Introduction
Although the prognosis of non-small cell lung cancer
(NSCLC) has been improved by several advancements in its diagnosis
and treatment, more effective and novel strategies for therapies
and prevention need to be developed. Non-steroidal
anti-inflammatory drugs (NSAIDs) have a chemopreventive effect.
NSAIDs inhibit two COX isoforms, COX-1 and COX-2. Inhibition of
COX-2 activity is thought to be the primary mechanism by which
NSAIDs exert their antitumor effects (1). It has been reported that COX-2 is
overexpressed in NSCLC (2). Tumor
cells with elevated levels of COX-2 are highly apoptosis-resistant,
angiogenic, invasive and suppressive of host immunity (3). The highly selective COX-2 inhibitors
are thought to be a preferable chemopreventive agent for NSCLC.
Despite epidemiological and experimental evidence indicating an
important role for the use of celecoxib, a highly selective COX-2
inhibitor, in the treatment and prevention of NSCLC (4,5), the
exact mechanism remains unclear. Conversely, however, recent
studies have shown that even the highly selective COX-2 inhibitors
have potential side effects (6).
These agents should, therefore, be used at a low dosage.
The insulin-like growth factor (IGF) axis is an
important growth-regulatory pathway that is prevalent in a variety
of cancer types, including NSCLC. The IGF axis is composed of
ligands IGF-1 and -2, their receptors, IGF-binding proteins
(IGFBPs) and other regulatory factors. The IGF axis is activated by
the IGFs via the type 1 IGF receptor (IGF-1R) and it is also
inhibited by IGFBPs via a variety of IGF-dependent or -independent
ways (7,8). The complex balance between IGFs and
IGFBP-3 determines the outcome for tumor cells between survival,
growth or death. Abundant data garnered from clinical studies have
confirmed that increased serum IGF-1 and/or decreased IGFBP-3
levels are risk factors for growth, invasion and metastasis of many
malignancies, including NSCLC (9),
colon (10) and breast cancer
(11). In order to prove that
increased serum IGF-1 levels are a risk factor, studies have shown
that systemic recombinant human IGF-1 (rhIGF-1) may stimulate the
growth of tumors directly by stimulating mitosis in athymic mice
(12), while the reduced
circulating IGF-1 levels delayed the onset of chemical and genetic
factors in induced mouse mammary tumors (13). Another study showed that IGFBP-3 may
play a role in tumorigenesis and that IGFBP-3 levels could be used
in future in cancer risk assessment/prevention or as markers of
response to cancer treatments (14).
IGF-1R, a protein tyrosine-kinase cell surface
receptor (two extracellular 125-KDa α-subunits and two
transmembrane 95 KDa β-subunits). Like COX-2, IGF-1R mediates many
cellular processes, including proliferation, survival and
metabolism (15). IGF-1R expression
is independently related to the outcomes of patients with NSCLC.
Overexpression of IGF-1R may be a useful predictor of lymph node
metastasis, recurrence and post-surgical outcomes in patients with
NSCLC (16). Under normal
conditions, the binding of IGFs to IGF-1R leads to activation of
downstream signaling pathways, such as the phosphatidylinositol
3′-kinase/AKT-kinase (PI3K/AKT) signaling pathway, and increasing
proliferation and survival (17).
As previously described, NSCLC cells frequently
harbor high levels of COX-2 and PI3K/AKT. The antitumor effect of
celecoxib partially depends on PI3K/AKT and the function of the IGF
axis is closely related with the PI3K/AKT signaling pathway. As
these factors appear to be so similar in their signaling
mechanisms, it raises the possibility that the IGF axis may be
involved in the anticancer effect of celecoxib on NSCLC. In the
present study, the effects of celecoxib on IGF-1-induced growth and
invasion in A549 cells were investigated. To clarify the underlying
mechanism of action, the effects of celecoxib, especially at a low
dosage, on the expression of phosphorylated IGF-1R and IGFBP-3 were
examined. Whether the AKT signaling pathway is involved in the
antitumor effect of celecoxib was examined.
