Introduction
Interleukin 21 (IL-21), a member of the common
γ-chain (γc) receptor cytokine family, has been shown to have
structural homology to IL-2 and IL-15. IL-21 is mainly secreted by
activated CD4+ T cells and NK T cells (1–4). Its
receptor (IL-21R) is widely expressed on various cell types within
the immune system, including NK cells, B cells, T cells,
macrophages and dendritic cells (DCs) (5–9). The
widespread lymphoid distribution of the IL-21R leads to pleiotropic
action of IL-21 in the innate and adaptive immune responses. A
number of preclinical studies have shown that IL-21 has important
anti-tumor effects (10–12). The antitumor activity of IL-21 has
been shown to mainly depend on CD8+ T cells and NK
cells. IL-21 promotes the activation and antigen-dependent
proliferation of CD8+ T cells and enhances their
cytolytic activity (13–15). It also regulates the proliferation,
survival, differentiation and effector functions of NK cells
(1,5,16). As
a promising cytokine for cancer immunotherapy, IL-21 has been
undergoing Phase I and II testing in clinical trials for the
treatment of early phase renal cell carcinoma and melanoma
(17–19).
Accumulated data have shown that tumors may exploit
certain inhibitory checkpoints and pathways to escape immune
attack, even in the face of a strongly induced antitumor immune
response. In these tumor-escaping mechanisms, the expression of
programmed death ligand 1 (PD-L1) by the tumor may play an
important role in its resistance to immune destruction (20). PD-L1 is normally expressed in a
broad spectrum of cell types and plays a crucial role in the
maintenance of peripheral tolerance. Upregulated expression of
PD-L1 has been found in certain solid tumors, including
hepatocellular carcinoma. Its overexpression is significantly
associated with tumor aggressiveness (21). The binding of PD-L1 to its receptor
PD-1, expressed on activated T cells, was found to inhibit the
proliferation of antigen-specific T cells, particularly cytotoxic T
cells, to induce T-cell apoptosis, and to decrease the secretion of
IL-2 and IFN-γ (20,22). Previous studies (23,24)
have demonstrated that blockade of the PD-1 pathway may enhance
antitumor immunity and inhibit tumor growth. Further studies have
revealed that soluble PD-1 (sPD-1) blocks the PD-1 pathway and
augments the antitumor immune response (25,26).
In this study, we used a gene transfer method to
determine whether sPD-1 is able to enhance the effects of IL-21 in
the treatment of hepatocellular carcinoma.
Materials and methods
Mice and cell lines
Female BALB/c mice (6–8 weeks old) were purchased
from the Center of Medical Experimental Animals of Xuzhou Medical
College (Xuzhou, China). The animals were maintained under
pathogen-free conditions. The mouse protocol was approved by the
Animal Care and Use Committee of Xuzhou Medical College. The mouse
H22 hepatocarcinoma and 293T cell lines were purchased from the
Institute of Oncology (Beijing, China). The mouse H22 cell line was
maintained by intraperitoneal (i.p.) passage in BALB/c mice.
Plasmids
The murine sPD-1 expression plasmid vector psPD-1
has been described previously (27). Briefly, the cDNA of the PD-1
extracellular domain was obtained from the whole cDNA of murine
splenocytes by reverse transcription-PCR (RT-PCR). The sequences of
the PCR primers specific to sPD-1 are as follows: sense
5′-GGTTCATAGAATTCCTGA AGGCGACACTGCC-3′,
(underlined nucleotides indicate EcoR I site) containing a
restriction site for endonuclease EcoRI, and antisense
5′-CCTGGTAAGCTTATTGAAA CCGGCCTTCTGG-3′
(underlined nucleotides indicate HindIII. site) containing a
restriction site for endonuclease HindIII. The plasmid
vector psPD-1 was constructed by insertion of the purified sPD-1
cDNA into plasmid pcDNA3.1 and then sequenced by Shenggong
Biotechnology Co. (Shanghai, China). Plasmid pcDNA3.1 was a gift
from Professor Chenzhi (Institute of Infectious Disease, Zhejiang
University, China). The murine IL-21 expression plasmid vector
pmIL-21 was provided by Professor Doujun (Department of
Microbiology and Immunology, Southeast University, China).
Cell transfection in vitro and in
vivo
In the in vitro culture, the transfection of
psPD-1 or pmIL-21 into 293T cells was performed using Lipofectamine
2000 liposomes (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Stably transfected clones were
selected using G418 (500 μg/ml).
In the in vivo animal studies, the
transfection was performed by direct local injection. Briefly,
BALB/c mice received an intramuscular (i.m.) injection of 100
μg naked plasmid in 100 μl saline at the inoculation
site. The plasmids were injected every 3 days.
Tumor animal model and treatment
protocol
The BALB/c mice (8 per group) received a
subcutaneous injection of 1×105 H22 cells in the right
hind limb to establish the hepatoma model. Day 1 post H22 cell
inoculation, 100 μg plasmid DNA was injected directly into
the muscle at the inoculation site in all treatment groups. In the
combination treatment group, the mice received 50 μg pmIL-21
and 50 μg psPD-1 by i.m. injection. The plasmid or saline
was injected every 3 days. The mice in the control groups received
an equal amount of pcDNA3.1 or equal volume of saline. The mice
were sacrificed on day 28 after injection of the H22 cells.
Tumor volume calculation and tumor growth
inhibition (TGI)
The sizes of the implanted tumors were measured
every week with a ruler. The tumor volume was calculated on days 7,
14, 21 and 28 using the formula: V=1/2(a×b2) (V, tumor
volume; a, length, b, width). TGI was calculated using the formula:
(1−T/C) × 100% (T, tumor volume of the treated group; C, tumor
volume of the control group).
RT-PCR
Total RNA was obtained from the tumor marginal
tissues of the tumor-bearing mice using the TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. The
relative quantities of the mRNAs of IL-2, IFN-γ and IL-10 were
determined by RT-PCR using a Two Step RT-PCR kit (Tiangen Biotech
Co., Ltd., Beijing, China); 30 PCR cycles were used for each sample
and β-actin was used as the matched control. The primer sequences
were as follows: IL-2 sense, 5′-ACCTTGCTAATCACTCC-3′, antisense,
5′-AAGTCCACC ACAGTTGCT-3′; IFN-γ sense, 5′-ATTGGCATAGATGTG GAA-3′,
antisense, 5′-TCAAACTTGGCAATACTC-3′; IL-10 sense,
5′-ACCTGGTAGAAGTGATGC-3′, antisense, 5′-AAG GAGTTGTTTCCGTTA-3′; and
β-actin sense, 5′-AGCGAG CATCCCCCAAAGTT-3′, antisense,
5′-GGGCACGAAGGC TCATCATT-3′.
