Introduction
Systemic antitumor chemotherapy induces
treatment-associated adverse effects in various organs, including
the skin and nails (1). To date,
skin and nail alterations have attracted a great deal of interest
due to their potential as surrogate markers for molecular-targeted
therapies, in particular epidermal growth factor receptor
(EGFR)-targeted treatment (2). A
close correlation has been observed between the severity of
skin/nail alterations, treatment efficacy and the survival benefits
of EGFR-targeted treatment (3).
An alternative approach to systemic chemotherapy is
low-dose metronomic (LDM) chemotherapy, which is focused on the
treatment of various neoplastic diseases (4–6). LDM
chemotherapy involves frequent administration of a cytotoxic drug
in a low dose to exert an alternative antitumor effect, in addition
to a direct cytotoxic effect. More specifically, studies have
revealed that LDM chemotherapy exerts an antitumor effect via
vascular mechanisms (7–9).
Docetaxel, a semi-synthetic taxoid drug made from a
precursor extracted from the needles of the European yew, Taxus
baccata, enhances microtubule assembly and is recognized as an
active metronomic agent. LDM docetaxel chemotherapy was used in a
pilot study as a therapy for previously treated non-small cell lung
cancer (NSCLC) (10). Docetaxel is
also a well-known causative agent of nail alterations (11–13).
Therefore, we hypothesized that nail alterations associated with
treatment may be a surrogate marker for the efficacy of LDM
docetaxel chemotherapy. In this study, we retrospectively analyzed
the correlation between nail alterations and survival benefit. The
results presented in this study demonstrate the favorable impact of
treatment-associated nail alterations during LDM docetaxel
chemotherapy on survival.
Patients and methods
Data collection
The medical records of all NSCLC patients who were
treated between January 1999 and December 2010 in Kansai Medical
University Takii Hospital (Moriguchi-City, Japan) were
retrospectively reviewed after Institutional Review Board approval.
Patients were included in this study if they had advanced stage
(III or IV) or relapsed NSCLC that was treated with LDM
chemotherapy using docetaxel. A clinical stage was assigned
according to the sixth edition of the TNM Classification for Lung
Cancer (14). Data with regard to
gender, age, clinical stage, histological typing of cancer, Eastern
Cooperative Oncology Group (ECOG) performance status (PS),
progression-free survival (PFS) and overall survival (OS) were
obtained retrospectively from the medical records. Adverse effects
on the finger- and toenails observed during the treatment period
were also ascertained retrospectively from the medical records and
photographs of the nails of patients were retrieved with informed
consent. All patients provided written informed consent before
undergoing chemotherapy. The study was carried out according to the
Declaration of Helsinki and was approved by the Institutional
Ethics Review Board (Subcommittee for the Epidemiologic Study,
Institutional Review Board of Kansai Medical University).
Treatment
Docetaxel (15 mg/m2) diluted with 250 ml
of a 5% glucose solution was administered intravenously over 60 min
each week. The treatment was administered on a weekly basis without
any intervals. To prevent a hyper-sensitive reaction, 2 mg of
dexamethasone or H1-blocker was administered as a premedication to
all patients. The treatment was repeated, with the exception of
cases where disease progression was observed. Responses were
evaluated using a chest CT every month during the treatment period.
Administration of docetaxel was bypassed if the patient experienced
unacceptable toxicity (e.g., grade 3 or worse
hematological/non-hematological toxicity, excluding appetite loss,
constipation and nausea/vomiting). Patients who refused to continue
this regimen and those who showed a decline in performance status
(PS; PS 4) were also allowed to avoid LDM chemotherapy.
