | 2 | | |
| Pathological
type | | | | |
| Cribriform | 28 | 11 | 0.79 | 0.67 |
| Tubular | 4 | 1 | | |
| Solid | 10 | 5 | | |
| T stage | | | | |
|
T1 | 14 | 3 | 10.5 | 0.015 |
|
T2 | 15 | 4 | | |
|
T3 | 9 | 7 | | |
|
T4 | 4 | 2 | | |
| Lymph node
metastasis | | | | |
| Yes | 22 | 10 | 0.48 | >0.05 |
| No | 20 | 7 | | |
| Resection margin
positive | | | | |
| Yes | 9 | 8 | | 0.001 |
| No | 33 | 9 | | Fisher’s Exact
Test |
| Perineural
invasion | | | | |
| Yes | 19 | 13 | 11.2 | 0.001 |
| No | 23 | 4 | | |
| Treatment | | | | |
| Surgery | 30 | 15 | 3.9 | 0.047 |
| Surgery +
radiotherapy | 12 | 2 | | |
Immunohistochemical analysis
For immunohistochemical evaluation, paraffin blocks
of formalin-fixed biopsies were retrieved from pathology archives.
In addition to tumor biopsies of the patients included in the
current analysis, 15 paraffin-embedded archival tissue blocks from
patients with nasal polyps, 15 from patients with vocal cord
polyps, 5 from patients with nasal benign schwannomas and 20 tissue
blocks from patients with nasal inverted papilloma were also
obtained.
Sections were immunostained for GLUT-1, PI3K and
p-Akt proteins using an EliVision™ plus IHC kit (Maixin Biological,
Fuzhou, China), according to the standard protocol (17). The antibodies, dilutions,
antigen-retrieval methods, sources and positive controls which were
used are detailed in Table II.
Antigen retrieval was performed after sections were deparaffinized
with xylene and dehydrated through an ethanol series. Endogenous
peroxidase activity was blocked by incubating slides in 1.5%
hydrogen peroxide in absolute methanol at room temperature for 10
min. Primary antibodies were applied for 1 h at room temperature,
followed by 50 μl polymer enhancer for 20 min and 50
μl polymerized horseradish peroxidiseantimouse
immunoglobulin G (IgG) for 30 min. The reaction products were
visualized using diaminobenzidine (DAB kit; Maixin Biological) and
sections were counterstained with hematoxylin and eosin (H&E),
dehydrated and evaluated by light microscopy. Tris-buffered saline
solution was used instead of the primary antibody for negative
controls. Erythrocytes, which were present in every section, served
as internal controls for GLUT-1 to ensure constant immunostaining
intensity.
 | Table II.Antibodies used in the
immunohistochemical analysis. |
Table II.
Antibodies used in the
immunohistochemical analysis.
| Antibody | Dilution | Manufacturer | Antigen retrieval
method | Positive
control |
|---|
| GLUT-1 | 1:50 | Dako, Carpinteria,
CA, USA | Enzyme | Erythrocytes |
| PI3K | 1:100 | Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA | Microwave | Breast
carcinoma |
| p-Akt | 1:50 | Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA | Microwave | Glioblastoma
multiforme |
Evaluation of stained sections
GLUT-1, PI3K and p-Akt immunostaining was evaluated
by the same investigator (H.T.Y.), who was blinded to the clinical
and follow-up data. GLUT-1 expression was considered positive only
if distinct membrane staining was present. PI3K and p-Akt proteins
were observed in the nucleus and cytoplasm. Protein analysis was
performed in 10 random high-power fields. A total of 100 tumor
cells were counted from each high-power field for each case and for
all antibodies analyzed. The percentage of positive cells was
calculated by dividing the number of positive tumor cells by the
total number of tumor cells counted. Staining was considered to be
negative when <25% of the cells were positive.
Statistical analysis
Analyses were performed using the SPSS for Windows
software (version 19.0; SPSS Inc., Chicago, IL, USA). Correlations
among GLUT-1, PI3K and p-Akt protein expression and the other
pretreatment parameters were analyzed using the Chi-square test and
Fisher’s exact test. The logistic regression test was used for the
multivariate analysis. P<0.05 was considered to indicate a
statistically significant result. The correlation analysis was
performed using Spearman’s Rho.