ELISA
The IL-21- and sPD-1-expressing 293T clones
(2.0×106/well) were cultured in 24-well plates for 24 h.
The supernatants of the cultures were collected for IL-21 and sPD-1
detection. Murine IL-21 and sPD-1 ELISA kits (R&D Systems,
Minneapolis, MN, USA) were used to identify the expression of IL-21
and sPD-1 proteins, respectively.
Mouse blood serum was collected on day 28 after
injection of the H22 cells. We used murine IL-21, sPD-1, IFN-γ,
IL-2 and IL-10 ELISA kits (R&D Systems) to assess the levels of
IL-21, sPD-1, IFN-γ, IL-2 and IL-10 in murine serum.
Western blot analysis
Muscle tissues, isolated 72 h after the i.m.
injection of psPD-1 or pmIL-21, were incubated with lysis buffer
and a protease inhibitor cocktail (EMD Biosciences, Inc., San
Diego, CA, USA) at 4°C for 20 min. Western blot analyses were
performed using standard techniques. Protein levels were
quantitated using a Bradford assay (Bio-Rad Laboratories, Hercules,
CA, USA). Total protein (30 μg per lane) was run on 12%
SDS-PAGE gel and transferred to a PVDF membrane. After blocking and
washing, the membranes were incubated with antibodies against
either IL-21 or sPD-1 in TBS-5% milk overnight at 4°C and then
incubated with the appropriate secondary antibody. The proteins
were detected using an enhanced chemiluminescence ECL kit (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The expression of
IL-21 or sPD-1 protein in the tumor tissues of the tumor-bearing
mice treated with the sPD-1 and/or IL-21 gene were also detected as
described above. Anti-mouse PD-1 polyclonal antibody was purchased
from BioLend (USA) and anti-mouse IL-21 polyclonal antibody was
purchased from ReliaTech GmbH (Wolfenbüttel, Germany).
CTL cytotoxicity assay
A lactate dehydrogenase (LDH) release assay was
performed to determine the cytotoxicity of the mouse splenocytes.
The splenocytes were co-cultured with irradiated H22 cells for 7
days in the presence of 20 U/ml rIL-2 and then used as effector
cells for the cytotoxicity assay. The H22 target cells were plated
at 5×103 cells/well in 96-well round-bottom plates and
co-cultured with effector cells at various effector/target (E:T)
ratios for 4 h at 37°C. After incubation, cytotoxic activity was
detected by LDH release using the CytoTox 96 Nonradioactive
Cytotoxicity assay (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. The rate of specific target cell lysis
was calculated using the following formula: [(Sample release
-spontaneous release)/(Total release-spontaneous release)] ×
100.
Flow cytometry
Flow cytometry was used to detect the numbers of
CD8+ T cells and NK cells in the spleen. A single-cell
suspension of splenocytes was prepared from the spleens of the
tumor-bearing mice. The cells were then stained with fluorescein
isothiocyanate (FITC)-labeled anti-CD8, phycoerythrin (PE)-Cy5
anti-CD3 and PE-anti-NK1.1. These fluorochromes were detected using
a flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed
using CellQuest software. All antibodies used were purchased from
BioLend (San Diego, CA, USA).
Histopathological analysis of tumors
The mice were sacrificed 28 days after injection of
the H22 cells. The tumors were surgically excised and fixed in 10%
formalin. The formalin-fixed tissues were embedded in paraffin and
then sectioned for H&E staining. The slides were viewed with a
microscope at a magnification of ×400.
Statistical analysis
For descriptive statistics, values are expressed as
the mean ± standard deviation. The statistical significance of
differences between groups was assessed using the Student’s t-test.
P<0.05 were considered to indicate a statistically significant
difference. Statistical analysis was performed using the GraphPad
Prism 4.0 statistical software package (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
IL-21 and sPD-1 proteins were expressed
in transfected cells and in vivo
Following the transfection of the recombinant
plasmids into 293T cells and the establishment of stably IL-21- and
sPD-1-expressing 293T cells, an ELISA was performed to detect the
secretion of IL-21 and sPD-1 proteins by the transfected cells. We
successfully detected high expression levels of IL-21 (839.98±56.38
pg/ml) and sPD-1 (764.64±61.25 pg/ml) proteins in the culture
supernatants. We also examined the expression of IL-21 and sPD-1
proteins in the peripheral blood of the tumor-bearing mice. The
levels of IL-21 protein were higher in the mice treated with the
IL-21 gene alone (357.54±60.36 pg/ml) or in combination with sPD-1
(238±53.44 pg/ml) than those in the mice treated with the sPD-1
gene (98.37±27.64 pg/ml) or plasmid pcDNA3.1 (82.15±19.87 pg/ml),
and the levels of sPD-1 protein were significantly elevated in the
mice treated with the sPD-1 gene alone (369.53±97.37 pg/ml) or in
combination with the IL-21 gene (217.38±65.64 pg/ml) than those in
the mice treated treated with the IL-21 gene (57.86±21.30 pg/ml) or
plasmid pcDNA3.1 (49.31±17.25 pg/ml). Later, we examined the
expression of IL-21 and sPD-1 in vivo following the
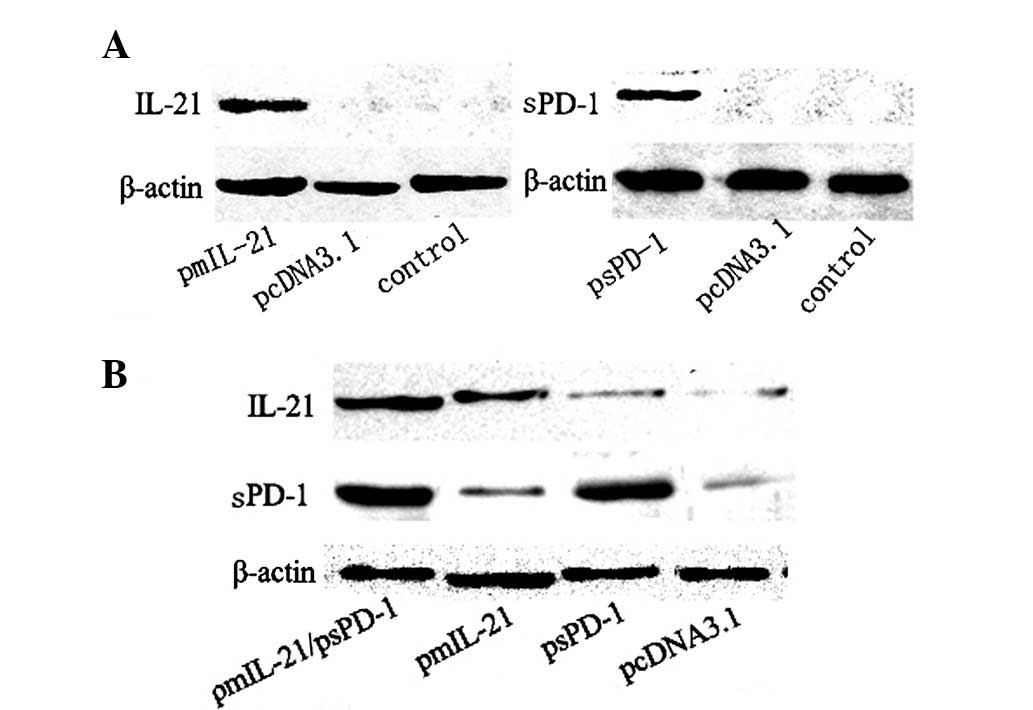
injection of naked recombinant plasmid DNA. As shown in Fig. 1, the IL-21 and sPD-1 proteins were
detected in the injected muscle tissues or tumor tissues by western
blot analysis.