Materials and methods
Cells and culture
Non-small cell lung cancer A549 cells were purchased
from The Chinese Academy of Science, Shanghai Cell Preservation
Center, China. The cells were cultured at 37°C with 5%
CO2 in RPMI-1640 medium containing 10% fetal bovine
serum (FBS). The cells were subcultured every 2–3 days and cells in
the logarithmic growth phase were used. This study was approved by
the ethics committee of The Affiliated Nanjing Hospital of Nanjing
Medical University. Informed consent was obtained from all
patients.
Drugs and reagents
Celecoxib was purchased from Pfizer Inc. (New York,
NY, USA). IGF-1 was obtained from Pepro Tech (London, UK). FBS,
RPMI-1640 medium, DMSO and penicillin-streptomycin were obtained
from Gibco BRL (Gaithersburg, MD, USA). The rabbit anti-IGF-1R
β-subunit polyclonal antibody (anti-IGF-1R/ phospho-Tyr1131) and
rabbit anti-human p-AKT polyclonal antibody were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The
anti-phosphorylated AKT antibody was purchased from Cell Signaling
Technology (Beverly, MA, USA).
Cell growth assay
Approximately 2×104 cells per well were
seeded into 96-well plates with 10% FBS and RPMI-1640 and cultured
for 48 h. The cells were washed twice with PBS and cultured with
0.5% FBS and RPMI-1640 for 3 days. After serum starvation, the
cells were treated with different doses of IGF-1 for 4 days.
Treatment with different concentrations of celecoxib was started 24
h after the IGF-1 treatment was started and continued for 5 days.
The medium was changed every day. After 4-day treatment, the net
numbers of viable cells were detected using a water-soluble
tetrazolium
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] colorimetric assay. The cell growth rate was
calculated as the ratio of absorbance on day 4 to that on day
0.
Cell invasion assay
To determine the invasion potential of A549 cells
with/without celecoxib, in addition to IGF-1, an invasion assay was
carried out, using a BD BioCoat Matrigel invasion chamber (Becton
Dickinson, Frankin Lakes, NJ, USA). Cells were cultured with
different concentrations of celecoxib for 36 h. The cells
(2×104) were then plated with 0.5% FBS RPMI-1640 in the
upper chamber, containing the same dose of celecoxib. The lower
chamber was filled with the same medium and IGF-1 was added as a
chemoattractant. After another 12 h incubation with 5%
CO2 at 37°C, the cells in the upper chamber were
removed. The cells invading through the filter were manually
counted.
Western blot analysis
The cells were trypsinized and ∼1.5×105
cells were plated in 4.0 ml of 10% FBS and RPMI-1640 in T25 flasks
for 48 h. At this 48 h time point, the cells were washed twice with
PBS. The celecoxib treatment at low dosage (12.5 μmol/l)
and/or IGF-1 (40 ng/ml) was started at plating. For the western
blot analysis, the cells were washed twice with PBS, lysed in
ice-cold lysis buffer [50 mmol/l Tris-HCl (PH 7.4), 150 mmol/l
NaCl, 1% NP40 and 0.25% sodium deoxycholate] containing protease
(Complete mini; Roche, Basel, Switzerland) and phosphatase (NaF and
NaVO3 at 1 mmol/l; Sigma-Aldrich, MO, USA). The total
protein concentrations were quantified using BCA Protein Assay kit
(Pierce, IL, USA). Centrifuged lysates were separated on 10%
SDS-polyacrylamide gels. After electrophoresis, the proteins were
transferred to nitrocellulose, the filters probed with the primary
antibodies and western blotting was carried out using the
electrogenerated chemiluminescence system.
IGFBP-3 ELISA
For detection of IGFBP-3, the cells and their
treatments were the same as those used for western blot analysis.
The concentrations of IGFBP-3 in the supernatants were detected by
IGFBP-3 Active ELISA kit (Diagnostic System Laboratories, TX, USA).
Supernatants (25 μl) diluted in 50 μl of assay buffer
were used to quantify IGFBP-3 as specified by the manufacturer.
Statistical analysis
The results were evaluated by one-way ANOVA to
detect significant differences among the treatment groups using
SPSS 17.0 statistical software. P<0.05 was considered to
indicate a statistically significant result.