Statistical analysis
Statistically significant differences between groups
were compared using Student’s t-test, a Chi-square test or Fisher’s
exact test. Spearman’s correlation coefficients (two-tailed) were
used to evaluate whether the number of docetaxel administration
cycles correlated with the level of nail alterations. Logistic
regression was used to test the univariate dose-response
correlation and multivariate associations with the development of
nail alterations. Variables were considered for the multivariate
models if their univariate P-value was <0.25. Overall survival
(OS) was defined as the time from the start of LDM chemotherapy to
the time of death from any cause or to the date the patient was
last known to be alive. Progression-free survival was defined as
the time between the start of LDM treatment and disease
progression, death or last known follow-up. Objective tumor
responses to chemotherapy were evaluated using the Response
Evaluation Criteria in Solid Tumors version 1.0 (15). The objective response rate (ORR) was
defined as the number of patients showing a complete response (CR)
and a partial response (PR) relative to the total number of
patients evaluated. The disease control rate (DCR) was defined as
the number of patients showing CR, PR and stable disease (SD)
relative to the total number of patients evaluated. The minimum
time interval between the 2 measurements required for the
determination of SD was 6 weeks. The 95% confidence intervals (95%
CI) for the ORR and DCR were calculated using binomial
distribution. Univariate and multivariate analysis of PFS and OS
were performed using the Kaplan-Meier product-limit method using
the log-rank test and the Cox proportional hazards model,
respectively. The 95% CI for the survival rate was calculated using
Greenwood’s method. To calculate the 95% CI of the median survival
time (MST), the Brookmeyer and Crowley method was used.
All statistical analysis was conducted using the JMP
(version 9.0.2) software program for Windows (SAS Institute Inc.,
Cary, NC, USA). All statistical tests were two-sided and P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Between January 1999 and December 2010, 49 patients
with NSCLC who met the eligibility criteria were enrolled in this
study. Patient characteristics are summarized in Table I. All patients were Japanese and the
median age was 69 years (range, 32–85 years). Ten patients were
female and 39 were male. Adenocarcinoma and squamous cell carcinoma
were present in 30 and 19 patients, respectively. Twenty-two
patients had stage III disease, whereas 27 patients had stage IV
disease. The ECOG PS was 0 and 1 in 21 and 28 patients,
respectively. Twenty-seven patients had received at least 1 regimen
of cytotoxic chemotherapy prior to this LDM regimen, but the