Antitumor efficacy induced by IL-21 alone
or in combination with sPD-1 in tumor-bearing mice
We measured the length and width of the implanted
tumors and calculated the tumor volume on days 7, 14, 21 and 28
after the injection of the H22 hepatoma cells into the BALB/c mice.
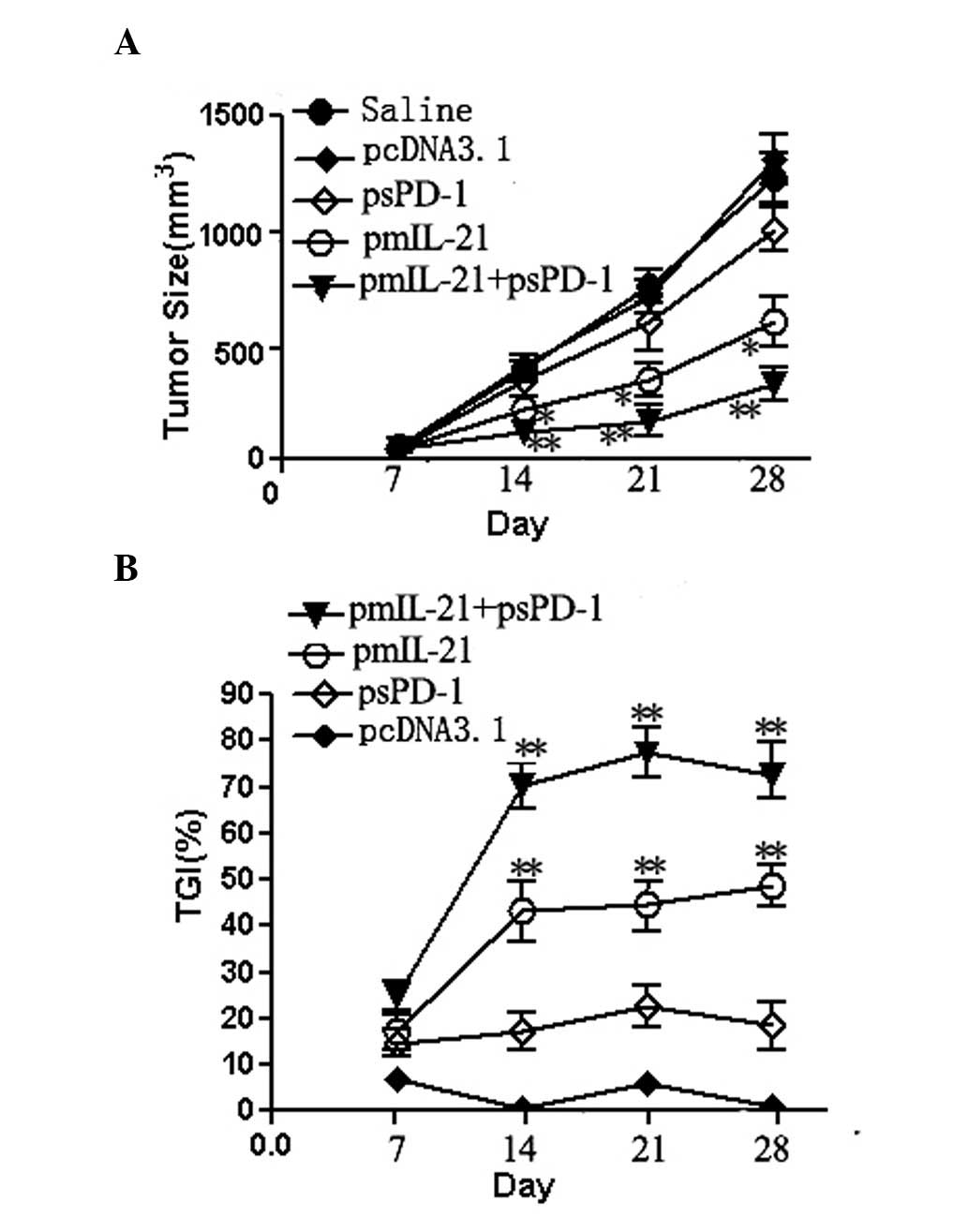
As shown in Fig. 2, treatment with
the IL-21 gene alone significantly inhibited tumor growth at 14, 21
and 28 days after H22 cell injection, compared with tumor growth in
mice receiving sPD-1 gene therapy or naked plasmid pcDNA3.1
(P<0.05). However, the combination of IL-21 and sPD-1 treatment
showed the most potent suppression of tumor growth with a TGI of
≥70%. The sPD-1 treatment also resulted in a slight inhibition of
tumor growth with a TGI of ≤20%. Therefore, the suppression of
tumor growth by combined IL-21 and sPD-1 treatment was much
stronger than that by IL-21 treatment alone (P<0.05). These data
suggest that sPD-1 enhanced the IL-21-mediated antitumor
responses.