Results
Patient characteristics
Of the 42 ACC tumor tissues, 9 (21.5%) were located
in the submandibular gland, 6 (14.3%) in the parotid gland, 5
(11.9%) in the palate, 4 (9.5%) in the paranasal sinus, 4 (9.5%) in
the external auditory canal, 3 (7.1%) in the nasal cavity, 3 (7.1%)
in the larynx, 3 (7.1%) in the floor of the mouth, one (2.4%) in
the sublingual gland, one (2.4%) in the tongue, one (2.4%) in the
pterygomandibular space, one (2.4%) in the buccal mucosa and one
(2.4%) in the trachea (Table I).
Among these, 16 (38.1%) were located in the major salivary gland,
22 (52.4%) were in the minor salivary gland and 4 (9.5%) were in
the external auditory canal. The median age of the 42 patients was
52.4 years (range, 32–83 years). Thirteen patients were male and 29
were female. Surgery was performed in all patients. Twenty-two
patients had enlarged ipsilateral lymph nodes (N1) and
received neck dissection. Thirty patients did not receive
postoperative radiotherapy following initial surgery (Table I).
Tumor-node-metastasis (TNM) classification and stage
were assigned based on the sixth edition of the American Joint
Committee on Cancer (AJCC) staging manual. Fourteen of the patients
(33.3%) had cancer classified as T1 stage, 15 (35.7%)
had T2 stage, 9 (21.4%) had T3 stage and 4
(9.6%) had T4a stage cancer.
The patients’ average follow-up period was 51.9
months (range, 6–293 months). Four patients were lost to follow-up.
Four patients (9.5%) developed distant metastases and 2 (4.8%)
succumbed to distant metastases. Thirty-three patients were alive
at the last follow-up. The median overall survival (OS) was 187
months [95% confidence interval (CI), 118–257]. The 5- and 10-year
OS probabilities were 79% (95% CI, 61.9–83.2%) and 53% (95% CI,
41.1–66.7%), respectively. There was no significant clinical factor
identified as a prognostic predictor (P>0.05). Of the 42
patients with follow-up, 17 (40.5%) developed recurrence following
initial surgery, with a median follow-up period of 30.3 months
(range, 5–70 months). Univariate analyses showed that T stage, a