Results
Celecoxib protects A549 cells against
IGF-1 induced cell growth
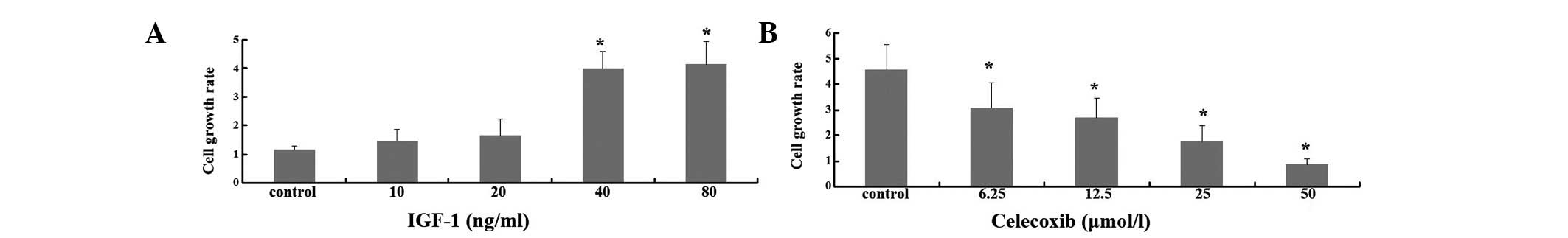
After being serum-starved with 0.5% FBS for 3 days,
the A549 cells were treated with IGF-1 in the presence or absence
of celecoxib. The water-soluble tetrazolium colorimetric assay was
then used. The absorbance on day 4 divided by the absorbance on day
0 was defined as the growth rate. IGF-1 stimulation produced a
2-fold growth of A549 cells and the maximum effective dose of IGF-1
was ∼40 ng/ml (Fig. 1A). Celecoxib
significantly protected A549 cells against IGF-1-induced cell
growth in a dose-dependent manner (Fig.
1B).
Celecoxib protects A549 cells against
IGF-1-induced cell invasion
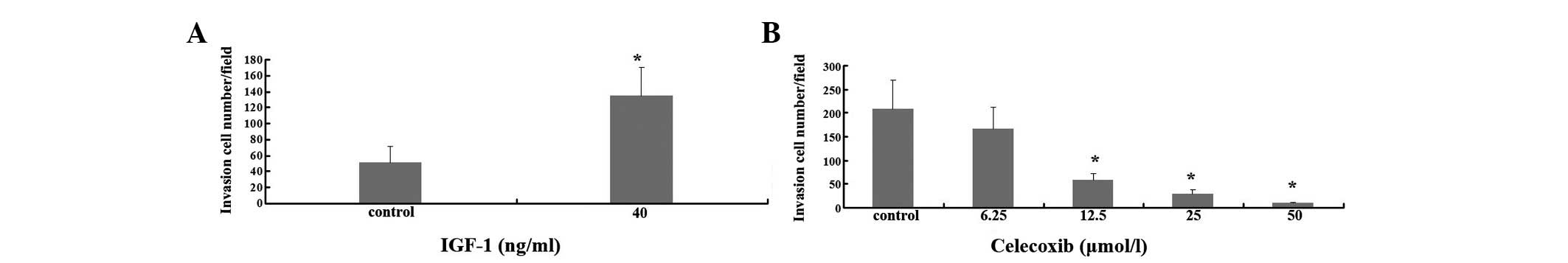
The invasion assay was carried out using a BioCoat
Matrigel invasion chamber. The cells were cultured with different
doses of celecoxib for 36 h. The cells were plated with 0.5% FBS,
RPMI-1640 and celecoxib in the upper chamber. The same medium and
IGF-1 were added to the lower chamber. The cell invasive potential
was significantly (3-fold) enhanced by IGF-1 (ng/ml) as compared
with the control (Fig. 2A).
Consistent with the results of the cell growth assay, celecoxib
significantly inhibited A549 cells against IGF-1-induced cell
invasion (Fig. 2B).