remaining 22 patients had never been treated. The median number of
cycles administered to the 49 patients was 16 (range, 2–60). Eleven
patients received at least 1 further chemotherapy regimen,
including EGFR-tyrosine kinase inhibitor, following this LDM
regimen.
 | Table IPatient and disease
characteristics. |
Table I
Patient and disease
characteristics.
| Characteristics | Total patients
(n=49) | Patients with nail
alterations (n=17) | Patients without nail
alterations (n=32) | P-value |
|---|
| Gender | | | | 0.0212a |
| Male | 39 | 10 | 29 | |
| Female | 10 | 7 | 3 | |
| Age (years) | | | | 0.8457 |
| Median | 69 | 71 | 68.5 | |
| Range | 32–85 | 49–85 | 32–83 | |
| ECOG PS | | | | 0.0351a |
| 0 | 21 | 11 | 10 | |
| 1 | 28 | 6 | 22 | |
| Histology
(cytology) | | | | 0.1347 |
| Ad | 30 | 13 | 17 | |
| Sq | 19 | 4 | 15 | |
| Disease stage | | | | 1.0000 |
| III | 22 | 8 | 14 | |
| IV | 27 | 9 | 18 | |
| Prior systemic
therapies | | | | 0.5480 |
| 0 regimen | 22 | 8 | 19 | |
| ≥1 regimen | 27 | 9 | 13 | |
| Treatment cycles | 1,003 | 557 | 446 | <0.0001a |
| Median | 16 | 31 | 12 | |
| Range | 2–60 | 9–60 | 2–48 | |
| DTX dose
(mg/body/week) | | | | 0.5073 |
| Median | 20 | 20 | 20.5 | |
| Range | 17–28 | 17–27 | 18–28 | |
| Post-systemic
therapies | | | | 0.1562 |
| 0 regimen | 38 | 11 | 27 | |
| ≥1 regimen | 11 | 6 | 5 | |
| Pleural effusion | | | | 0.0058a |
| Observed | 30 | 15 | 15 | |
| Not observed | 19 | 2 | 17 | |
| ORR (%) | 8.2 | 17.6 | 3.1 | 0.1139 |
| DCR (%) | 53.1 | 70.6 | 43.8 | 0.1317 |
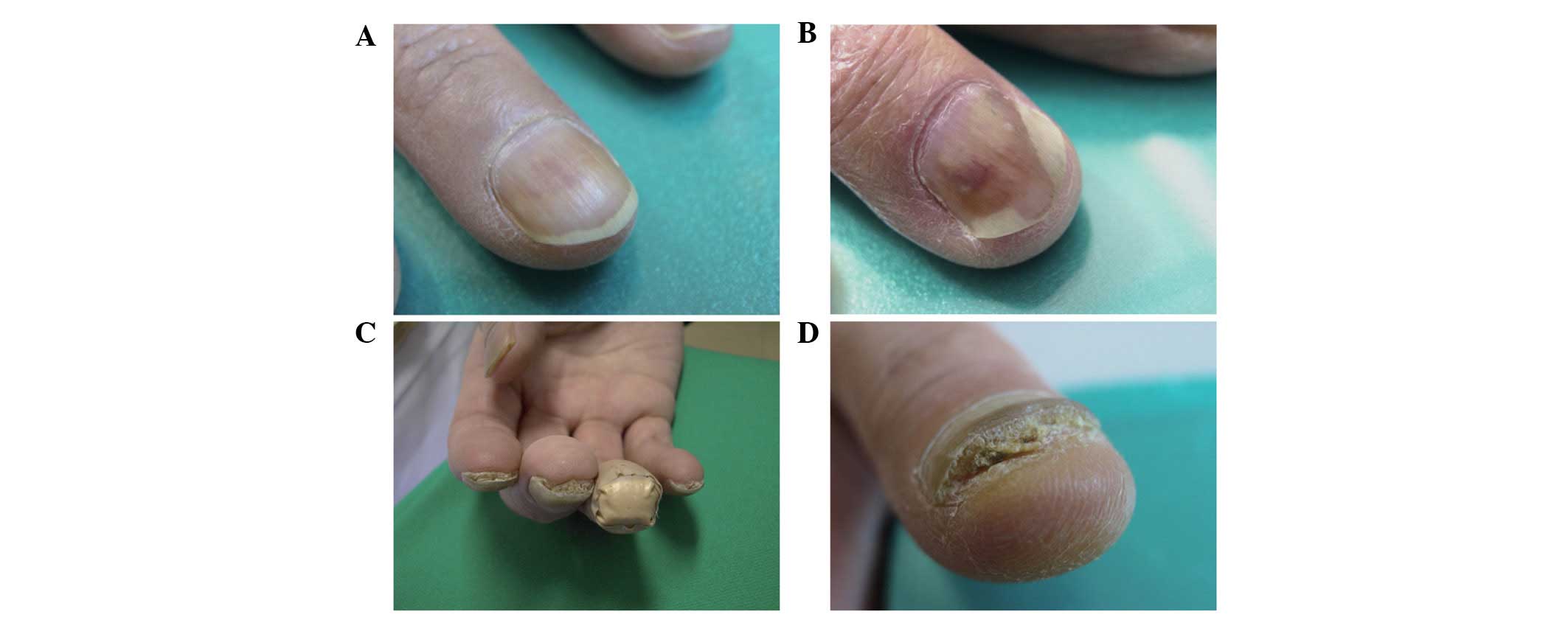
Nail alterations
No nail alterations were observed at the beginning
of this LDM regimen. In the present study, various nail alterations
were observed in 17 out of 49 patients (34.7%) during and after
treatment. As previously described, docetaxel is known to induce
various nail alterations. Examples include nail discoloration
(including subungual hemorrhage), onycholysis, subungual
hyperkeratosis, aseptic subungual abscess, loss of transverse line
and Beau’s line (1). In this study,
dark nail discoloration, onycholysis, subungual hyperkeratosis and
aseptic subungual abscess were observed (Fig. 1), but Beau’s line and loss of
transverse line were not. The metronomic schedule, as opposed to
the conventional schedule of the maximum tolerated dose every three
weeks, may be responsible for the observed spectrum of nail
alterations. Nail alterations developed 8 weeks from the first
administration of docetaxel. A spontaneous remission of nail
alterations was observed after the withdrawal of LDM docetaxel
treatment. Recovery of nail discoloration and reattachment of the
nail to the nail bed was observed ∼8 weeks after the last docetaxel
administration. These findings indicate that nail alterations due
to the LDM docetaxel treatment are reversible.