positive resection margin, perineural invasion and surgery without
postoperative radiotherapy were significantly associated with a
higher recurrence risk (χ2=10.5, P=0.015; P=0.001,
Fisher’s exact test; χ2=11.2, P=0.001;
χ2=3.9, P=0.047, respectively). Site, tumor stage,
pathological type and gender did not influence the risk of
recurrence in the present study (Table
I). Patients who had local recurrence received radical surgery
and those who did not received radiotherapy following surgery, or
salvage surgical treatment plus chemotherapy.
GLUT-1, PI3K and Akt protein expression
in ACC
The positive rates of GLUT-1, PI3K and p-Akt protein
in ACC were 38.1% (16/42), 38.1% (16/42) and 50.0% (21/42),
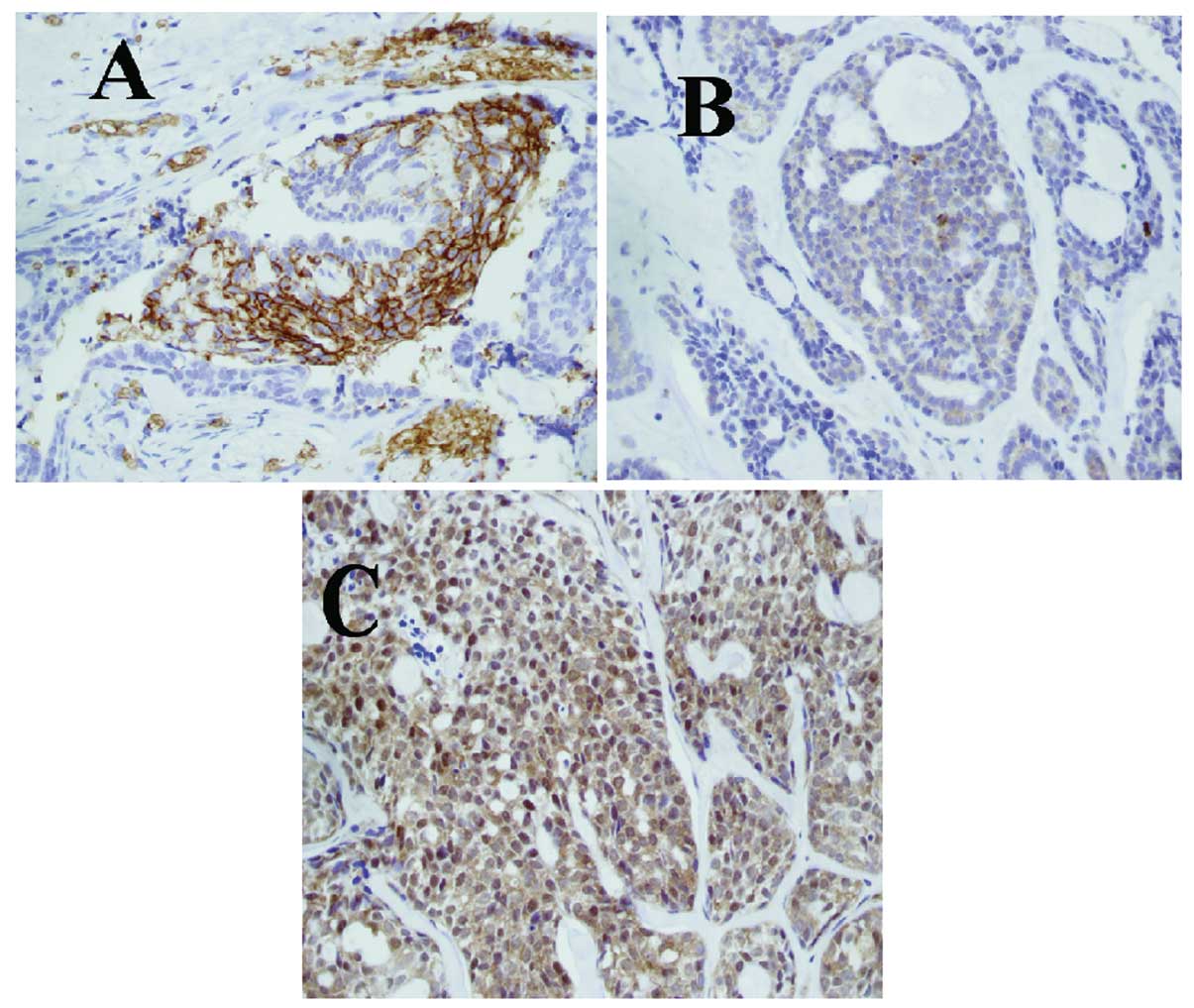
respectively (Table III, Fig. 1). The expression of GLUT-1, p-Akt
and PI3K protein in ACC was higher than that in inflammatory
lesions or benign tumors (p<0.001; Table III).
 | Table III.Immunohistochemical results of
GLUT-1, PI3K and p-Akt in adenoid cystic carcinomas, inflammatory
lesions and benign tumors. |
Table III.
Immunohistochemical results of
GLUT-1, PI3K and p-Akt in adenoid cystic carcinomas, inflammatory
lesions and benign tumors.
| | GLUT-1
| | PI3K
| | p-Akt
| |
|---|
| Group | N | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Inflammatory
lesions | 30 | 0 | 30 | <0.001 | 0 | 30 | <0.001 | 0 | 30 | <0.001 |
| Benign tumors | 25 | 0 | 25 | <0.001 | 1 | 24 | <0.001 | 3 | 22 | <0.001 |
| ACC | 42 | 16 | 26 | <0.001 | 16 | 26 | 0.001 | 21 | 21 | <0.001 |
Correlation among GLUT-1, PI3K and p-Akt
protein expression and PI3K/p-Akt combinatorial groups
The Spearman’s Rho analysis showed significant
correlations between GLUT-1 and PI3K expression (r=0.394, P=0.01),
GLUT-1 and p-Akt expression (r=0.528, P<0.001) and p-Akt and
PI3K expression (r=0.528, P<0.001). According to the expression
of p-Akt and PI3K, the prevalence of the four phenotypic groups was
31.0% p-Akt+/PI3K+, 21.4%
p-Akt+/PI3K−, 7.1%
p-Akt−/PI3K+ and 40.5%
p-Akt−/PI3K−. A significant positive
correlation was noted between the phenotypic groups, T stage
(p=0.022) and recurrence (P=0.000).
