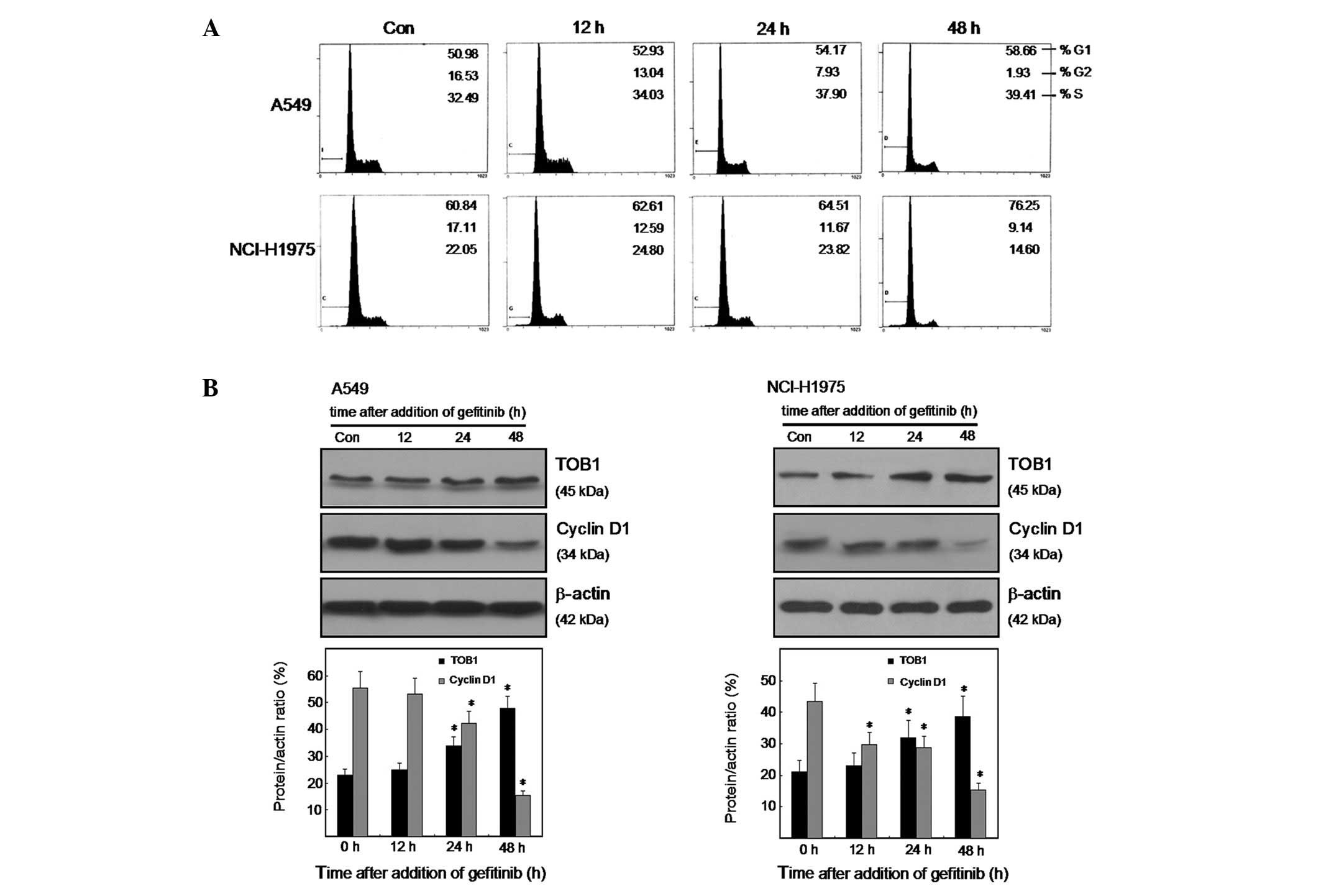
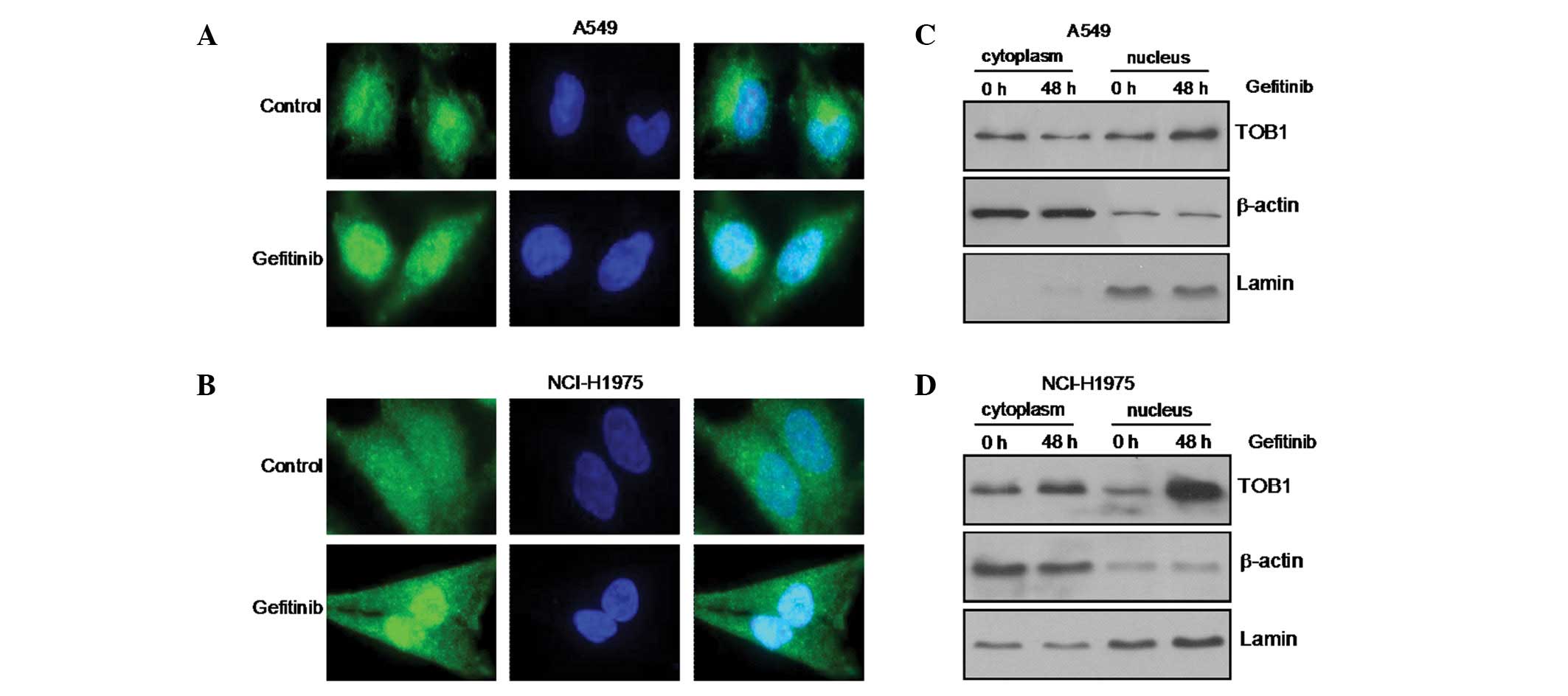
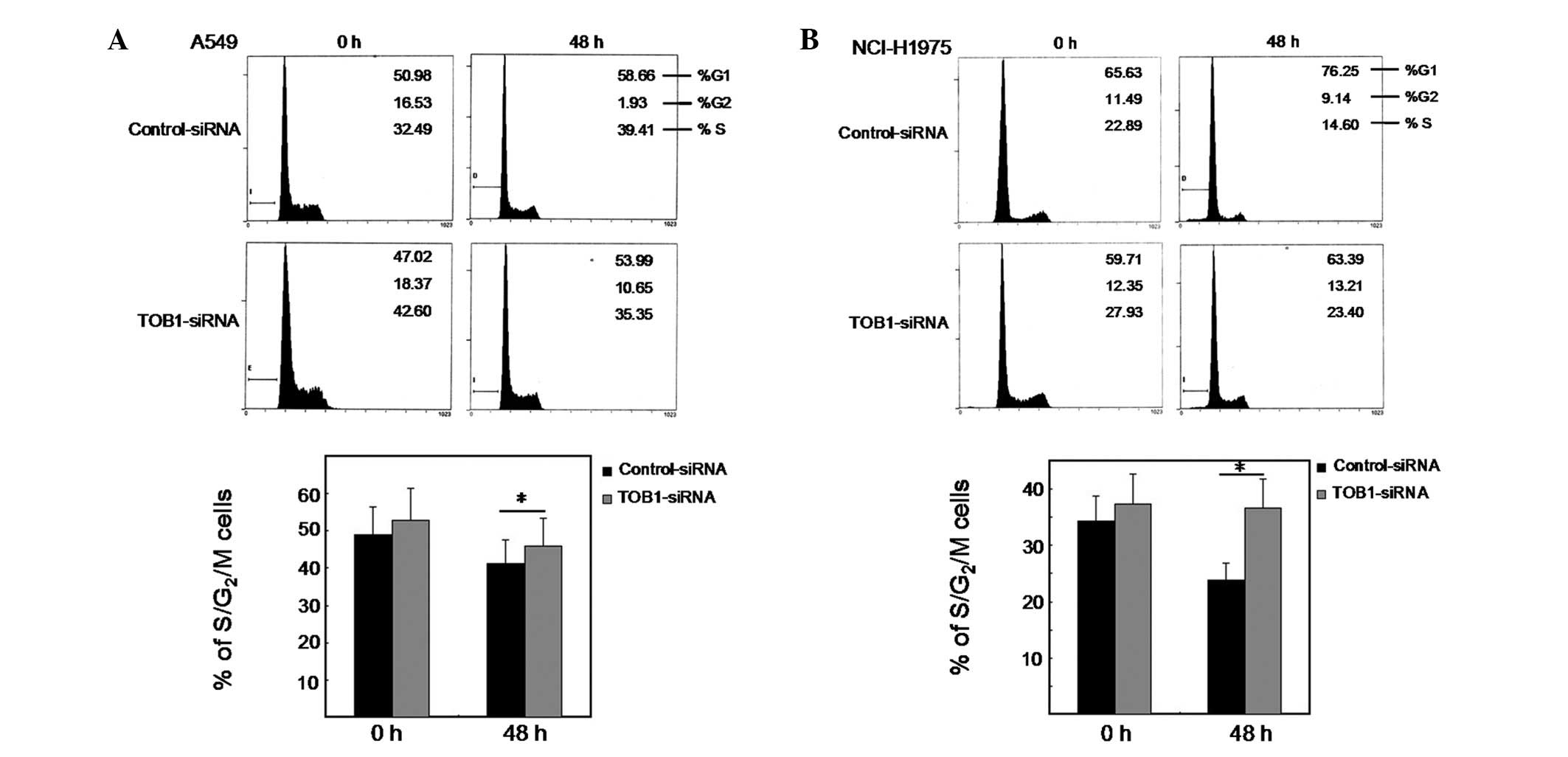
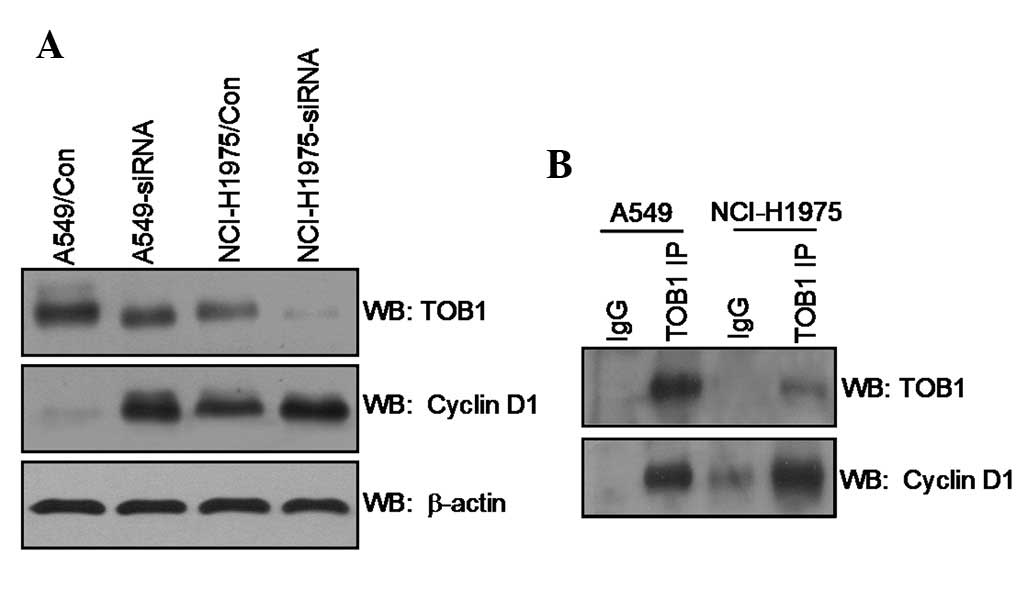
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 2:69–90. 2011. View Article : Google Scholar
|
|
2
|
Inamura K and Ishikawa Y: Lung cancer
progression and metastasis from the prognostic point of view. Clin
Exp Metastasis. 6:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakeling AE, Guy SP, Woodburn JR, et al:
ZD1839 (Iressa): an orally active inhibitor of epidermal growth
factor signaling with potential for cancer therapy. Cancer Res.