The nail alterations appeared to develop in a
step-wise manner. Nail discoloration occurred first, followed by
onycholysis. Finally, subungual hyperkeratosis and aseptic pus were
observed. Since the most recent version of the common toxicity
criteria (CTCAE v.4.03) for nail alterations is abstract and has
only a few grades, a novel grading scale for nail alterations was
developed in this study (16). Nail
alterations were classified into 4 subsets according to severity
(Table II): No evident nail
alterations (level 0, 32 cases), nail discoloration only (level 1,
6 cases), nail discoloration with onycholysis (level 2, 6 cases)
and nail discoloration and onycholysis with subungual
hyperkeratosis (level 3, 5 cases). Subsequently, we attempted to
determine whether the cycle number of docetaxel administration
correlated with a particular level of nail alterations. Spearman’s
correlation coefficients (2-tailed) were used to evaluate the
correlation between cycle number and the level of nail alterations.
Statistical analysis revealed that a larger number of docetaxel
administrations correlated with a higher degree of nail alterations
(Spearman’s ϱ=0.6409, P<0.0001; Fig.
2).
 | Table IISummary of nail alterations observed
(n=49). |
Table II
Summary of nail alterations observed
(n=49).
| Level | Definition | Patients, n
(%) |
|---|
| 0 | No evident nail
alterations | 32 (65.3) |
| 1 | Discoloration | 6 (12.2) |
| 2 | Level 1 +
onycholysis | 6 (12.2) |
| 3 | Level 2 + subungual
hyperkeratosis | 5 (10.2) |
| 1–3 | Total nail
alterations | 17 (34.7) |
Risk factors
The risk factors for the development of nail
alterations were assessed. Univariate analysis showed that the
number of treatment cycles (P<0.0001), female gender (P=0.0212),
PS 0 (P=0.0351) and treatment-associated pleural effusion
(P=0.0058) were statistically significant risk factors for the
development of nail alterations (Table
I). However, multiple logistic regression analysis indicated
that the number of treatment cycles [P=0.0005; odds ratio (OR),
1.148/cycle], PS 0 (P=0.0195; OR, 8.907) and treatment-associated
pleural effusion (P=0.0492, OR, 10.138) were independent risk
factors for the development of nail alterations (Table III).
 | Table IIIMultiple logistic regression analysis
of the clinical parameters associated with nail alteration. |
Table III
Multiple logistic regression analysis
of the clinical parameters associated with nail alteration.
| Clinical
parameters | OR (95% CI) | P-value |
|---|
| Number of cycles
(/cycle) | 1.148
(1.053–1.296) | 0.0005a |
| Female vs.
male | 8.736
(0.986–128.278) | 0.0515 |
| PS 0 vs. PS 1 | 8.907
(1.393–97.050) | 0.0195a |
| Sq vs. Ad | 3.450
(0.381–49.322) | 0.2803 |
| Pleural effusion
vs. absent | 10.138
(1.007–253.901) | 0.0492a |
Effect of nail alterations on
survival
The effect of nail alterations in this LDM regimen
(Table IV) on survival was
evaluated. The MST for the patients with and without nail
alterations was 30.6 and 6.7 months, respectively. Univariate
analysis indicated that the patients with nail alterations showed
significantly longer survival than those without nail alterations
(P=0.0006; Fig. 3A). Additionally,
female gender (P=0.0154), age <70 years (P=0.0108), the presence
of adenocarcinoma (P=0.0049), prior chemotherapy (P=0.0099), PS 0
(P=0.0400) and treatment-associated pleural effusion (P= 0.0245)
were significant prognostic factors for OS (Fig. 3B). By contrast, multivariate
analysis revealed that the development of nail alterations [hazard
ratio (HR), 0.2138; 95% CI, 0.0809–0.5515; P=0.0014) and prior
systemic chemotherapy (HR, 0.3485; 95% CI, 0.1613–0.7459; P=0.0068)
were independent favorable prognostic factors for OS. The patients
with nail alterations also showed a significantly longer PFS than
those without nail alterations (P=0.0037; Fig. 3C).
 | Table IVUnivariate and multivariate analysis
for OS. |
Table IV
Univariate and multivariate analysis
for OS.
| Univariate analysis
| Multivariate
analysis
|
|---|
| Covariate | MST (Mo) | P-value | HR (95% CI) | P-value |
|---|
| Female vs.