Association of GLUT-1, PI3K and p-Akt
with clinicopathological parameters
The correlation between GLUT-1, PI3K and p-Akt
expression and clinicopathological features is shown in Table III. Univariate analyses revealed
that there was a significant correlation between GLUT-1 expression,
T stage (P=0.003), distant metastasis (P=0.016) and local
recurrence (P=0.001; Table IV). The
expression of GLUT-1 was not significantly correlated with other
clinicopathological factors (gender, age, pathological type, lymph
node metastasis, site and survival). A significant positive
correlation was noted between PI3K expression (P<0.001), p-Akt
expression (P=0.003) and local recurrence. The expression of PI3K
and p-Akt was not significantly correlated with other
clinicopathological factors (gender, age, pathological type, TNM
stage, site and survival), therefore these markers were not
predictors of OS.
 | Table IV.Correlation between the
clinicopathological features and expression of GLUT-1, PI3K and
p-Akt. |
Table IV.
Correlation between the
clinicopathological features and expression of GLUT-1, PI3K and
p-Akt.
| Clinicopathological
features | N | GLUT-1 + | χ2 | P-value | p-Akt + | χ2 | P-value | PI3K + | χ2 | P-value |
|---|
| Gender | | | | | | | | | | |
| Female | 29 | 11 | 0.12 | >0.05 | 15 | 0.20 | >0.05 | 10 | 0.52 | >0.05 |
| Male | 13 | 5 | | | 6 | | | 6 | | |
| Age (years) | | | | | | | | | | |
| <60 | 27 | 9 | 0.73 | >0.05 | 14 | 0.10 | >0.05 | 11 | 0.22 | >0.05 |
| ≥60 | 15 | 7 | | | 7 | | | 5 | | |
| Histopathological
type | | | | | | | | | | |
| Cribriform | 28 | 11 | 0.54 | >0.05 | 12 | 2.17 | >0.05 | 10 | 0.32 | >0.05 |
| Tubular | 4 | 2 | | | 2 | | | 2 | | |
| Solid | 10 | 3 | | | 7 | | | 4 | | |
| T stage | | | | | | | | | | |
|
T1 | 14 | 1 | 14.15 | 0.003 | 4 | 4.64 | >0.05 | 4 | 4.84 | >0.05 |
|
T2 | 15 | 5 | | | 8 | | | 4 | | |
|
T3 | 9 | 7 | | | 6 | | | 5 | | |
|
T4 | 4 | 3 | | | 3 | | | 3 | | |
| Lymph node
metastasis | | | | | | | | | | |
| Yes | 22 | 11 | 2.77 | >0.05 | 10 | 0.38 | >0.05 | 10 | 1.06 | >0.05 |
| No | 20 | 5 | | | 11 | | | 6 | | |
| Site | | | | | | | | | | |
| Major salivary
gland | 16 | 6 | 0.27 | >0.05 | 8 | 1.18 | | 7 | | |
| Minor salivary
gland | 22 | 8 | | | 10 | | | 9 | | |
| Other | 4 | 2 | | | 3 | | >0.05 | 0 | 2.75 | >0.05 |
| Metastasis | | | | | | | | | | |
| Yes | 4 | 4 | Fisher’s Exact
test | 0.016 | 3 | Fisher’s Exact
Test | 0.61 | 2 | 0.27 | >0.05 |
| No | 38 | 12 | | 18 | | 14 | | |
| Recurrence | | | | | | | | | | |
| Yes | 17 | 12 | Fisher’s Exact
test | 0.001 | 15 | 16.7 | <0.001 | 11 | 8.58 | 0.003 |
| No | 25 | 4 | | 6 | | | 5 | | |
In multivariate analyses, perineural invasion
(P=0.021), a positive resection margin (P=0.032) and p-Akt
(P=0.006) were significant predictors of recurrence.
Discussion
In agreement with previous studies (1,7,8,10),
we found that ACC in our patients had a high recurrence rate,
similar to that observed in the study of Chen et al
(18). The recurrence rate was up
to 40.5%, and mostly occurred within 5 years of initial treatment.
The mean time to recurrence was 40 months (range, 9-70 months). The
patients’ average follow-up period was 51.9 months (range, 6–293
months). Four patients were lost to follow-up. Four patients (9.5%)
developed distant metastases and 2 succumbed to distant metastases.
Thirty-three patients were alive at the last follow-up. The median
OS was 187 months. There were no significant factors, such as the
expression of GLUT-1, p-Akt and PI3K, identified as prognostic
predictors (P>0.05).
