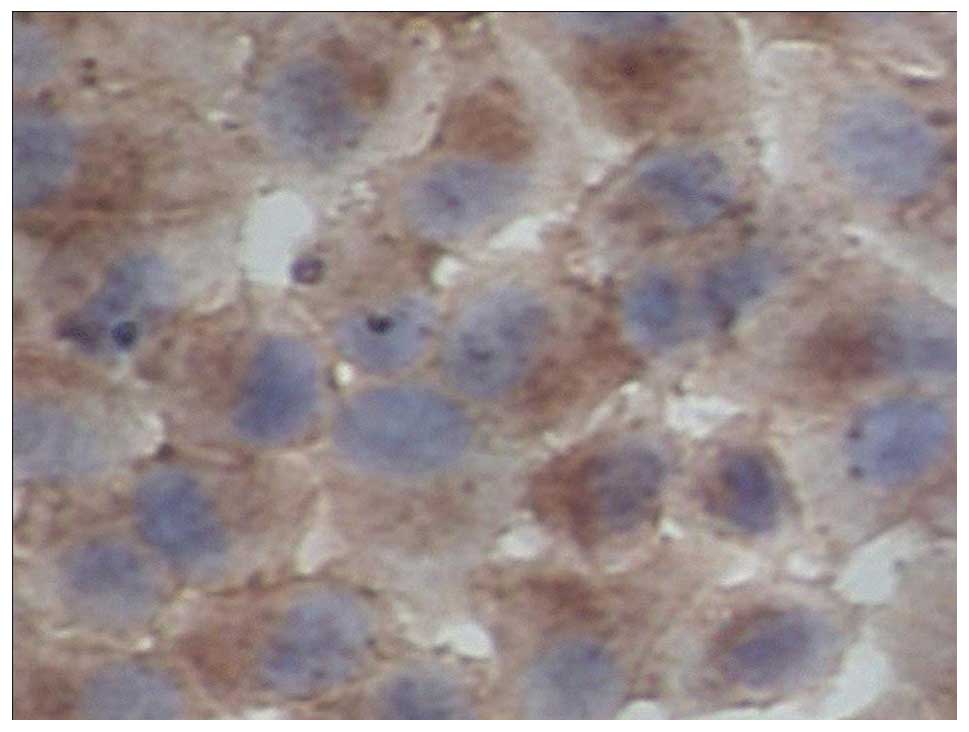
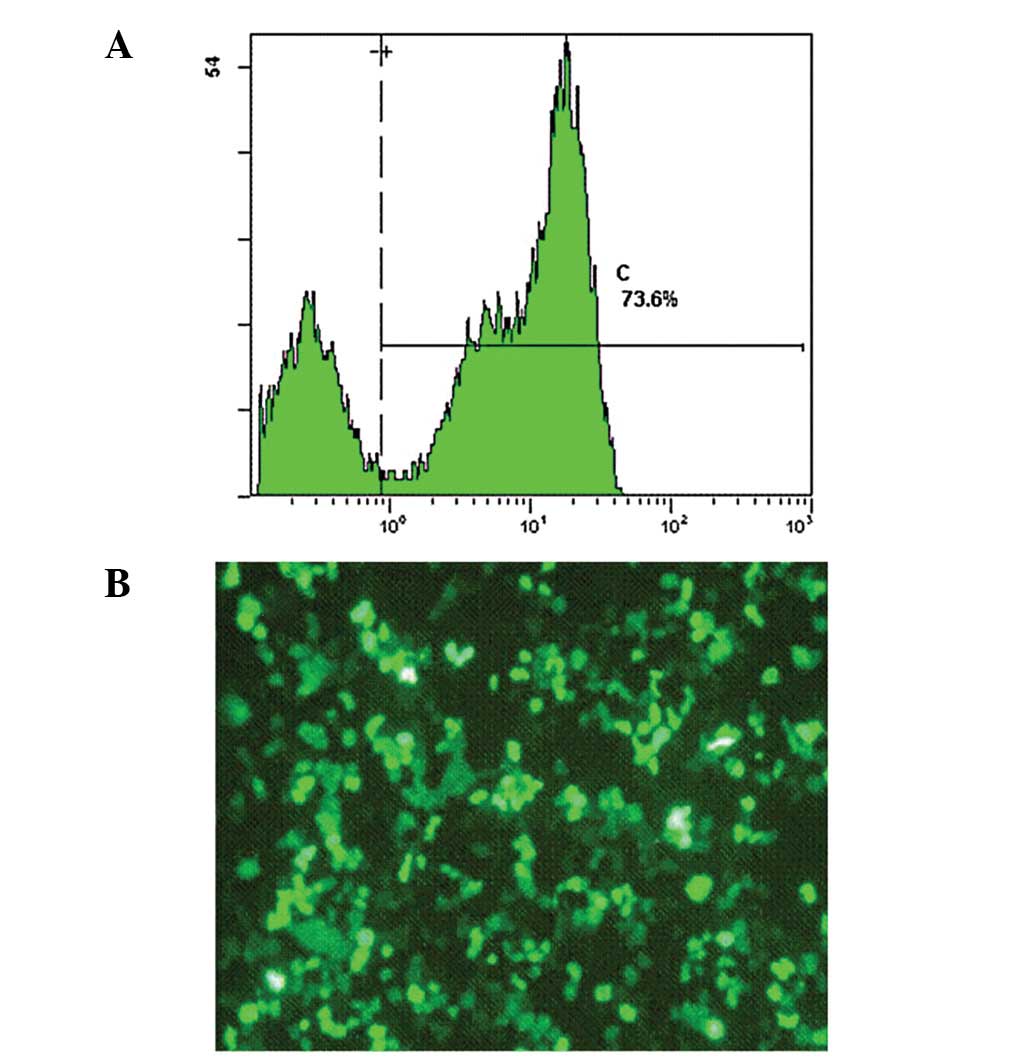
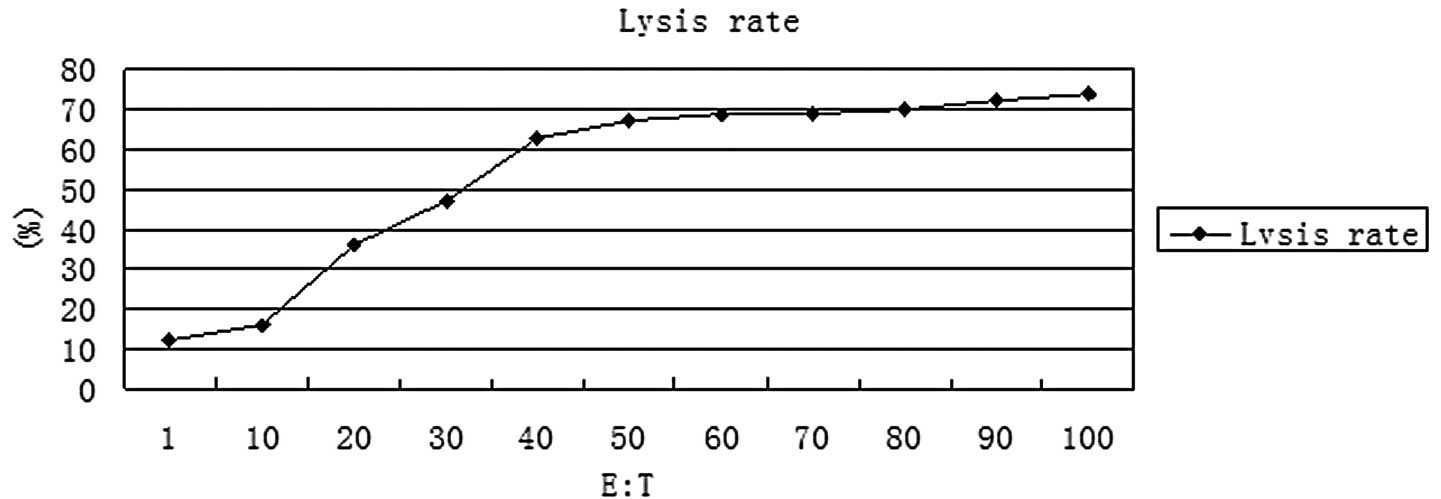
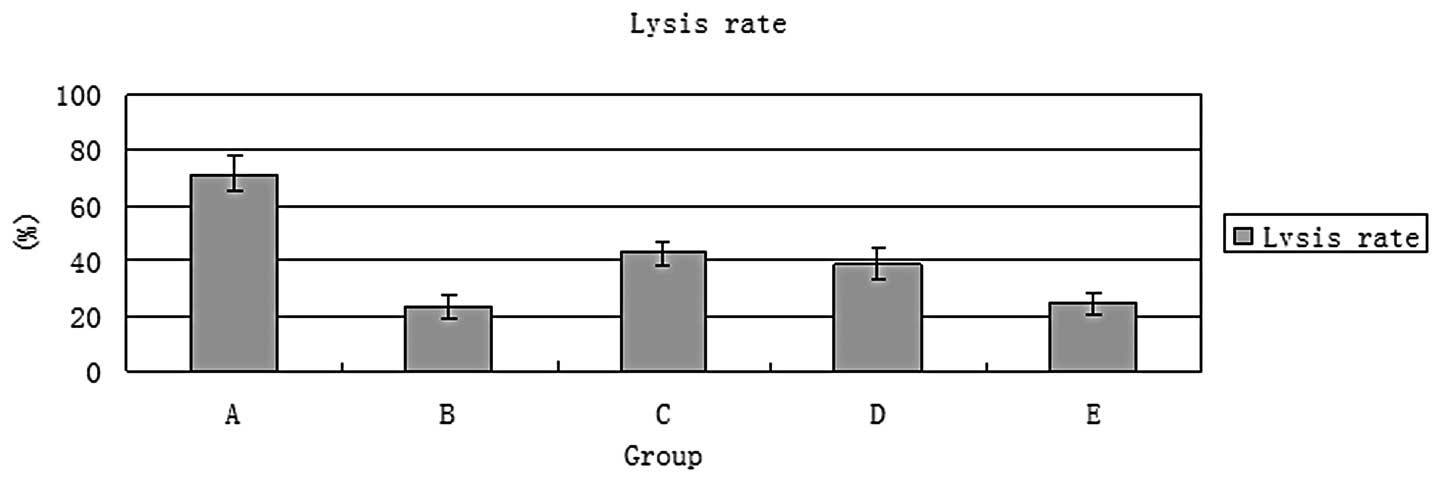
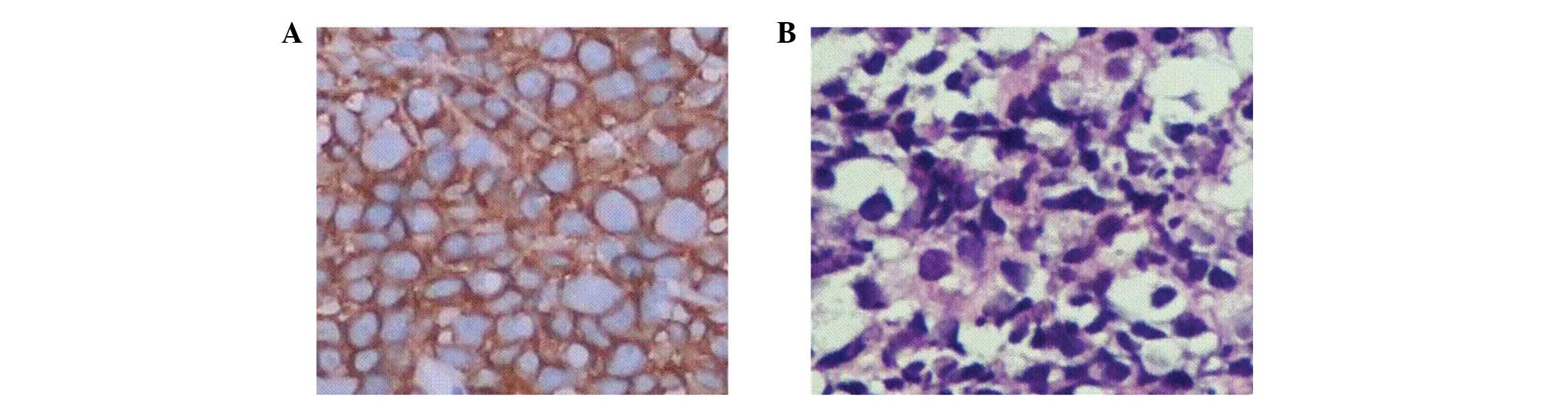
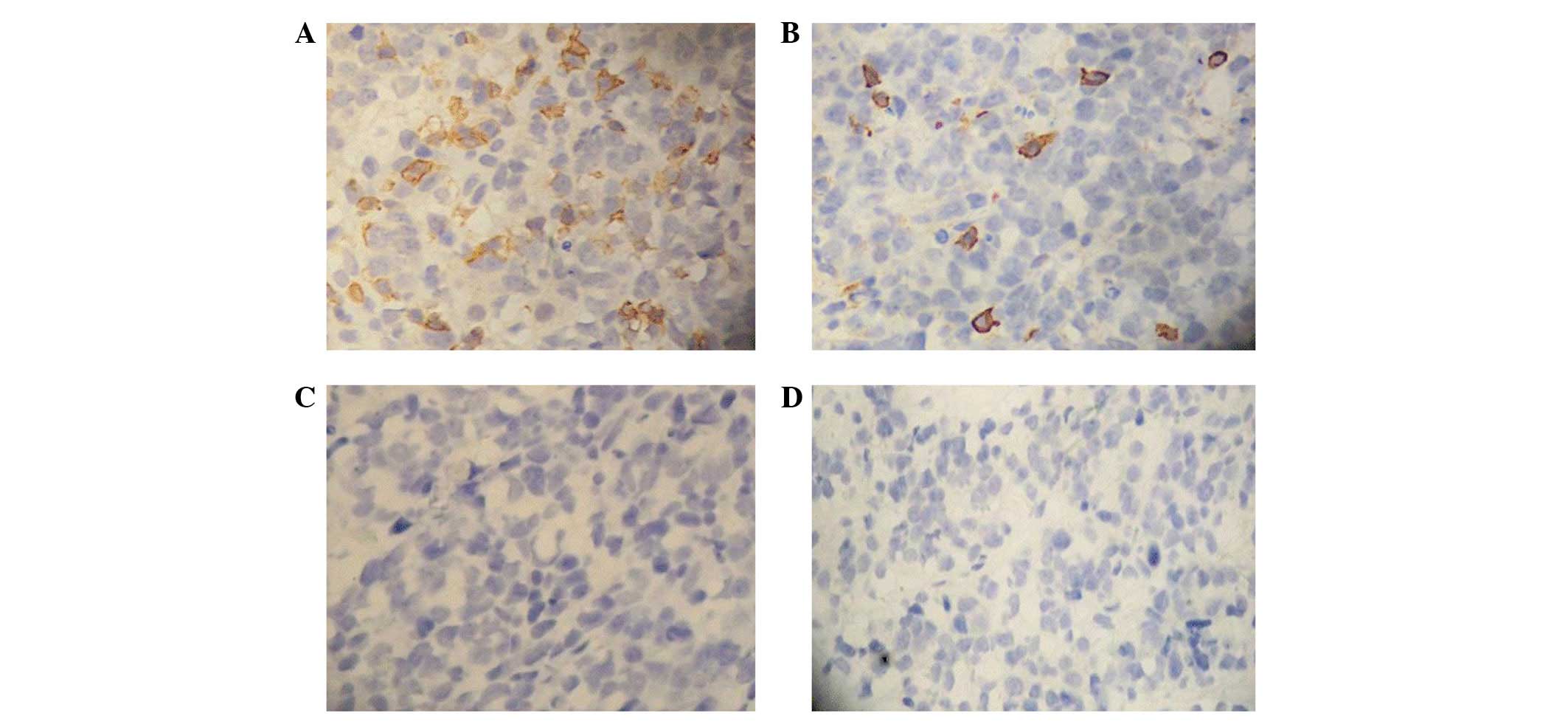
|
1
|
Parkin DM, Poisani P and Ferlay: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar
|
|
2
|
Stein HJ, Sendler A, Fink U and Siewert
JR: Multidisciplinary approach to esophageal and gastric cancer.
Surg Clin North Am. 80:659–682. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu PH and Negrin RS: A novel population of
expanded human CD3+CD56+ cells derived from T
cells with potent in vivo antitumor activity in mice with severe
combined immunodeficiency. J Immunol. 153:1687–1696.
1994.PubMed/NCBI
|
|
4
|
Schmidt-Wolf IG, Lefterova P, Mehta BA, et
al: Phenotypic characterization and identification of effector
cells involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
5
|
Märten A, Ziske C, Schöttker B, et al:
Interactions between dendritic cells and cytokine-induced killer
cells lead to an activation of both populations. J Immunother.