male | 36.4 vs. 8.5 | 0.0154a | 1.0949
(0.3495–2.9692) | 0.8673 |
| Age <70 vs. ≥70
years | 16.4 vs. 7.5 | 0.0108a | 0.6855
(0.3262–1.4259) | 0.3107 |
| Ad vs. Sq | 16.4 vs. 7.2 | 0.0049a | 0.6409
(0.3146–1.3271) | 0.2266 |
| Relapsed vs.
naïve | 16.4 vs. 7.6 | 0.0099a | 0.3485
(0.1613–0.7459) | 0.0068a |
| PS 0 vs. PS 1 | 25.7 vs. 7.6 | 0.0400a | 0.9548
(0.4395–2.0791) | 0.9065 |
| Stage III vs.
IV | 9.0 vs. 9.8 | 0.3758 | 0.7655
(0.3792–1.5090) | 0.4424 |
| Nail alterations
(observed vs. absent) | 30.6 vs. 6.7 | 0.0006a | 0.2138
(0.0809–0.5515) | 0.0014a |
| Pleural effusion
(observed vs. absent) | 11.6 vs. 7.0 | 0.0245a | 0.5613
(0.2908–1.0860) | 0.0858 |
Discussion
LDM chemotherapy targets the tumor vascular system
(7–9). This suggests that the deterioration of
constitutive remodeling of the microvascular network surrounding
the tumor tissue may lead to a decrease in tumor burden. However,
this type of chemotherapy may also damage non-tumor-associated
microvascular networks in normal tissue, as tumor vessels are
derived from the normal vascular system and composed of genetically
normal endothelial cells. Microvessels in the nail bed or pleural
architecture are also likely be affected. Consequently, nail
alterations or capillary leakage resulting in pleural effusion may
be induced. This may explain the close correlation observed in this
study between nail alterations and pleural effusion. If a common
pathogenic procedure is introduced by LDM chemotherapy, one might
become a surrogate marker for the other. In this study, we showed
that the development of nail alterations during LDM docetaxel
chemotherapy was an independent favorable prognostic factor for OS.
Therefore, nail alterations observed during treatment may be a
potential surrogate marker for the efficacy of LDM docetaxel
chemotherapy.
LDM chemotherapy has been shown to induce nail
alterations more frequently than other treatments, particularly
onycholysis, which is characterized by nail plate detachment from
the nail bed and often results from subungual hemorrhage. In
conventional chemotherapy with docetaxel (100 mg/m2
every 3 weeks), onycholysis was identified in 19% of patients
(17). By contrast, onycholysis was
observed in 12 out of 49 patients (24.5%) treated with a metronomic
low dose of docetaxel (15 mg/m2 every week). Onycholysis
and subungual hyperkeratosis have also been observed in patients
with psoriasis, onychomycosis and certain autoimmune diseases, in
addition to malignant tumor patients receiving chemotherapy
(18–20). Although their pathological etiology
remains to be elucidated, a number of studies support the
possibility of a neurological involvement in the pathogenesis of
these nail alterations. It has been reported that onycholysis or
subungual hyperkeratosis does not extend to the nails of the
paretic limb due to cerebrovascular events or metastatic brain
tumors (21–24). These findings suggest that the
neurological network is likely to play an essential transmissive
role in the pathogenesis of nail alterations.
Another common pathogenic process for nail
alterations may be capillary leakage, an alternative antitumor
effect due to LDM docetaxel chemotherapy. A few studies have
indicated that certain neuropeptides are able to induce an increase
in vascular permeability and consequent plasma leakage (25,26).
Moreover, docetaxel enhances microtubule assembly, leading to an
interference of axonal flow and an impediment to the innervation of
the microvascular network (27,28).
According to these findings, treatment-associated adverse
neurological effects may also be a favorable predictive factor for
LDM docetaxel chemotherapy. However, no survival benefit was able
to be associated with neurotoxicities in this study, as no
neurological events occurred in our study population. Additionally,
no significant results have been reported to support the effect of
neurotoxicities associated with taxane-based chemotherapy on
survival. A possible explanation for this is that neurosensory and
neurovascular networks might be independently affected by taxanes.
A recent study showed that there was no association between
intraepidermal nerve fiber density and neurological symptoms during
chemotherapy with neurotoxic agents (29).