Celecoxib suppresses phosphorylation of
IGF-1R
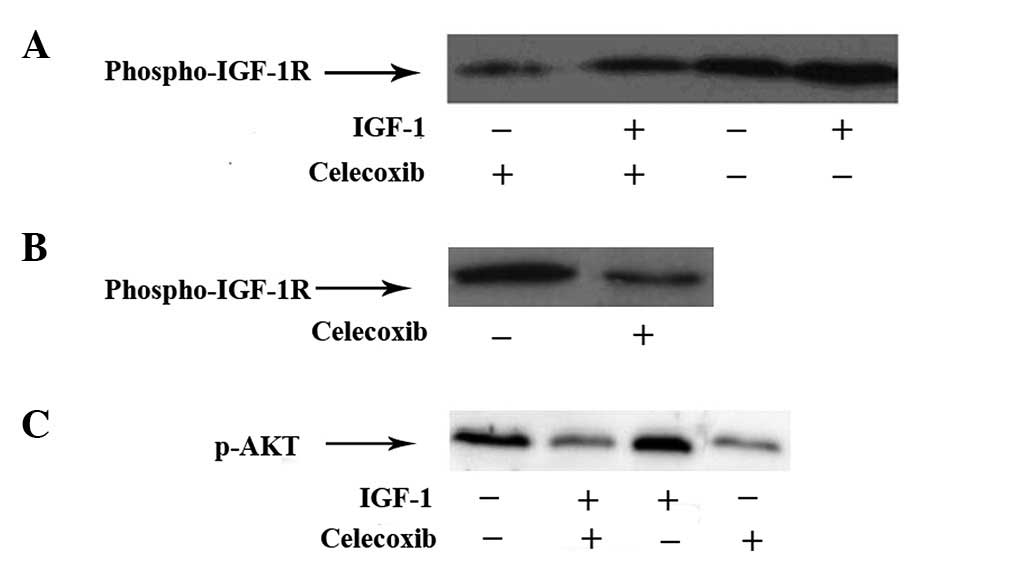
It was postulated that celecoxib protected A549
cells against IGF-1-induced cell growth and invasion via the IGF-1R
pathway. Furthermore, phosphorylation of the triple tyrosine
cluster Tyr1131/1135/1136 in the intracellular kinase domain of
IGF-IR is caused by autophosphorylation and is required for
activation of the intracellular kinase domain of IGF-IR (3). The phosphorylation of Tyr1131 of
IGF-1R in A549 cells by western blot analysis was investigated. As
shown in Fig. 3A, celecoxib
reversed the IGF-1 activated phosphorylation of IGF-1R. The
pIGF-1R-Tyr1131 level was decreased after 48 h treatment of
celecoxib at low dosage (Fig.
3B).
Celecoxib inhibits expression of
p-AKT
The AKT signaling pathway plays a crucial role in
proliferation, invasion and metastasis of tumor cells. AKT is the
key regulatory factor of the AKT signaling pathway. p-AKT is the
phosphorylated state of AKT. Only p-AKT has a biological function.
The expression of p-AKT was examined using western blot analysis.
As shown in Fig. 3C, IGF-1
stimulation increased expression of p-AKT. Treatment with celecoxib
at low dosage (12.5 μmol/l) decreased the level of p-AKT and
reversed the action of IGF-1-induced phosphorylation of AKT.
Celecoxib upregulates IGFBP-3
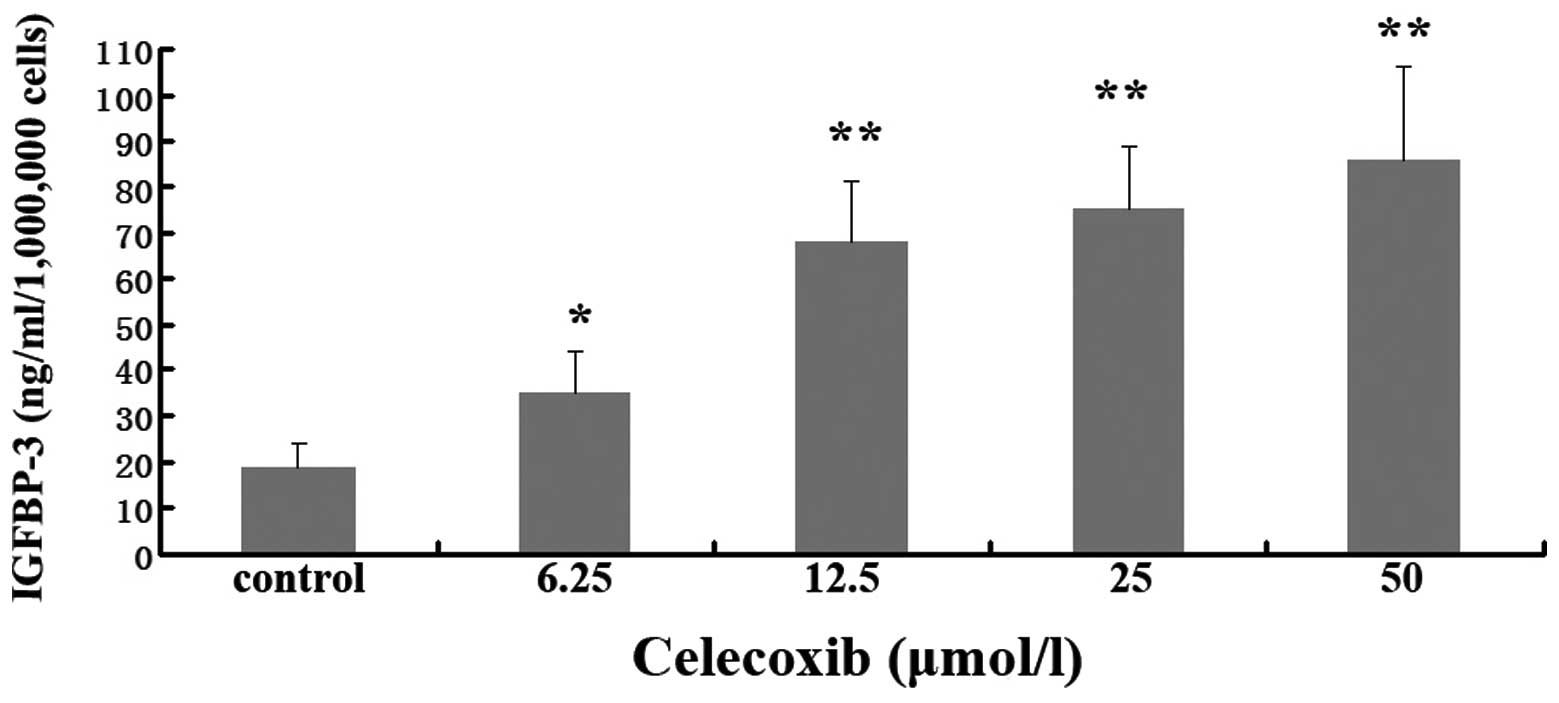
The influence of celecoxib on the expression of
IGFBP-3 was detected using ELISA. As shown in Fig. 4, exposure of A549 cells to celecoxib
significantly elevated the levels of IGFBP-3 in tumor cell culture