62:5749–5754. 2002.PubMed/NCBI
|
|
4
|
Ciardiello F: Epidermal growth factor
receptor inhibitors in cancer treatment. Future Oncol. 1:221–234.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maione P, Gridelli C, Troiani T, et al:
Combining targeted therapies and drugs with multiple targets in the
treatment of NSCLC. Oncologist. 11:274–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polychronis A, Sinnett HD, Hadjiminas D,
et al: Preoperative gefitinib versus gefitinib and anastrozole in
postmenopausal patients with oestrogen-receptor positive and
epidermal-growt h-factor-receptor-positive primary breast cancer: a
double-blind placebo-controlled phase II randomised trial. Lancet
Oncol. 6:383–391. 2005. View Article : Google Scholar
|
|
7
|
Montemurro F, Valabrega G and Aglietta M:
Lapatinib: a dual inhibitor of EGFR and HER2 tyrosine kinase
activity. Expert Opin Biol Ther. 7:257–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranson M, Hammond LA, Ferry D, et al:
ZD1839, a selective oral epidermal growth factor receptor-tyrosine
kinase inhibitor, is well tolerated and active in patients with
solid, malignant tumors: results of a phase I trial. J Clin Oncol.
20:2240–2250. 2002. View Article : Google Scholar
|
|
9
|
Krol J, Francis RE, Albergaria A, et al:
The transcription factor FOXO3a is a crucial cellular target of
gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther.
6:3169–3179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda S, Kawamura-Tsuzuku J, Ohsugi M,
et al: Tob, a novel protein that interacts with p185erbB2, is
associated with anti-proliferative activity. Oncogene. 4:705–713.
1996.PubMed/NCBI
|
|
11
|
Winkler GS: The mammalian
anti-proliferative BTG/Tob protein family. J Cell Physiol. 1:66–72.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XM, Gao X, Zhang XH, et al: The
negative cell cycle regulator, Tob (transducer of ErbB-2), is
involved in motor skill learning. Biochem Biophys Res Commun.
4:1023–1027. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia S and Meng A: Tob genes in development
and homeostasis. Dev Dyn. 4:913–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Helms MW, Kemming D, Contag CH, et al:
TOB1 is regulated by EGF-dependent HER2 and EGFR signaling, is
highly phosphorylated, and indicates poor prognosis in
node-negative breast cancer. Cancer Res. 12:5049–5056. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiao Y, Sun KK, Zhao L, et al: Suppression
of human lung cancer cell proliferation and metastasis in vitro by
the transducer of ErbB-2.1 (TOB1). Acta Pharmacol Sin. 33:250–260.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Astsaturov I, Cohen RB and Harari P:
Targeting epidermal growth factor receptor signaling in the
treatment of head and neck cancer. Expert Rev Anticancer Ther.
6:1179–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scaltriti M and Baselga J: The epidermal
growth factor receptor pathway: a model for targeted therapy. Clin
Cancer Res. 12:5268–5272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnston JB, Navaratnam S, Pitz MW, et al:
Targeting the EGFR pathway for cancer therapy. Curr Med Chem.
13:3483–3492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida A, Hirano S, Kitao H, et al:
Activation of downstream epidermal growth factor receptor (EGFR)
signaling provides gefitinib-resistance in cells carrying EGFR
mutation. Cancer Sci. 98:357–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwanaga K, Sueoka N, Sato A, et al:
Alteration of expression or phosphorylation status of tob, a novel
tumor suppressor gene product, is an early event in lung cancer.
Cancer Lett. 1:71–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito Y, Suzuki T, Yoshida H, et al:
Phosphorylation and inactivation of Tob contributes to the
progression of papillary carcinoma of the thyroid. Cancer Lett.
2:237–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yanagie H, Tanabe T, Sumimoto H, et al:
Tumor growth suppression by adenovirus-mediated introduction of a
cell-growth-suppressing gene tob in a pancreatic cancer model.
Biomed Pharmacother. 4:275–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lapenna S and Giordano A: Cell cycle
kinases as therapeutic targets for cancer. Nat Rev Drug Discov.
8:547–566. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Felsher DW: Cancer revoked: oncogenes as
therapeutic targets. Nat Rev Cancer. 3:375–380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki T, K-Tsuzuku J, Ajima R, et al:
Phosphorylation of three regulatory serines of Tob by Erk1 and Erk2
is required for Ras-mediated cell proliferation and transformation.
Genes Dev. 11:1356–1370. 2002. View Article : Google Scholar : PubMed/NCBI
|