Although we have clearly demonstrated the favorable
impact of treatment-associated nail alterations on survival and
shown for the first time that these nail alterations act as a
surrogate marker for the efficacy of LDM docetaxel chemotherapy,
this study had a number of limitations. The study population was
small and heterogeneous, containing previously untreated and
relapsed patients. In addition, the arbitrary nail alteration
severity index defined in this study was qualitative, not
quantitative.
The quality of life for patients undergoing
antineoplastic treatment is becoming a more important issue. The
quality and length of life for an individual should not be
considered in mutually exclusive terms. Numerous physicians have
pointed out that nail alterations are not a life-threatening
adverse effect and are therefore not enough of a reason to abstain
from chemotherapy (13). In the
present study, we have demonstrated for the first time that nail
alterations may act as a surrogate marker for the efficacy of LDM
chemotherapy. Our preliminary findings provide a basis for further
investigation into LDM chemotherapy.
References
|
1
|
Gilbar P, Hain A and Peereboom VM: Nail
toxicity induced by cancer chemotherapy. J Oncol Pharm Pract.
15:143–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robert C, Soria JC, Spatz A, Le Cesne A,
Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V,
Armand JP and Le Chevalier T: Cutaneous side-effects of kinase
inhibitors and blocking antibodies. Lancet Oncol. 6:491–500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tiseo M, Rossi G, Capelletti M, Sartori G,
Spiritelli E, Marchioni A, Bozzetti C, De Palma G, Lagrasta C,
Campanini N, Camisa R, Boni L, Franciosi V, Rindi G and Ardizzoni
A: Predictors of gefitinib outcomes in advanced non-small cell lung
cancer (NSCLC): study of a comprehensive panel of molecular
markers. Lung Cancer. 67:355–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Görn M, Habermann CR, Anige M, Thöm I,
Schuch G, Andritzky B, Brandl S, Burkholder I, Edler L, Hossfeld
DK, Bokemeyer C and Laack E: A pilot study of docetaxel and
trofosfamide as second-line ‘metronomic’ chemotherapy in the
treatment of metastatic non-small cell lung cancer (NSCLC).
Onkologie. 31:185–189. 2008.PubMed/NCBI
|
|
5
|
Young SD, Lafrenie RM and Clemons MJ:
Phase ii trial of a metronomic schedule of docetaxel and
capecitabine with concurrent celecoxib in patients with prior
anthracycline exposure for metastatic breast cancer. Curr Oncol.
19:75–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ladoire S, Eymard JC, Zanetta S, Mignot G,
Martin E, Kermarrec I, Mourey E, Michel F, Cormier L and
Ghiringhelli F: Metronomic oral cyclophosphamide prednisolone
chemotherapy is an effective treatment for metastatic
hormone-refractory prostate cancer after docetaxel failure.
Anticancer Res. 30:4317–4323. 2010.
|
|
7
|
Browder T, Butterfield CE, Kräling BM,
Marshall B, O’Reilly MS and Folkman J: Antiangiogenic scheduling of
chemotherapy improves efficacy against experimental drug-resistant
cancer. Cancer Res. 60:1878–1886. 2000.PubMed/NCBI
|
|
8
|
Bertolini F, Paul S, Mancuso P,
Monestiroli S, Gobbi A, Shaked Y and Kerbel RS: Maximum tolerable
dose and low-dose metronomic chemotherapy have opposite effects on
the mobilization and viability of circulating endothelial
progenitor cells. Cancer Res. 63:4342–4346. 2003.PubMed/NCBI
|
|
9
|
Man S, Bocci G, Francia G, Green SK, Jothy
S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G and Kerbel RS:
Antitumor effects in mice of low-dose (metronomic) cyclophosphamide
administered continuously through the drinking water. Cancer Res.