The analysis of clinicopathological factors
influencing recurrence revealed that T stage, a positive resection
margin, perineural invasion and surgery without postoperative
radiotherapy were predictive of recurrence by univariate analyses.
However, only perineural invasion (P=0.021) and a positive
resection margin showed a higher risk of recurrence of ACC of the
head and neck by multivariate analysis. Most authors have suggested
that routine postoperative radiotherapy is a factor for the local
control of ACC of the head and neck (18–21).
Chen et al suggested that postoperative radiotherapy was an
effective factor in controlling the local recurrence of ACC in the
head and neck (18). The 5- and
10-year rates of local control were 92 and 84%, respectively, for
patients treated with postoperative radiation compared with 80 and
61%, respectively, for those treated with surgery alone. They also
found that stage T4 disease, positive surgical margins,
perineural invasion and major (named) nerve involvement were
factors predicting local recurrence (18). Mendenhall et al reported
higher local control rates of ACC of the head and neck for surgery
combined with postoperative radiotherapy than most other studies
(21). The 5- and 10-year rates of
local control were 94 and 91%, respectively, for patients treated
with postoperative radiation compared with 56 and 43%,
respectively, for those treated with surgery alone. If radiation
therapy is administered to patients, the dose should be no less
than 60 Gy (21). Mendenhall et
al also found that T stage influenced local control (21). Prokopakis et al reported that
the tumor site was the single most important factor predicting the
development of locally recurrent disease and was correlated with
primary tumor stage and resection margins (20). However, Iseli et al proposed
that no significant correlation was evident between improved local
control in patients with ACC of the head and neck receiving surgery
plus postoperative radiotherapy compared with patients receiving
surgery alone (22).
In the present study, we analyzed additional
recurrence factors of ACC of the head and neck at the molecular
biomarker level by assessing glucose metabolism by
immunohistochemistry. We found that the GLUT-1 expression rate was
38.1%, the p-Akt expression rate was 50.0% and the PI3K expression
rate was 38.1%. The expression of these markers was higher in ACC
than in inflammatory tissues and tissues of benign tumors
(P<0.001). Our results indicate that GLUT-1, PI3K and p-Akt may
be useful in predicting recurrence in patients with ACC of the head
and neck. In multivariate analyses, only p-Akt was a significant
predictor of recurrence. We also found that GLUT-1 was associated
with T stage and distant metastasis of ACC. This result was similar
to our previous study as well as to other studies (23–26).
We found that GLUT-1 expression was higher in head and neck
carcinoma than in normal tissues and adjacent carcinoma tissues
(23). As early as 1996, Younes
et al demonstrated that GLUT-1 expression was higher in
various malignant tumors than in benign counterparts using
immunohistochemistry and suggested that GLUT-1 played a crucial
role in carcinogenesis (24). Pizzi
et al confirmed that GLUT-1 was involved in a relatively
early phase of pancreatic carcinogenesis (25). Semaan et al showed that
GLUT-1 expression was correlated with tumor proliferation and
microvessel density in epithelial ovarian carcinoma (26). In ACC, Campistron et al
reported a high uptake of 18F-FDG by the lung and a case
of liver metastasis in a patient with lung ACC by PET/CT. The liver
18F-FDG uptake was confirmed by GLUT-1-positive
immunostaining (9). These findings
showed that GLUT-1 expression was correlated with the
transformation of benign tissue from borderline to invasive cancer.
Völker et al (27) found
that the expression of p-Akt was an independent predictor of higher
relapse risk in ACC, as was incomplete surgical resection (27); however, GLUT-1 was not associated
with recurrence and metastasis. Thus, the significance of GLUT-1
expression in ACC of the head and neck should be further
studied.
GLUT-1 expression reflects the hypoxia of malignant
tumors (12,23,26)
and may be activated by the PI3K/Akt pathway. The PI3K/Akt pathway
has been shown to promote both GLUT-1 cell-surface trafficking and
activity (14,15). The activation and phosphorylation of
PI3K/Akt are not only well-recognized regulators of cell growth,
survival and angiogenesis, but they also play an important role in
promoting glucose metabolism (28).