supernatants, even when the dose of celecoxib was very low (6.25
μmol).
Discussion
Lung cancer is a malignant tumor with a high
incidence worldwide, and NSCLC accounts for 75–80% of cases. The
five-year survival rate has not exceeded 15% in the past few
decades (18). Surgical excision
remains the mainstay of treatment for NSCLC. Paradoxically, the
perioperative period represents a high risk of tumor cell
metastasis (19). Celecoxib, a
highly selective COX-2 inhibitor, is commonly administered to
patients in order to relieve postoperative pain. One study
demonstrated that celecoxib suppresses cancer progression, in
addition to its analgesic and anti-inflammatory effect (20). Furthermore, it has been shown that
perioperative COX-2 inhibition reduces the adhesion and metastatic
potential of disseminating tumors in murine models and improves
cancer recurrence-free survival rates in mice undergoing primary
tumor excision (21). Celecoxib may
exert its antitumor effect via COX-2-dependent and -independent
pathways.
Despite many studies indicating that celecoxib plays
an important role in the prevention and treatment of tumors, the
detailed molecular mechanisms are not well understood. It was
postulated that there is a functional association between COX-2
inhibitors and the IGF axis in NSCLC. These could be attributed to
three possible mechanisms.
The first is that celecoxib suppresses
phosphorylation of IGF-1R. The results of the present study
indicate that celecoxib downregulates the expression of
phosphorylated IGF-1R (Fig. 3A).