Enhanced CTL cytotoxicity in pmIL-21- and
psPD-1-injected mice
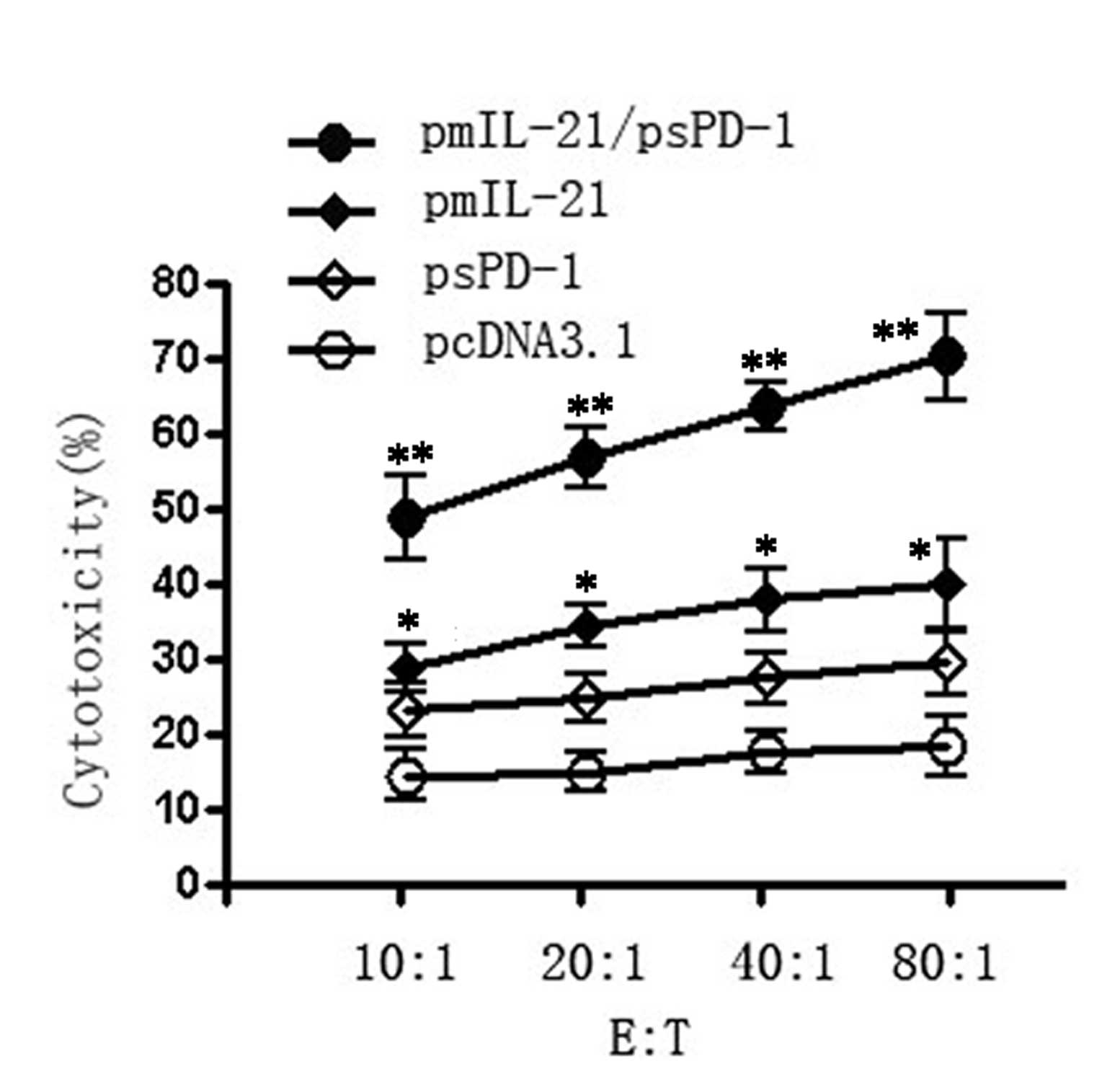
We next examined the CTL cytotoxic activities of
splenocytes from the tumor-bearing mice injected with pmIL-21 alone
or with a combination of pmIL-21 and psPD-1 on day 28 after tumor
inoculation. Fig. 3 shows that the
cytotoxic activities of the splenocytes to H22 tumor cells were
significantly enhanced in the pmIL-21 group and in the psPD-1 group
compared with the control vector pcDNA3 treatment group
(P<0.01). However, the cytotoxicity of the splenocytes was
further enhanced in the IL-21/sPD-1 combination group, compared
with the IL-21 and sPD-1 gene single treatment groups (P<0.01).
Thus, IL-21 and sPD-1 each mediate the cytotoxic function of
splenocytes, and a combination of IL-21 and sPD-1 showed
synergistic antitumor CTL cytotoxicity.