The activation of Akt may be responsible for the metabolic
processes occurring during the Warburg effect (29). Melstrom et al found that PI3K
inhibitors downregulated both GLUT-1 mRNA and protein expression
and suggested that the PI3K/Akt pathway is involved in mediating
GLUT-1 (15). In the present study,
the correlations between GLUT-1 and PI3K expression, between GLUT-1
and p-Akt expression and between p-Akt and PI3K expression were
significant. For further scrutiny of the effect of the PI3K/Akt
pathway in mediating GLUT-1 expression, tumors were redefined
according to their combined GLUT-1 and p-Akt immunohistochemical
status, resulting in 4 phenotypes:
p-Akt+/GLUT+,
p-Akt+/GLUT−,
p-Akt−/GLUT+ and
p-Akt−/GLUT−. We observed that the prevalence
of the 4 phenotypic groups was 31.0%
p-Akt+/GLUT+, 16.7%
p-Akt+/GLUT−, 9.4%
p-Akt−/GLUT+ and 42.9%
p-Akt−/GLUT−. The consistency of GLUT-1
expression and p-Akt expression was 59.6%, suggesting that p-Akt
may regulate GLUT-1 expression. One mechanism may be that Akt
stimulates GLUT-1 transcription via the phosphorylation of a
particular protein(s) (30).
However, another possible mechanism may be that Akt does not affect
the expression of the GLUT-1 gene, but Akt-driven glucose uptake
and phosphorylation by GLUT-1 causes an accumulation of
glucose-6-phosphate, which acts to provide cell survival signals,
and Akt maintains cell-surface GLUT-1 localization (31). However, an inconsistency of
expression between GLUT-1 and p-Akt existed
(p-Akt+/GLUT− and
p-Akt−/GLUT+). This suggested that other
signaling pathways may play a role in regulating the expression of
GLUT-1, such as MAPK pathways (32). Thus, the mechanism by which the
PI3K/Akt signaling pathway affects GLUT-1 expression remains
unclear.
In ACC, few studies exist regarding the expression
of signaling proteins of the PI3K/Akt pathway (27,33).
These studies have only analyzed the expression of p-Akt (27,33).
Although our study may be limited by the small number of patients,
to our knowledge, this study is the first to detect the two
signaling proteins, p-Akt and PI3K, in ACC. In our cohort, the
prevalence of the 4 phenotypic groups was 31.0%
p-Akt+/PI3K+, 21.4%
p-Akt+/PI3K−, 7.1%
p-Akt−/PI3K+ and 40.5%
p-Akt−/PI3K−. A significant positive
correlation was found between these phenotypic groups, T stage
(p=0.022) and recurrence (p=0.000). The PI3K+ groups
(p-Akt−/PI3K+ and
p-Akt+/PI3K+) showed higher recurrence rates
than the PI3K− groups
(p-Akt+/PI3K− and
p-Akt−/PI3K−). Aleskandarany et al
demonstrated that the prevalence of overexpressed p-Akt was 76% and
that of PI3K was 75% in breast cancer (33). This relatively high prevalence of
p-Akt may be a reflection of the oncogenetic role that was reported
to occur early during the cascade of carcinogenic events, as
observed in in situ stage carcinomas prior to their
progression to invasive carcinomas (33). They also found that significant
differences among these combinatorial phenotypic groups were noted
for breast cancer-specific survival and metastasis-free survival.
In our study, the proportion of p-Akt+/PI3K−
was 21.4% and p-Akt−/PI3K+ was 7.1%. These
results show that the expression of PI3K and Akt was incoordinate
and suggest that the activation of Akt in ACC may occur through
mechanisms of Akt activation rather than through PI3K (34).
In summary, our study has demonstrated that T stage,
a positive resection margin, perineural invasion, surgery without
postoperative radiotherapy and expression of GLUT-1, PI3K and p-Akt
were factors predictive of ACC recurrence by univariate analyses.
In multivariate analyses, perineural invasion, a positive resection
margin and p-Akt were significant predictors of recurrence. Thus,
initial surgery is very important for the recurrence of ACC of the
head and neck. The expression of GLUT-1, p-Akt or PI3K protein in
ACC was higher than that in inflammatory lesions or benign tumors;
therefore, overexpression of these biomarkers may play a role in
the development of ACC.
Acknowledgements
This study was supported by grants
from the Department of Health Bureau, Zhejiang, China (No.
2009B042), the Science and Technology Department of Zhejiang
Province (No. 2009C33026), and the National Natural Science
Foundation of China (No. 81172562).