24:502–510. 2001.PubMed/NCBI
|
|
6
|
Hui D, Qiang L, Jian W, et al: A
randomized, controlled trial of postoperative adjuvant
cytokine-induced killer cells immunotherapy after radical resection
of hepatocellular carcinoma. Dig Liver Dis. 41:36–41. 2009.
View Article : Google Scholar
|
|
7
|
Li H, Wang C, Yu J, et al: Dendritic
cell-activated cytokine-induced killer cells enhance the anti-tumor
effect of chemotherapy on non-small cell lung cancer in patients
after surgery. Cytotherapy. 11:1076–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beun GD, van de Welde CJ and Fleuren GJ:
T-cell based cancer immunotherapy: direct or redirected tumor-cell
recognition? Immunol Today. 15:11–15. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fury MG, Lipton A, Smith KM, Winston CB
and Pfister DG: A phase-I trial of the epidermal growth factor
receptor directed bispecic antibody MDX-447 without and with
recombinant human granulocytecolony stimulating factor in patients
with advanced solid tumors. Cancer Immunol Immunother. 57:155–163.
2008. View Article : Google Scholar
|
|
10
|
Gall JM, Davol PA, Grabert RC, Deaver M
and Lum LG: T cells armed with anti-CD3 × anti-CD20 bispecific
antibody enhance killing of CD20+ malignant B cells and
bypass complement-mediated rituximab resistance in vitro. Exp
Hematol. 33:452–459. 2005.
|
|
11
|
Stanglmaier M, Faltin M, Ruf P,
Bodenhausen A, Schröder P and Lindhofer H: Bi20 (fBTA05), a novel
trifunctional bispecific antibody (anti-CD20 3 anti-CD3), mediates
efficient killing of B-cell lymphoma cells even with very low CD20
expression levels. Int J Cancer. 123:1181–1189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wimberger P, Xiang W, Mayr D, Diebold J,
Dreier T, Baeuerle PA and Kimmig R: Efficient tumor cell lysis by
autologous, tumor-resident T-lymphocytes in primary ovarian cancer
samples by an EP-CAM-/CD3-bispecific antibody. Int J Cancer.
105:241–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morecki S, Lindhofer H, Yacovlev E,
Gelfand Y, Ruf P and Slavin S: Induction of long-lasting antitumor
immunity by concomitant cell therapy with allogeneic lymphocytes
and trifunctional bispecific antibody. Exp Hematol. 36:997–1003.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sen M, Wankowski DM, Garlie NK, et al: Use
of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting
cytotoxicity of activated T cells toward HER2/neu+
tumors. J Hematother Stem Cell Res. 10:247–260. 2001.
|
|
15
|
Tita-Nwa F, Moldenhauer G, Herbst M,
Kleist C, Ho AD and Kornacker M: Cytokine-induced killer cells
targeted by the novel bispecific antibody CD19xCD5 (HD37xT5.16)
efficiently lyse B-lymphoma cells. Cancer Immunol Immunother.
56:1911–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoyle C, Bangs CD, Chang P, et al:
Expansion of Philadelphia chromosome-negative CD3(+) CD56(+)
cytotoxic cells from chronic myeloid leukemia patients: in vitro
and in vivo efficacy in severe combined immunodeficiency disease
mice. Blood. 92:3318–33127. 1998.PubMed/NCBI
|
|
17
|
Chan JK, Hamilton CA, Cheung MK, Karimi M,
Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, Lum LG
and Negrin RS: Enhanced killing of primary ovarian cancer by
retargeting autologous cytokine-induced killer cells with
bispecific antibodies: a preclinical study. Clin Cancer Res.
12:1859–1867. 2006. View Article : Google Scholar
|
|
18
|
Mehta BA, Schmidt-Wolf IG, Weissman IL and
Negrin RS: Two pathways of exocytosis of cytoplasmic granule
contents and target cell killing by cytokine-induced
CD3+ CD56+ killer cells. Blood. 86:3493–3499.
1995.PubMed/NCBI
|
|
19
|
Schmidt-Wolf GD, Negrin RS and
Schmidt-Wolf IG: Activated. T cells and cytokine-induced
CD3+CD56+ killer cells. Ann Hematol.