The IGF axis plays an important role in tumor growth, invasion and
metastasis. IGF-1 is a potent mitogen for both normal and tumor
cells. Vlachostergios et al measured the baseline IGF-1
plasma levels in 77 patients who were diagnosed with metastatic
NSCLC. Their results showed that IGF-1 was correlated with systemic
inflammation and appeared to play an independent predictive role in
metastatic NSCLC (22). The present
study confirms that IGF-1, a major ligand for IGF-1R, has an
important role in the growth and invasion in NSCLC cells (Fig. 1A and 2A). The function of IGF-1 is mediated
primarily by the IGF-1R. As previously described, IGF-1R is a
heterotetramer containing two α-and two β-subunits. Binding of the
ligand (IGFs) to the α-subunit triggers a conformational change
that leads to the autophosphorylation of a triple tyrosine cluster
Tyr1131/1135/1136 of the intracellular kinase domain in the
β-subunit (3,23). Autophosphorylation strongly enhances
the activity of the IGF-1R catalytic domain. Activation of IGF-IR
upregulates PI3K/AKT signaling and increases proliferation and
survival. Combined with previous findings, this suggests that in
future NSCLC targeted therapies, COX-2 and IGF-1R inhibitors could
be combined.
The second mechanism may be that celecoxib
upregulates the expression of IGFBP-3. The activities of the IGF
axis are strictly regulated by a family of IGFBPs, especially
IGFBP-3. IGFBP-3, a major serum carrier protein for IGFs, is a
multi-functional protein known to inhibit cellular growth and
induce apoptosis of various cancer cells (24). IGFBP-3 inhibits IGF-induced
biological effects by binding to IGFs, thereby blocking IGF binding
(25). Furthermore, overexpression
of COX-2 by tumor cells downregulates IGFBP-3 mRNA expression
(26). In the present study,
celecoxib upregulated expression of IGFBP-3, even when the dose was
low (Fig. 4). Thus,
celecoxib-mediated upregulation IGFBP-3 in NSCLC cells could
decrease the mitogenic and invasive potential of IGF-1.
The third possible mechanism is that celecoxib
down-regulates expression of p-AKT. As previously described, AKT is
a serine/threonine protein kinase also known as protein kinase B,
which is one of the key pathways modulating cell growth,
proliferation, metabolism, survival and angiogenesis (27). p-AKT is the activated state of AKT
that is highly expressed in most tumors. Only p-AKT has biological
characteristics (28). Uddin et
al reported that inhibition of COX-2 by NS398, a highly
selective COX-2 inhibitor, impaired phosphorylation of AKT,
resulting in decreased downstream signaling leading to cell growth
inhibition and induction of apoptosis in the epithelial ovarian
carcinoma (EOC) cell line (29).
Another study suggested that celecoxib exerted its antitumor
activities in human osteosarcoma cell line MG-63 through
COX-2-independent mechanisms, which may be PI3K/AKT-dependent. PI3K
may be at the center of the celecoxib effects (30). In the current study, western blot
analysis was used to determine the p-AKT protein expression in A549
cells. The results show that the p-AKT protein was decreased in
A549 cells with the treatment of celecoxib (Fig. 3C). These results illustrate that in
A549 cells, celecoxib-inhibited cell growth and invasion may be
related to inhibition of the PI3K/AKT pathway. p-AKT downregulation
is another potential target for the future prevention and treatment
of NSCLC.
There were limitations in the present study. The
detailed mechanisms of celecoxib on the IGF-1R and AKT signaling
pathway need to be studied. It remains unclear whether a longer
treatment period would improve the outcome.
In summary, celecoxib inhibits the growth and
invasion of NSCLC cells via the IGF axis and AKT pathway. Celecoxib
suppresses phosphorylation of IGF-1R, upregulates the expression of
IGFBP-3 and downregulates the AKT signaling pathway. This suggests
a close correlation between COX-2 inhibitors, the IGF axis and the
PI3K/AKT pathway (Fig. 5). It
suggests that celecoxib administration as adjuvant therapy or in
combination with PI3K/AKT inhibitors and/or IGF-1R inhibitors could
be of therapeutic benefit for patients with NSCLC.
Acknowledgements
This study was supported by Grant
Number 201108028 from the Nanjing City Committee of Science and
Technology.