References
|
1.
|
E ZvrkoM GolubovićLaryngeal adenoid cystic
carcinomaActa Otorhinolaryngol Ital292792822009
|
|
2.
|
AD LupinettiDB RobertsMD WilliamsSinonasal
adenoid cystic carcinoma: the M.D. Anderson Cancer Center
experienceCancer11027262731200710.1002/cncr.2309617960615
|
|
3.
|
DE Cruz PerezFR PiresMA LopesOP de
AlmeidaLP KowalskiAdenoid cystic carcinoma and mucoepidermoid
carcinoma of the maxillary sinus: report of a 44-year experience of
25 cases from a single institutionJ Oral Maxillofac
Surg6415921597200617052584
|
|
4.
|
F DongPW GidleyT HoAdenoid cystic
carcinoma of the external auditory
canalLaryngoscope11815911596200810.1097/MLG.0b013e31817c42a818677277
|
|
5.
|
JE van der WalAG BeckingGB SnowI van der
WaalDistant metastases of adenoid cystic carcinoma of the salivary
glands and the value of diagnostic examinations during
follow-upHead Neck24779783200212203804
|
|
6.
|
DR GomezBS HoppeSL WoldenOutcomes and
prognostic variables in adenoid cystic carcinoma of the head and
neck: a recent experienceInt J Radiat Oncol Biol
Phys7013651372200810.1016/j.ijrobp.2007.08.00818029108
|
|
7.
|
YH KoMA LeeYS HongPrognostic factors
affecting the clinical outcome of adenoid cystic carcinoma of the
head and neckJpn J Clin
Oncol37805811200710.1093/jjco/hym11918057012
|
|
8.
|
J ShangL ShengK WangY ShuiQ WeiExpression
of neural cell adhesion molecule in salivary adenoid cystic
carcinoma and its correlation with perineural invasionOncol
Rep1814131416200717982624
|
|
9.
|
M CampistronI RouquetteF CourbonAdenoid
cystic carcinoma of the lung: interest of 18FDG PET/CT in the
management of an atypical presentationLung
Cancer59133136200810.1016/j.lungcan.2007.06.00217640764
|
|
10.
|
LF LiSH ZhouK ZhaoClinical significance of
FDG single-photon emission computed tomography: computed tomography
in the diagnosis of head and neck cancers and study of its
mechanismCancer Biother
Radiopharm23701714200810.1089/cbr.2008.0510
|
|
11.
|
IS WoodP TrayhurnGlucose transporters
(GLUT and SGLT): expanded families of sugar transport proteinsBr J
Nutr8939200310.1079/BJN200276312568659
|
|
12.
|
JK ChungYJ LeeSK KimJM JeongDS LeeMC
LeeComparison of [18F] fluorodeoxyglucose uptake with glucose
transporter-1 expression and proliferation rate in human glioma and
non-small-cell lung cancerNucl Med Commun2511172004
|
|
13.
|
SH ZhouJ FanXM ChenKJ ChengSQ
WangInhibition of cell proliferation and glucose uptake in human
laryngeal carcinoma cells by antisense oligonucleotides against
glucose transporter-1Head Neck3116241633200910.1002/hed.21137
|
|
14.
|
SR JacobsCE HermanNJ MaciverGlucose uptake
is limiting in T cell activation and requires CD28-mediated
Akt-dependent and independent pathwaysJ
Immunol18044764486200810.4049/jimmunol.180.7.447618354169
|
|
15.
|
LG MelstromMR SalabatXZ DingApigenin
inhibits the GLUT-1 glucose transporter and the phosphoinositide
3-kinase/Akt pathway in human pancreatic cancer
cellsPancreas37426431200810.1097/MPA.0b013e3181735ccb18953257
|
|
16.
|
J BussinkAJ van der KogelJH
KaandersActivation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancerLancet
Oncol9288296200810.1016/S1470-2045(08)70073-118308254
|
|
17.
|
J FangXM LuoHT YaoSH ZhouLX RuanSX
YanExpression of glucose transporter-1, hypoxia-inducible
factor-1α, phosphatidylinositol 3-kinase and protein kinase B (Akt)
in relation to [(18)F] fluorodeoxyglucose uptake in nasopharyngeal
diffuse large B-cell lymphoma: a case report and literature reviewJ
Int Med Res38216021682010
|
|
18.