Increased CD8+ T cell and NK
cell numbers in splenocytes following pmIL-21 and psPD-1
treatment
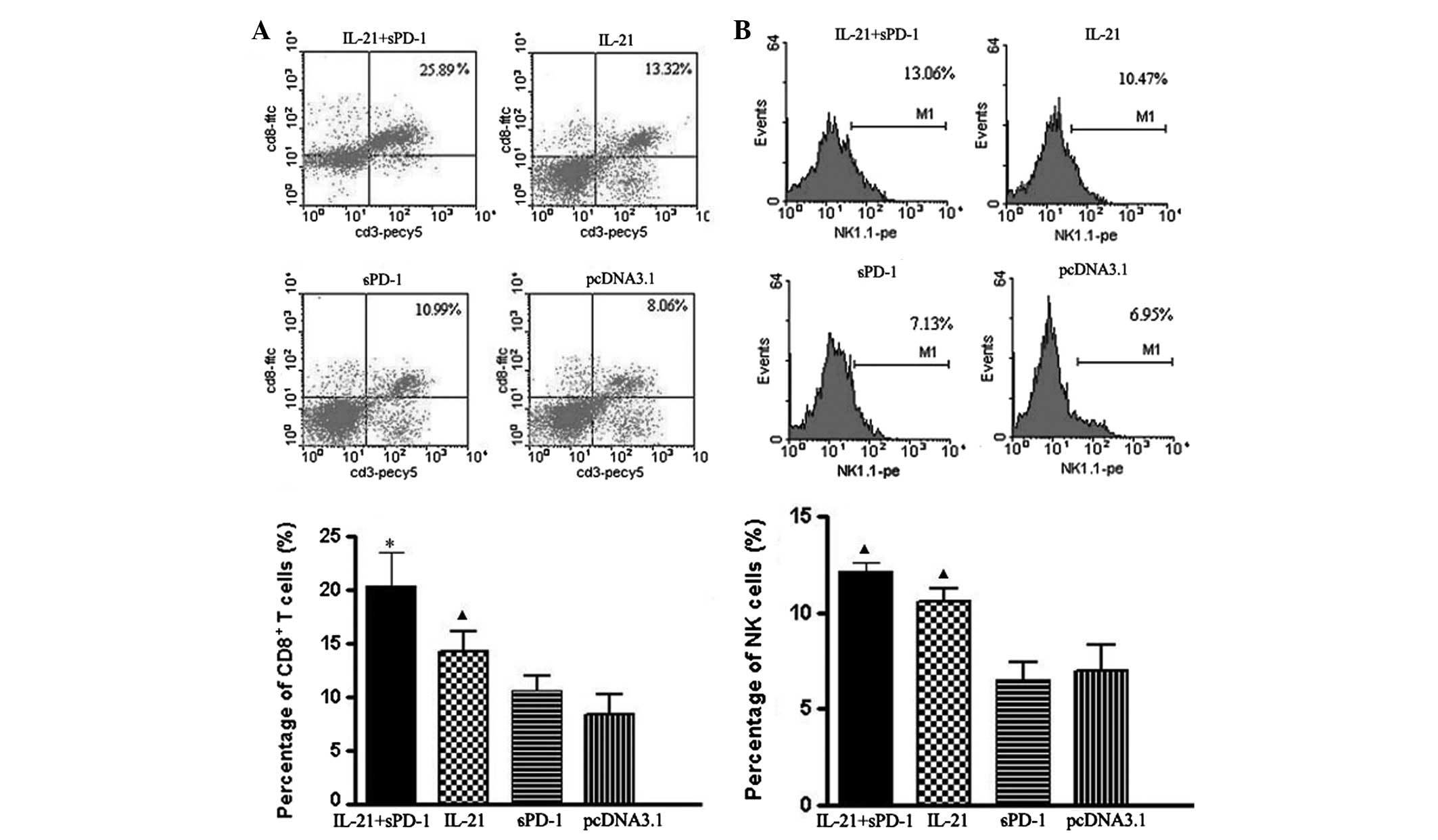
To further study the antitumor immunity trigged by
pmIL-21 gene therapy, we counted the numbers of CD8+ T
cells and NK cells in the splenocytes of the tumor-bearing mice.
Fig. 4 demonstrates that the
percentages of CD8+ T cells and NK cells were
significantly increased in the pmIL-21/ psPD-1 combination group
compared with the single-gene (IL-21 or sPD-1) treated groups
(P<0.05). Treatment with IL-21 gene alone also resulted in
significantly increased quantities of CD8+ T cells and
NK cells compared with the control group (P<0.05). Moreover, the
percentage of CD8+ T cells in the combined treatment
group was higher than that in the IL-21 treatment group
(P<0.05), which supports the synergistic efficacy of the sPD-1
and IL-21 treatments.
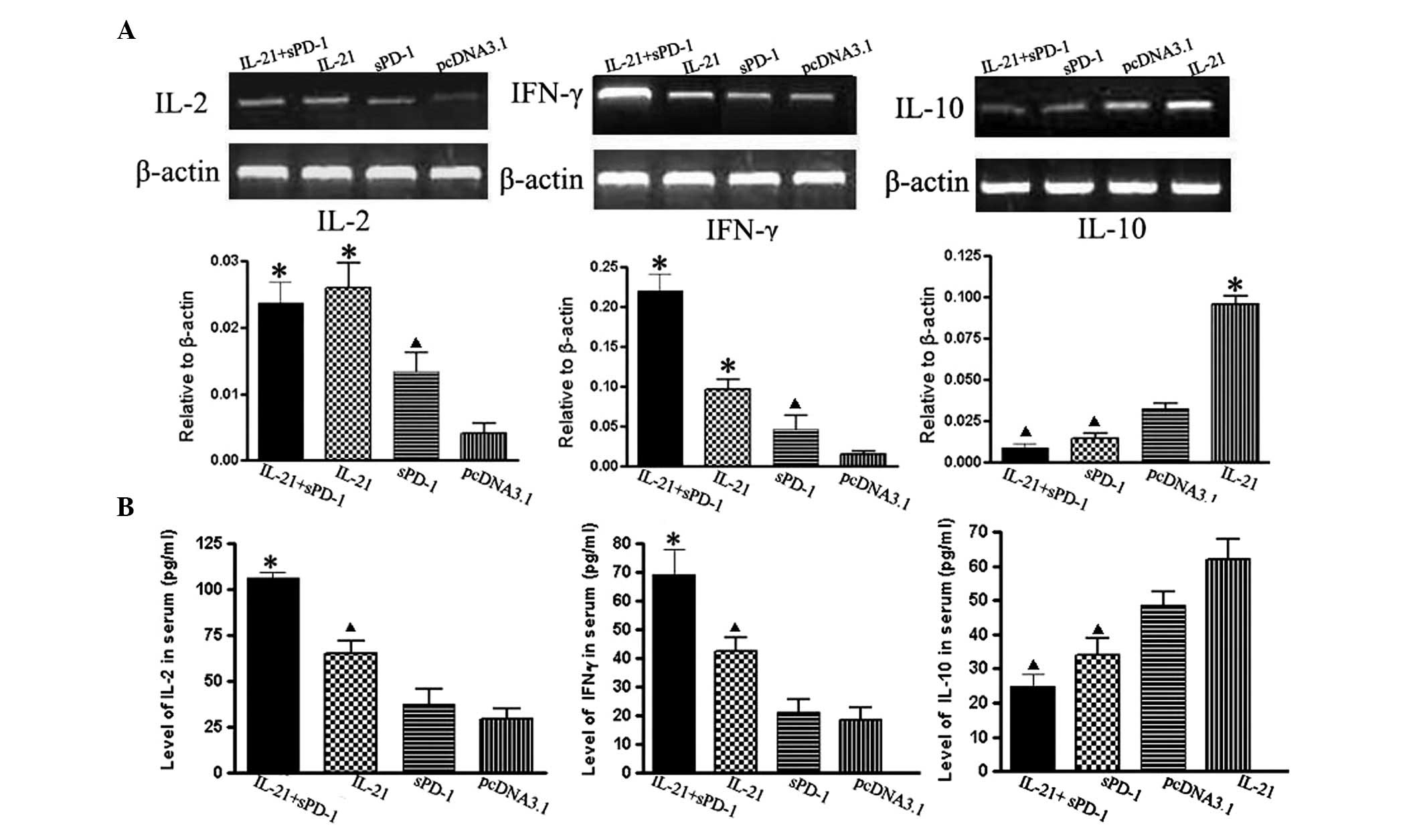
Effects of IL-21 and sPD-1 treatment on
the induction of cytokine expression
Cytokines are important in immune responses. To
assess the expression of cytokines associated with the antitumor
immunity induced by IL-21 alone or in combination with sPD-1
treatment in tumor-bearing mice, we examined not only the serum
levels of IL-2, IFN-γ and IL-10 by ELISA but also the transcription
activities of IL-2, IFN-γ and IL-10 genes in tumor marginal tissues
by RT-PCR. We found that in the combination treatment group the
expression level of IFN-γ mRNA was markedly upregulated, but the
expression level of IL-10 mRNA was significantly decreased compared
with those in the single-gene treatment and control groups
(P<0.05; Fig. 5). The changes of
IL-2 and IFN-γ expression in the IL-21 treatment group showed
similar trends to those in the control group. This was confirmed by
ELISA detection of the cytokine levels in serum (Fig. 5). The sPD-1 blockade of PD-1
resulted in slight increases of IL-2 or IFN-γ expression levels but
a significant decrease in IL-10 expression level relative to those
in the control group (P<0.05).