References
|
1
|
Yasumaru M, Tsuji S, Tsujii M, Irie T,
Komori M, Kimura A, Nishida T, Kakiuchi Y, Kawai N, Murata H,
Horimoto M, Sasaki Y, Hayashi N, Kawano S and Hori M: Inhibition of
angiotensin II activity enhanced the antitumor effect of
cyclooxygenase-2 inhibitors via insulin-like growth factor I
receptor pathway. Cancer Res. 63:6726–6734. 2003.PubMed/NCBI
|
|
2
|
Mukhopadhyay P, Ali MA, Nandi A, Carreon
P, Choy H and Saha D: The cyclin-dependent kinase 2 inhibitor
down-regulates interleukin-1 beta-mediated induction of
cyclooxygenase-2 expression in human lung carcinoma cells. Cancer
Res. 66:1758–1766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Põld M, Krysan K, Põld A, Dohadwala M,
Heuze-Vourc’h N, Mao JT, Riedl KL, Sharma S and Dubinett SM:
Cyclooxygenase-2 modulates the insulin-like growth factor axis in
non-small-cell lung cancer. Cancer Res. 64:6549–6555.
2004.PubMed/NCBI
|
|
4
|
Koch A, Bergman B, Holmberg E, Sederholm
C, Ek L, Kosieradzki J, et al: Effect of celecoxib on survival in
patients with advanced non-small cell lung cancer: a double blind
randomised clinical phase III trial (CYCLUS study) by the Swedish
Lung Cancer Study Group. Eur J Cancer. 47:1546–1555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Yue P, Zhou Z, Khuri FR and Sun SY:
Death receptor regulation and celecoxib-induced apoptosis in human
lung cancer cells. J Natl Cancer Inst. 96:1769–1780. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Wen JK, Li BH, Fang XM, Wang JJ,
Zhang YP, Shi CJ, Zhang DQ and Han M: Celecoxib and
acetylbritannilactone interact synergistically to suppress breast
cancer cell growth via COX-2-dependent and -independent mechanisms.
Cell Death Dis. e1852011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jogie-Brahim S, Feldman D and Oh Y:
Unraveling insulin-like growth factor binding protein-3 actions in
human disease. Endocr Rev. 30:417–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodon J, DeSantos V, Ferry RJ Jr and
Kurzrock R: Early drug development of inhibitors of the
insulin-like growth factor-I receptor pathway: lessons from the
first clinical trials. Mol Cancer Ther. 7:2575–2588. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masago K, Fujita S, Togashi Y, Kim YH,
Hatachi Y, Fukuhara A, Nagai H, Irisa K, Sakamori Y, Mio T and
Mishima M: Clinical significance of epidermal growth factor
receptor mutations and insulin-like growth factor 1 and its binding
protein 3 in advanced non-squamous non-small cell lung cancer.
Oncol Rep. 26:795–803. 2011.PubMed/NCBI
|
|
10
|
Gao Y, Katki H, Graubard B, Pollak M,
Martin M, Tao Y, Schoen RE, Church T, Hayes RB, Greene MH and
Berndt SI: Serum IGF1, IGF2 and IGFBP3 and risk of advanced
colorectal adenoma. Int J Cancer. 131:E105–E113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teas J, Irhimeh MR, Druker S, Hurley TG,
Hébert JR, Savarese TM and Kurzer MS: Serum IGF-1 concentrations
change with soy and seaweed supplements in healthy postmenopausal
American women. Nutr Cancer. 63:743–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butler AA, Blakesley VA, Poulaki V, Tsokos
M, Wood TL and LeRoith D: Stimulation of tumor growth by
recombinant human insulin-like growth factor-I (IGF-I) is dependent
on the dose and the level of IGF-I receptor expression. Cancer Res.
58:3021–3027. 1998.PubMed/NCBI
|
|
13
|
Wu Y, Cui K, Miyoshi K, Hennighausen L,
Green JE, Setser J, LeRoith D and Yakar S: Reduced circulating
insulin-like growth factor I levels delay the onset of chemically
and genetically induced mammary tumors. Cancer Res. 63:4384–4388.