74:51–56. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt-Wolf IG, Lefterova P, Johnston V,
Huhn D, Blume KG and Negrin RS: Propagation of large numbers of T
cells with natural killer cell markers. Br J Haematol. 87:453–458.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canevari S, Stoter G, Arienti F, Bolis G,
Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers
CH, et al: Regression of advanced ovarian carcinoma by
intraperitoneal treatment with autologous T lymphocytes retargeted
by a bispecific monoclonal antibody. J Natl Cancer Inst.
87:1463–1469. 1995. View Article : Google Scholar
|
|
22
|
de Gast GC, Haagen IA, van Houten AA,
Klein SC, Duits AJ, de Weger RA, Vroom TM, Clark MR, Phillips J and
van Dijk AJ: CD8 T cell activation after intravenous administration
of CD3 × CD19 bispecific antibody in patients with non-Hodgkin
lymphoma. Cancer Immunol Immunother. 40:390–396. 1995.
|
|
23
|
Kroesen BJ, Bakker A, van Lier RA, et al:
Bispecific antibody-mediated target cell-specific costimulation of
resting T cells via CD5 and CD28. Cancer Res. 55:4409–4415.
1995.PubMed/NCBI
|
|
24
|
Marmé A, Strauss G, Bastert G, Grischke EM
and Moldenhauer G: Intraperitoneal bispecific antibody
(HEA125xOKT3) therapy inhibits malignant ascites production in
advanced ovarian carcinoma. Int J Cancer. 101:183–189.
2002.PubMed/NCBI
|
|
25
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
et al: Cetuximab for the treatment of colorectal cancer. N Engl J
Med. 357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar
|
|
27
|
Katzenwadel A, Schleer H, Gierschner D,
Wetterauer U and Elsässer-Beile U: Construction and in vivo
evaluation of an anti-PSA x anti-CD3 bispecific antibody for the
immunotherapy of prostate cancer. Anticancer Res. 20:1551–1555.
2000.PubMed/NCBI
|
|
28
|
de Leij L, Molema G, Helfrich W and
Kroesen BJ: Bispecific antibodies for treatment of cancer in
experimental animal models and man. Adv Drug Deliv Rev. 31:105–129.
1998.PubMed/NCBI
|
|
29
|
Verneris MR, Arshi A, Edinger M, Kornacker
M, Natkunam Y, Karami M, Cao YA, Marina N, Contag CH and Negrin RS:
Low levels of Her2/neu expressed by Ewing's family tumor cell lines
can redirect cytokine-induced killer cells. Clin Cancer Res.
11:4561–4570. 2005.PubMed/NCBI
|
|
30
|
Scambia G, Benedetti Panici P, Ferrandina
G, et al: Significance of epidermal growth factor receptor
expression in primary human endometrial cancer. Int J Cancer.
56:26–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mukohara T, Kudoh S, Yamauchi S, et al:
Expression of epidermal growth factor receptor (EGFR) and
downstream-activated peptides in surgically excised non-small-cell
lung cancer (NSCLC). Lung Cancer. 41:123–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie FH and Wang RX: The research progress
of the relationship between the way of EGFR family’s signal
transfer and breast cancer. Int J Lab Med. 27:1004–1008. 2006.
|
|
33
|
Kameda T, Yasui W, Tsujino T, et al:
Tyrosine kinase activity of epidermal growth factor receptor in
human gastric carcinomas. Pathol Res Pract. 188:37–43. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yasui W, Hata T, Yokozaki H, et al:
Interaction between epidermal growth factor and its receptor in
progression of human gastric carcinoma. Int J Cancer. 41:211–217.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pinto C, Difabio F, Siena S, et al: Phase
II study of cetuximab in combination with FOLFIRI in patients with
untreated advanced gastric or gastroesophageal junction
adenocarcinoma (FOLCETUX study). Ann Oncol. 18:510–517. 2007.
View Article : Google Scholar
|
|
36
|
Han SW, Oh DY, Im SA, et al: Phase II
study and biomarker analysis of cetuximab combined with modified
FOLFOX6 in advanced gastric cancer. Br J Cancer. 100:298–304. 2009.
View Article : Google Scholar : PubMed/NCBI
|