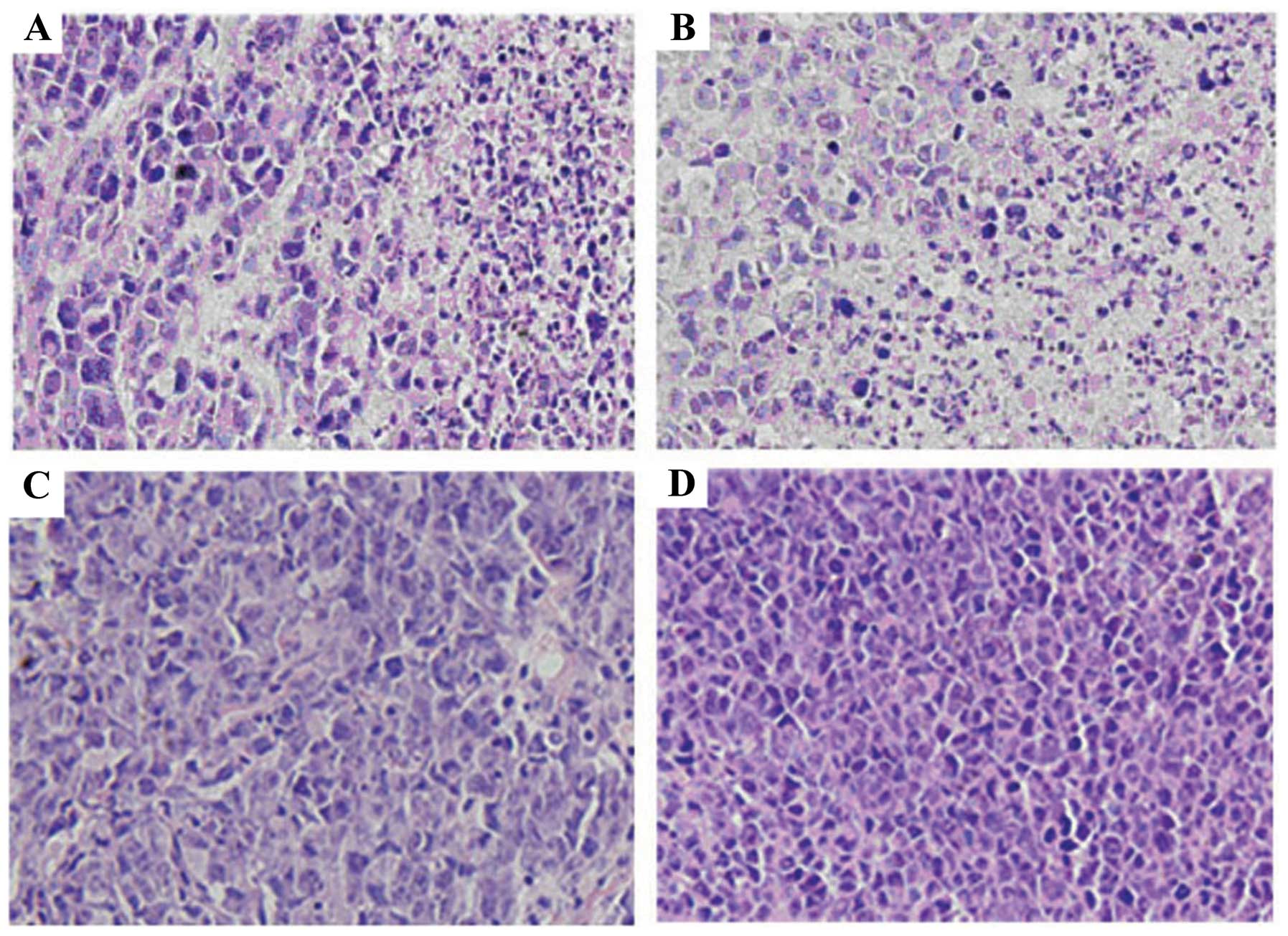
Tumor histopathology analysis
To further investigate the antitumor effects of
IL-21 and sPD1 treatment, tumor tissues were fixed and stained.
Fig. 6A and B reveals that,
consistent with the increased number of tumor-infiltrating
immunocytes, increased numbers of necrotic or apoptotic tumor cells
were found in the tumor sites derived from the tumor-bearing mice
challenged with pmIL-21 alone or in combination with psPD-1. By
contrast, tumor cells showed active growth and evident nucleic
division in tumor sites derived from the tumor-bearing mice
challenged with psPD-1 or pcDNA3.1 (Fig. 6C and D). These findings suggest that
IL-21 alone or combination with sPD-1 is able to trigger the
cellular immune response to the H22 hepatocellular carcinoma.