2003.PubMed/NCBI
|
|
14
|
McCarthy K, Laban C, McVittie CJ,
Ogunkolade W, Khalaf S, Bustin S, Carpenter R and Jenkins PJ: The
expression and function of IGFBP-3 in normal and malignant breast
tissue. Anticancer Res. 29:3785–3790. 2009.PubMed/NCBI
|
|
15
|
Valenciano A, Henríquez-Hernández LA,
Moreno M, Lloret M and Lara PC: Role of IGF-1 receptor in radiation
response. Transl Oncol. 5:1–9. 2012. View Article : Google Scholar
|
|
16
|
Yamamoto T, Oshima T, Yoshihara K, Nishi
T, Arai H, Inui K, Kaneko T, Nozawa A, Adachi H, Rino Y, Masuda M
and Imada T: Clinical significance of immunohistochemical
expression of insulin-like growth factor-1 receptor and matrix
metalloproteinase-7 in resected non-small cell lung cancer. Exp
Ther Med. 3:797–802. 2012.PubMed/NCBI
|
|
17
|
Wang H, Zhang Q, Zhang L, Little PJ, Xie
X, Meng Q, Ren Y, Zhou L, Gao G, Quirion R and Zheng W:
Insulin-like growth factor-1 induces the phosphorylation of PRAS40
via the PI3K/Akt signaling pathway in PC12 cells. Neurosci Lett.
516:105–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu R, Chen J, Sima J, Shen X, Liu D and
Shen J: NS398 induces apoptosis in non-small cell lung cancer
cells. J Cancer Res Clin Oncol. 138:119–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shakhar G and Ben-Eliyahu S: Potential
prophylactic measures against postoperative immunosuppression:
could they reduce recurrence rates in oncological patients? Ann
Surg Oncol. 10:972–992. 2003. View Article : Google Scholar
|
|
20
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmaol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Backhus LM, Sievers E, Lin GY, Castanos R,
Bart RD, Starnes VA and Bremner RM: Perioperative cyclooxygenase 2
inhibition to reduce tumor cell adhesion and metastatic potential
of circulating tumor cells in non-small cell lung cancer. J Thorac
Cardiovasc Surg. 132:297–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vlachostergios PJ, Gioulbasanis I,
Kamposioras K, Georgoulias P, Baracos VE, Ghosh S, Maragouli E,
Georgoulias V and Papandreou CN: Baseline insulin-like growth
factor-I plasma levels, systemic inflammation, weight loss and
clinical outcome in metastatic non-small cell lung cancer patients.
Oncology. 81:113–118. 2011. View Article : Google Scholar
|
|
23
|
Kelly GM, Buckley DA, Kiely PA, Adams DR
and O’Connor R: Serine phosphorylation of the insulin-like growth
factor I (IGF-1) receptor C-terminal tail restrains kinase activity
and cell growth. J Biol Chem. 287:28180–28194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee DY, Yi HK, Hwang PH and Oh Y: Enhanced
expression of insulin-like growth factor binding protein-3
sensitizes the growth inhibitory effect of anticancer drugs in
gastric cancer cells. Biochem Biophys Res Commun. 294:480–486.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
26
|
Kozaki K, Koshikawa K, Tatematsu Y,
Miyaishi O, Saito H, Hida T, Osada H and Takahashi T: Multi-faceted
analyses of a highly metastatic human lung cancer cell line
NCI-H460-LNM35 suggest mimicry of inflammatory cells in metastasis.
Oncogene. 20:4228–4234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cappuzzo F, Magrini E, Ceresoli GL,
Bartolini S, Rossi E, Ludovini V, et al: Akt phosphorylation and
gefitinib efficacy in patients with advanced non-small-cell lung
cancer. J Natl Cancer Inst. 96:1133–1141. 2004. View Article : Google Scholar
|
|
29
|
Uddin S, Ahmed M, Hussain A, Assad L,
Al-Dayel F, Bavi P, Al-Kuraya KS and Munkarah A: Cyclooxygenase-2
inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian
cancer. Int J Cancer. 126:382–394
|
|
30
|
Liu B, Qu L, Yang Z and Tao H:
Cyclooxygenase-2 inhibitors induce anoikis in osteosarcoma via
PI3K/Akt pathway. Med Hypotheses. 79:98–100. 2012. View Article : Google Scholar : PubMed/NCBI
|