|
AM ChenMK BucciV WeinbergAdenoid cystic
carcinoma of the head and neck treated by surgery with or without
postoperative radiation therapy: prognostic features of
recurrenceInt J Radiat Oncol Biol
Phys66152159200610.1016/j.ijrobp.2006.04.01416904520
|
|
19.
|
YH KoSY RohHS WonPrognostic significance
of nuclear survivin expression in resected adenoid cystic carcinoma
of the head and neckHead Neck
Oncol230201010.1186/1758-3284-2-3021034499
|
|
20.
|
EP ProkopakisCH SnydermanEY HannaRL
CarrauJT JohnsonF D’AmicoRisk factors for local recurrence of
adenoid cystic carcinoma: the role of post-operative radiotherapyAm
J Otolarygo120281286199910.1016/S0196-0709(99)90028-5
|
|
21.
|
WM MendenhallCG MorrisRJ AmdurJW WerningRW
HinermanDB VillaretRadiotherapy alone or combined with surgery for
adenoid cystic carcinoma of the head and neckHead
Neck26154162200410.1002/hed.1038014762884
|
|
22.
|
TA IseliLH KarnellSM GrahamRole of
radiotherapy in adenoid cystic carcinoma of the head and neckJ
Laryngol Otol12311371144200910.1017/S002221510999033819573256
|
|
23.
|
S ZhouS WangQ WuQ WangExpression of
glucose transporter-1 and -3 in the head and neck carcinoma - The
correlation of the expression with the biological behaviorsORL J
Otorhinolaryngol Relat
Spec70189194200810.1159/00012429318401196
|
|
24.
|
M YounesLV LechagoJR SomoanoM MosharafJ
LechagoWide expression of the human erythrocyte glucose transporter
Glut1 in human cancersCancer Res561164116719968640778
|
|
25.
|
S PizziA PorzionatoC PasqualiGlucose
transporter-1 expression and prognostic significance in pancreactic
carcinogenesisHistol Histopathol24175185200919085834
|
|
26.
|
A SemaanAR MunkarahH ArabiExpression of
GLUT-1 in epithelial ovarian carcinoma: Correlation with tumor cell
proliferation, angiogenesis, survival and ability to predict
optimal cytoreductionGynecol
Oncol121181186201110.1016/j.ygyno.2010.11.019
|
|
27.
|
HU VölkerM ScheichA BerndtExpression of
p-AKT characterizes adenoid cystic carcinomas of head and neck with
a higher risk for tumor relapsesDiagn Patho1918200919545368
|
|
28.
|
JY PaikBH KoKH JungKH LeeFibronectin
stimulates endothelial cell 18F-FDG uptake through focal adhesion
kinase-mediated phosphatidylinositol 3-kinase/Akt signalingJ Nucl
Med50618624200910.2967/jnumed.108.059386
|
|
29.
|
RL ElstromDE BauerM BuzzaiAkt stimulates
aerobic glycolysis in cancer cellsCancer
Res6438923899200410.1158/0008-5472.CAN-03-290415172999
|
|
30.
|
A BarthelST OkinoJ LiaoRegulation of GLUT1
gene transcription by the serine/threonine kinase Akt1J Biol
Chem2742028120286199910.1074/jbc.274.29.2028110400647
|
|
31.
|
JC RathmellCJ FoxDR PlasPS HammermanRM
CinalliCB ThompsonAkt-directed glucose metabolism can prevent Bax
conformation change and promote growth factor-independent
survivalMol Cell
Biol2373157328200310.1128/MCB.23.20.7315-7328.200314517300
|
|
32.
|
YK LeeJE KimSH NamDifferential regulation
of the biosynthesis of glucose transporters by the PI3-K and MAPK
pathways of insulin signaling by treatment with novel compounds
from Liriope platyphyllaInt J Mol Med27319327201121165549
|
|
33.
|
MA AleskandaranyEA RakhaMA AhmedDG PoweIO
EllisAR GreenClinicopathologic and molecular significance of
phospho-Akt expression in early invasive breast cancerBreast Cancer
Res Treat127407416201110.1007/s10549-010-1012-y20617378
|
|
34.
|
JD CarptenAL FaberC HornA transforming
mutation in the pleckstrin homology domain of AKT1 in
cancerNature448439444200710.1038/nature0593317611497
|