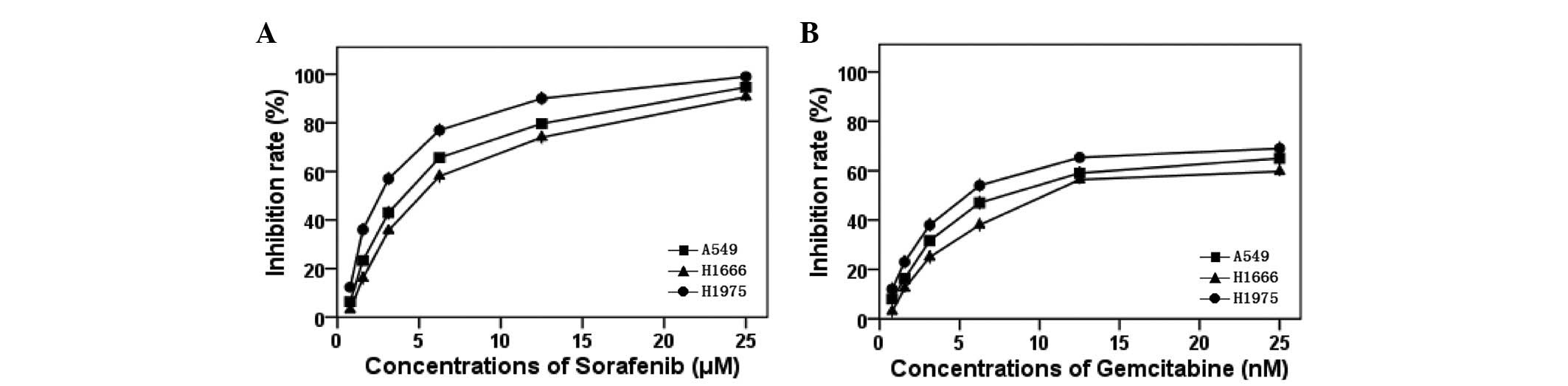
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Pao W, Wang TY, Riely GJ, et al: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Roles of the Raf/MEK/ERK pathway in cell growth, malignant
transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: role in clinical
response to EGFR tyrosine kinase inhibitors. Oncogene. 28(Suppl 1):
S24–S31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adjei AA: K-ras as a Target for Lung
Cancer Therapy. J Thorac Oncol. 3(Suppl 2): S160–S163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ladanyi M and Pao W: Lung adenocarcinoma:
guiding EGFR-targeted therapy and beyond. Mod Pathol. 21:S16–S22.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carter CA, Chen C, Brink C, et al:
Sorafenib is efficacious and tolerated in combination with
cytotoxic or cytostatic agents in preclinical models of human
non-small cell lung carcinoma. Cancer Chemother Pharmacol.
59:183–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dal Lago L, D’Hondt V and Awada A:
Selected combination therapy with sorafenib: a review of clinical
data and perspectives in advanced solid tumors. Oncologist.
13:845–858. 2008.PubMed/NCBI
|
|
10
|
Blumenschein GR Jr, Gatzemeier U, Fossella
F, et al: Phase II, multicenter, uncontrolled trial of single-agent
sorafenib in patients with relapsed or refractory, advanced
non-small-cell lung cancer. J Clin Oncol. 27:4274–4280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dy GK, Hillman SL, Rowland KM Jr, et al: A
front-line window of opportunity phase 2 study of sorafenib in
patients with advanced nonsmall cell lung cancer: North Central
Cancer Treatment Group Study N0326. Cancer. 116:5686–5693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dougherty DW and Friedberg JW: Gemcitabine
and other new cytotoxic drugs: will any find their way into primary
therapy? Curr Hematol Malig Rep. 5:148–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strumberg D, Clark JW, Awada A, et al:
Safety, pharmacokinetics, and preliminary antitumor activity of
sorafenib: a review of four phase I trials in patients with
advanced refractory solid tumors. Oncologist. 12:426–437. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masago K, Fujita S, Togashi Y, et al:
Clinicopathologic factors affecting the progression-free survival
of patients with advanced non-small-cell lung cancer after
gefitinib therapy. Clin Lung Cancer. 12:56–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrelli A and Giordano S: From single- to
multi-target drugs in cancer therapy: when aspecificity becomes an
advantage. Curr Med Chem. 15:422–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linardou H, Dahabreh IJ, Kanaloupiti D, et
al: Assessment of somatic K-Ras mutations as a mechanism associated
with resistance to EGFR-targeted agents: a systematic review and
meta-analysis of studies in advanced non-small cell lung cancer and
colorectal cancer. Lancet Oncol. 9:962–972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopez-Chavez A, Carter CA and Giaccone G:
The role of KRAS mutations in resistance to EGFR inhibition in the
treatment of cancer. Curr Opin Investig Drugs. 10:1305–1314.
2009.PubMed/NCBI
|
|
21
|
Kotoula V, Sozopoulos E, Litsiou H, et al:
Mutational analysis of the BRAF, RAS and EGFR genes in human
adrenocortical carcinomas. Endocr Relat Cancer. 16:565–572. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torres K and Horwitz SB: Mechanisms of
taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
23
|
Takezawa K, Okamoto I, Yonesaka K,
Hatashita E, Yamada Y, Fukuoka M and Nakagawa K: Sorafenib inhibits
non-small cell lung cancer cell growth by targeting B-RAF in KRAS
wild-type cells and C-RAF in KRAS mutant cells. Cancer Res.
69:6515–6521. 2009. View Article : Google Scholar : PubMed/NCBI
|