62:2731–2735. 2002.PubMed/NCBI
|
|
10
|
Yokoi T, Tamaki T, Shimizu T and Nomura S:
A pilot study of a metronomic chemotherapy regimen with weekly
low-dose docetaxel for previously treated non-small cell lung
cancer. Lung Cancer: Targets and Therapy. 3:15–20. 2012.
|
|
11
|
Hong J, Park SH, Choi SJ, Lee SH, Lee KC,
Lee JI, Kyung SY, An CH, Lee SP, Park JW, Jeong SH, Nam E, Bang SM,
Cho EK, Shin DB and Lee JH: Nail toxicity after treatment with
docetaxel: a prospective analysis in patients with advanced
non-small cell lung cancer. Jpn J Clin Oncol. 37:424–428. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roh MR, Cho JY and Lew W:
Docetaxel-induced onycholysis: the role of subungual hemorrhage and
suppuration. Yonsei Med J. 48:124–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Correia O, Azevedo C, Pinto Ferreira E,
Braga Cruz F and Polónia J: Nail changes secondary to docetaxel
(Taxotere). Dermatology. 198:288–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin L and Wittekind CH: TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss; New
York: pp. 99–103. 2002
|
|
15
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
16
|
National Cancer Institute (NCI): Common
Terminology Criteria for Adverse Events (CTCAE), version 4.0, 28
May 2009. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed October 9, 2012.
|
|
17
|
ten Bokkel Huinink WW, Prove AM, Piccart
M, Steward W, Tursz T, Wanders J, Franklin H, Clavel M, Verweij J,
Alakl M, Bayssas M and Kaye SB: A phase II trial with docetaxel
(Taxotere) in second line treatment with chemotherapy for advanced
breast cancer. A study of the EORTC Early Clinical Trials Group.
Ann Oncol. 5:527–532. 1994.PubMed/NCBI
|
|
18
|
Grover C, Reddy BS and Uma Chaturvedi K:
Diagnosis of nail psoriasis: importance of biopsy and
histopathology. Br J Dermatol. 153:1153–1158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scher RK, Tavakkol A, Sigurgeirsson B, Hay
RJ, Joseph WS, Tosti A, Fleckman P, Ghannoum M, Armstrong DG,
Markinson BC and Elewski BE: Onychomycosis: diagnosis and
definition of cure. J Am Acad Dermatol. 56:939–944. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richert B, André J, Bourguignon R and de
la Brassinne M: Hyperkeratotic nail discoid lupus erythematosus
evolving towards systemic lupus erythematosus: therapeutic
difficulties. J Eur Acad Dermatol Venereol. 18:728–730. 2004.
View Article : Google Scholar
|
|
21
|
Stevenson R and El-Modir A: Unilateral
onycholysis in a patient taking erlotinib (Tarceva). BMJ Case Rep.
2011. View Article : Google Scholar
|
|
22
|
Truchuelo M, Vano-Galvan S, Pérez B,
Muñoz-Zato E and Jaén P: Unilateral taxane-induced onychopathy in a
patient with a brain metastasis. Dermatol Online J.
15:72009.PubMed/NCBI
|
|
23
|
Badger J, Banerjee AK and McFadden J:
Unilateral subungual hyperkeratosis following a cerebrovascular
incident in a patient with psoriasis. Clin Exp Dermatol.
17:454–455. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wasner G, Hilpert F, Schattschneider J,
Binder A, Pfisterer J and Baron R: Docetaxel-induced nail changes -
a neurogenic mechanism: a case report. J Neurooncol. 58:167–174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujiwara H, Hashikawa-Hobara N, Wake Y,
Takatori S, Goda M, Higuchi H, Zamami Y, Tangsucharit P and
Kawasaki H: Neurogenic vascular responses in male mouse mesenteric
vascular beds. J Pharmacol Sci. 119:260–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brain SD and Williams TJ: Inflammatory
oedema induced by synergism between calcitonin gene-related peptide
(CGRP) and mediators of increased vascular permeability. Br J
Pharmacol. 86:855–860. 1985. View Article : Google Scholar
|
|
27
|
Fazio R, Quattrini A, Bolognesi A,
Bordogna G, Villa E, Previtali S, Canal N and Nemni R: Docetaxel
neuropathy: a distal axonopathy. Acta Neuropathol. 98:651–653.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
New PZ, Jackson CE, Rinaldi D, Burris H
and Barohn RJ: Peripheral neuropathy secondary to docetaxel
(Taxotere). Neurology. 46:108–111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koskinen MJ, Kautio AL, Haanpää ML,
Haapasalo HK, Kellokumpu-Lehtinen PL, Saarto T and Hietaharju AJ:
Intraepidermal nerve fibre density in cancer patients receiving
adjuvant chemotherapy. Anticancer Res. 31:4413–4416.
2011.PubMed/NCBI
|