Discussion
Cancer immunotherapies usually focus on enhancing
the ability of effector T cells to eradicate tumors. However, these
therapies rarely translate into clinically satisfactory patient
responses. It is now recognized that this failure is partially the
result of the presence of negative regulatory pathways in the tumor
microenvironment. These negative pathways dampen antitumor immune
responses. The PD-L1:PD-1 immunosuppressive pathway is able to
inhibit specific T-cell responses and may be associated with tumor
cell immune tolerance (20). In
this study, we investigated the antitumor therapeutic effects of
transfection with recombinant plasmids containing sPD-1 and IL-21
against H22 hepatocellular carcinoma in mice. We found that IL-21
alone was able to induce a powerful antitumor immune response and
that sPD-1 further increased the antitumor immunity mediated by
IL-21. This treatment slowed tumor growth, reduced tumor size and
resulted in increased tumor cell necrosis and tumor-infiltrating
immunocytes, particularly in tumor tissues derived from the mice
treated with IL-21 in combination with sPD-1. The current results
suggest that the combination of IL-21 and blockade of PD-1:PD-L1
signaling with sPD-1 has a synergistic antitumor effect.
Other studies have shown that IL-21 elicits
significant antitumor effects in mice with established tumors
(28,29). However, to date, no study has
demonstrated that IL-21 has antitumor activity in vivo in
murine hepatocellular carcinoma models. In the present study, we
first demonstrated that the administration of IL-21-gene expression
vectors alone significantly inhibited the growth of H22
hepatocellular carcinoma tumors and induced an antitumor immune
response. We found that CTL cytotoxicity and CD8+ T cell
frequency in the spleen were significantly increased. In addition,
the number of NK cells in splenocytes and the expression levels of
IFN-γ and IL-2 in the serum or in the tumor margin were also
enhanced. In addition to immunostimulatory effects, IL-21 has also
been shown to directly increase the expression of PD-1 in T cells
and of PD-1 ligands on APCs (30).
The increased expression of PD-1 on T cells may decrease the
antitumor effects of IL-21. In an attempt to address this issue, we
investigated whether the antitumor effect of IL-21 could be
synergistically enhanced by the blockade of the PD-L1:PD-1
pathway.
Previous studies have suggested that CD8+
T cells and NK cells are the main effector cells responsible for
the lysis of tumor cells (31–33).
The mechanisms underlying the CD8+ T-cell-mediated
killing activity are direct contact-mediated cytotoxicity and the
secretion of cytokines, including TNF-α and IFN-γ. NK cells not
only destroy tumor cells directly, but they also modulate the
development of adaptive immune responses. In this way, NK cells are
critical to antitumor immunity. In this study, a combination of
IL-21 and sPD-1 treatment led to significantly increased numbers of
CD8+ T cells and NK cells and enhanced specific CTL
cytotoxic activity in the splenocytes of the tumor-bearing mice as
compared with single-gene (IL-21 or sPD-1) treatment. Although we
did not determine whether immune stimulation by IL-21 in
combination with sPD-1 results in similar changes in
tumor-infiltrated lymphocytes, our findings suggest that the
combined treatment may trigger innate and acquired immune
responses, causing regression of the H22 hepatoma carcinoma in this
mouse model
Cytokines have been shown to play a critical role in
the modulation of innate and adaptive immune responses (34). In the present study, we found that
the administration of IL-21 was able to upregulate the cytokines
IFN-γ and IL-2, while sPD-1 treatment significantly reduced the
expression of IL-10 in the tumor-bearing mice. The expression
levels of IFN-γ and IL-2 were further enhanced in mice treated with
a combination of IL-21 and sPD-1. In addition, the expression of
IL-10, a negative regulatory cytokine, was also inhibited in the
mice treated with the combination. These data suggest that
increased levels of IL-2 and IFN-γ and reduced levels of IL-10 in
the tumor-bearing mice treated with a combination of IL-21 and
sPD-1 may all contribute to enhancements of the antitumor effects
of CD8+ T cells or NK cells.
These findings demonstrate that immunotherapy with
IL-21 in combination with sPD-1 is able to synergistically improve
the efficacy of antitumor immune responses. For this reason,
combination gene immunotherapy may be a valuable approach for the
treatment of hepatocellular carcinoma.
Abbreviations:
|
sPD-1
|
soluble programmed death
receptor-1;
|
|
IL-21
|
interleukin 21;
|
|
pmIL-21
|
plasmid carrying full-length cDNA of
murine interleukin 21;
|
|
psPD-1
|
plasmid carrying cDNA encoding the
extracellular domain of murine PD-1
|
Acknowledgements
This study was supported by the Open
Foundation of the Key Laboratory of Biological Cancer Therapy in
Jiangsu Province (2008C02).
References
|
1
|
Parrish-Novak J, Dillon SR, Nelson A, et
al: Interleukin 21 and its receptor are involved in NK cell
expansion and regulation of lymphocyte function. Nature. 408:57–63.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bryant VL, Ma CS, Avery DT, Li Y, Good KL,
Corcoran LM, de Waal Malefyt R and Tangye SG: Cytokine-mediated
regulation of human B cell differentiation into Ig-secreting cells:
predominant role of IL-21 produced by CXCR5+T follicular
helper cells. J Immunol. 179:8180–8190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kashiwakuma D, Suto A, Hiramatsu Y, Ikeda
K, Takatori H, Suzuki K, Kaqami S, Hirose K, Watanabe N, Iwamoto I
and Nakajima H: B and T lymphocyte attenuator suppresses IL-21
production from follicular Th cells and subsequent humoral immune
responses. J Immunol. 185:2730–2736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coquet JM, Kyparissoudis K, Pellicci DG,
Besra G, Berzins SP, Smyth MJ and Godfrey DI: IL-21 is produced by
NKT cells and modulates NKT cell activation and cytokine
production. J Immunol. 178:2827–2834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin H, Carrio R, Yu A and Malek TR:
Distinct activation signals determine whether IL-21 induces B cell
costimulation, growth arrest, or Bim-dependent apoptosis. J
Immunol. 173:657–665. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brandt K, Bulfone-Paus S, Foster DC and
Rückert R: Interleukin-21 inhibits dendritic cell activation and
maturation. Blood. 102:4090–4098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Distler JH, Jüngel A, Kowal-Bielecka O, et
al: Expression of interleukin-21 receptor in epidermis from
patients with systemic sclerosis. Arthritis Rheum. 52:856–864.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caruso R, Fina D, Peluso I, Stolfi C,
Fantini MC, Gioia V, Caprioli F, Del Vecchio Bianco G, Paoluzi OA,
Macdonald TT, et al: A functional role for interleukin-21 in
promoting the synthesis of the T-cell chemoattractant, MIP-3alpha,
by gut epithelial cells. Gastroenterology. 132:166–175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pesce J, Kaviratne M, Ramalingam TR,
Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M, Grusby
MJ and Wynn TA: The IL-21 receptor augments Th2 effector function
and alternative macrophage activation. J Clin Invest.
116:2044–2055. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Carlo E, Comes EA, Orengo AM, Rosso O,
Meazza R, Musiani P, Colombo MP and Ferrini S: IL-21 induces tumor
rejection by specific CTL and IFN-γ-dependent CXC chemokines in
syngeneic mice. J Immunol. 172:1540–1547. 2004.PubMed/NCBI
|
|
11
|
Dou J, Chen GB, Wang J, Zhao FS, Chen JS,
Fang XS, Tang Q and Chu LL: Preliminary study on mouse
interleukin-21 application in tumor gene therapy. Cell Mol Immunol.
1:461–466. 2004.PubMed/NCBI
|
|
12
|
Moroz A, Eppolito C, Li Q, Tao JM, Clegg
CH and Shrikant PA: IL-21 enhances and sustains CD8+ T
cell responses to achieve durable tumor immunity: comparative
evaluation of IL-2, IL-15, and IL-21. J Immunol. 173:900–909.
2004.PubMed/NCBI
|
|
13
|
Liu S, Lizée G, Lou Y, Liu CW, Overwijk
WW, Wang G and Hwu P: IL-21 synergizes with IL-7 to augment
expansion and anti-tumor function of cytotoxic T cells. Int
Immunol. 19:1213–1221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casey KA and Mescher MF: IL-21 promotes
differentiation of naive CD8 T cells to a unique effector
phenotype. J Immunol. 178:7640–7648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim-Schulze S, Kim HS, Fan Q, Kim DW and
Kaufman HL: Local IL-21 promotes the therapeutic activity of
effector T cells by decreasing regulatory T cells within the tumor
microenvironment. Mol Ther. 17:380–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Tschoi M, Spolski R, Lou YY, Ozaki
K, Feng C, Kim G, Leonard WJ and Hwu P: In vivo antitumor activity
of interleukin 21 mediated by natural killer cells. Cancer Res.
63:9016–9022. 2003.PubMed/NCBI
|
|
17
|
Schmidt H, Brown J, Mouritzen U, Selby P,
Fode K, Svane IM, Cook GP, Mollerup DH and Geertsen PF: Safety and
clinical effect of subcutaneous human interleukin-21 in patients
with metastatic melanoma or renal cell carcinoma: a phase I trial.
Clin Cancer Res. 16:5312–5319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grünwald V, Desar IM, Haanen J, Fiedler W,
Mouritzen U, Olsen MW and van Herpen CM: A phase I study of
recombinant human interleukin-21 (rIL-21) in combination with
sunitinib in patients with metastatic renal cell carcinoma (RCC).
Acta Oncol. 50:121–126. 2011.PubMed/NCBI
|
|
19
|
Hashmi MH and Van Veldhuizen PJ:
Interleukin-21: updated review of Phase I and II clinical trials in
metastatic renal cell carcinoma, metastatic melanoma and
relapsed/refractory indolent non-Hodgkin’s lymphoma. Expert Opin
Biol Ther. 5:807–817. 2010.PubMed/NCBI
|
|
20
|
Dong H, Strome SE and Salomao DR:
Tumor-associated B7-H1 promotes T-cell apoptosis: a potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M,
Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y and Fan J:
Overexpression of PD-L1 significantly associates with tumor
aggressiveness and postoperative recurrence in human hepatocellular
carcinoma. Clin Cancer Res. 15:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwai Y, Terawaki S and Honjo T: PD-1
blockade inhibits hematogenous spread of poorly immunogenic tumor
cells by enhanced recruitment of effector T cells. Int Immunol.
17:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Lau R, Yu D, Zhu WW, Korman A and
Weber J: PD-1 blockade reverses the suppression of melanoma
antigen-specific CTL by CD4+ CD25 (Hi) regulatory T
cells. Int Immunol. 21:1065–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onlamoon N, Rogers K, Mayne AE,
Pattanapanyasat K, Mori K, Villinger F and Ansari AA: Soluble PD-1
rescues the proliferative response of simian immunodeficiency
virus-specific CD4 and CD8 T cells during chronic infection.
Immunology. 124:277–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XH, Zhang GM, He YF, Zhang H and Feng
ZH: Soluble PD-1 can augment anti-tumor immunity induced by
HSP70-peptide complex in tumor bearing mice. Chin J Cell Mol
Immunol. 20:655–658. 2004.(In Chinese).
|
|
27
|
He YF, Zhang GM, Wang XH, Zhang H, Yuan Y,
Li D and Feng ZH: Eukaryotic expression and functional
characterization of PD-1 extracellular domain. Chin J Biotechnol.
20:699–703. 2004.(In Chinese).
|
|
28
|
Dou J, Wang Y, Wang J, Zhao F, Li Y, Cao
M, et al: Antitumor efficacy induced by human ovarian cancer cells
secreting IL-21 alone or combination with GM-CSF cytokines in nude
mice model. Immunobiolog. 214:483–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jauch D, Martin M, Schiechl G, Kesselring
R, Schlitt HJ, Geissler EK and Fichtner-Feigl S: Interleukin 21
controls tumour growth and tumour immunosurveillance in
colitis-associated tumorigenesis in mice. Gut. 60:1678–1686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kinter AL, Godbout EJ, McNally JP, Sereti
I, Roby GA, O’Shea MA and Fauci AS: The common γ-chain cytokines
IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed
death-1 and its ligands. J Immunol. 181:6738–6746. 2008.
|
|
31
|
Arens R and Schoenberger SP: Plasticity in
programming of effector and memory CD8 T-cell formation. Immunol
Rev. 235:190–205. 2010.PubMed/NCBI
|
|
32
|
Cerwenka A and Lanier LL: Natural killer
cells, viruses and cancer. Nat Rev Immunol. 1:41–49. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smyth MJ, Hayakawa Y, Takeda K and Yagita
H: New aspects of natural-killer-cell surveillance and therapy of
cancer. Nat Rev Cancer. 2:850–861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O’Garra A and Arai N: The molecular basis
of T helper 1 and T helper 2 cell differentiation. Trends Cell
Biol. 10:542–550. 2000.PubMed/NCBI
|