Introduction
The endothelial growth factor receptor (EGFR), which
is present in numerous cell types, is a transmembrane protein
consisting of an extracellular binding domain, a hydrophobic
transmembrane segment and a cytoplasmic tyrosine kinase domain, and
is considered one of the best characterized ligand-receptor systems
(1). Overexpression of EGFR has
been identified in a variety of solid tumors (2), and EGF has played a crucial role in
disease progression, poor prognosis and reduced sensitivity to
chemotherapy (3). Therefore,
blocking the signaling of EGF has been a major focus of new cancer
therapies.
Cetuximab is a human-murine monoclonal antibody
directed against the EGFR protein, which is expressed on the
surface of human tumor cells (4).
Cetuximab was approved by the Food and Drug Administration (FDA)
for use against metastatic colorectal cancer in February 2004
(5) and first gained approval in
Europe for use in the treatment of EGFR-expressing metastatic
colorectal cancer following the failure of irinotecan-containing
regimens (6). More recently, a
meta-analysis demonstrated an improved overall survival (OS) in
non-small cell lung cancer patients receiving chemotherapy plus
cetuximab compared with chemotherapy alone (7). The clinical efficacy of cetuximab in a
number of other malignancies, including head and neck cancer and
pancreatic cancer, is currently undergoing extensive
evaluation.
With the use of cetuximab, substantial adverse
events have been observed. Rashes, diarrhea, fatigue, neutropenia,
hypertension, nausea, infusion-related or hypersensitivity
reactions, and hand-foot skin reactions were extremely common when
cetuximab was first administrated for advanced cancer. In September
2005, the FDA released a warning about the possibility of severe
hypomagnesemia in relation to cetuximab therapy (8). A large number of patients with
metastatic colorectal cancer receiving cetuximab developed severe
hypomagnesemia that was refractory to oral magnesium
supplementation (9,10). However, no significant association
has yet been established between cetuximab and hypomagnesemia in
randomized controlled clinical trials (RCTs). Thus, we undertook a
systematic review of the relevant RCTs to evaluate the risk of
hypomagnesemia associated with cetuximab treatment for advanced
cancer.
Materials and methods
Data source
An extensive search of PubMed (up to March, 2012),
the Cochrane Central Register of Controlled Trials (up to Cochrane
Library Issue 3, 2012), and Embase (up to March, 2012) was
conducted to identify relevant RCTs for the meta-analysis, using
the keywords; ‘cetuximab’, ‘erbitux’, ‘cancer’ and
‘hypomagnesemia’. Abstracts and virtual meeting presentations from
the American Society of Clinical Oncology conferences held between
January 2000 and March 2012 were also searched for relevant RCTs.
The reference lists of articles, reviews, letters to the editor and
case reports were also searched to find those not yet included in
the computerized databases. The language of the research papers was
not restricted.
Study selection
RCTs that directly compared advanced cancer patients
treated with and without cetuximab, respectively, were selected for
the analysis. Phase I and single-arm phase II trials were excluded
due to the lack of control groups. Specifically, clinical trials
that met the following criteria were included in the meta-analysis:
i) prospective phase II and phase III RCTs in patients with
advanced cancer; ii) random assignment of participants to cetuximab
treatment or control group (placebo or best supportive care), in
addition to concurrent chemotherapy and/or treatment with a
biological agent; and iii) available data, including events or
incidences of hypomagnesemia and sample size for analysis.
Data extraction
Two researchers independently extracted data from
each identified trial using a predesigned review form. The
following data were included: authors of each study, publication
year, trial design, number of patients, number of patients eligible
for hypomagnesemia evaluation, age, gender, intervention, dose of
cetuximab administered, cancer type, phase of trial, follow-up
time, allocation concealment, blinded analysis and events or
incidences of hypomagnesemia.
Qualitative assessment
The studies were appraised independently by two
authors based on the standard criteria (randomization, blinding,
loss to follow-up and generation of allocation concealment), and
additional quantitative quality was assessed using the scoring
system developed by Jadad et al(11), appropriately modified according to
the treatments under study. The quality scoring system was as
follows: i) adequacy of randomization, coded as properly used with
detailed description of randomization (score 2), randomized but
details not reported (score 1) and inappropriate randomization
(score 0); ii) allocation concealment, coded as properly used
(score 2), unclear (score 1) and not used (score 0); iii) blinded
method, coded as double blind (score 2), single-blind (score 1) and
open label or unclear (score 0); iv) drop-outs and follow-ups,
coded as data given (score 1), and data not given (score 0). Any
disagreement was resolved by discussion.
Clinical end-points
The primary end-point was the incidence of
hypomagnesemia. Hypomagnesemia in these studies was assessed and
recorded according to the Common Terminology Criteria for Adverse
Events (version 2 or 3) (12,13).
Statistical analysis
Stata version 10.0 software (StataCorp., College
Station, TX, USA) was used for the statistical analysis. The
incidence of hypomagnesemia was calculated using the number of
patients with hypomagnesemia in the cetuximab group and the total
number of patients receiving cetuximab treatment. The proportion of
patients with hypomagnesemia was calculated and the 95% confidence
interval (CI) was derived for each trial.
The Chi-square test of heterogeneity and the
I2 measure of inconsistency were used to assess the
heterogeneity between trials. With an I2 value of
>50% indicating significant heterogeneity, the following
techniques were used as explanations: (a) subgroup analysis; (b)
sensitivity analysis performed by excluding the trials which
potentially biased the results; and (c) the random effects model
was used to explore the cause of the heterogeneity. The Begg’s test
was used to determine the presence of publication bias with regard
to the primary variable [relative risk (RR) of hypomagnesemia]. A
two-tailed P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Identification of included studies
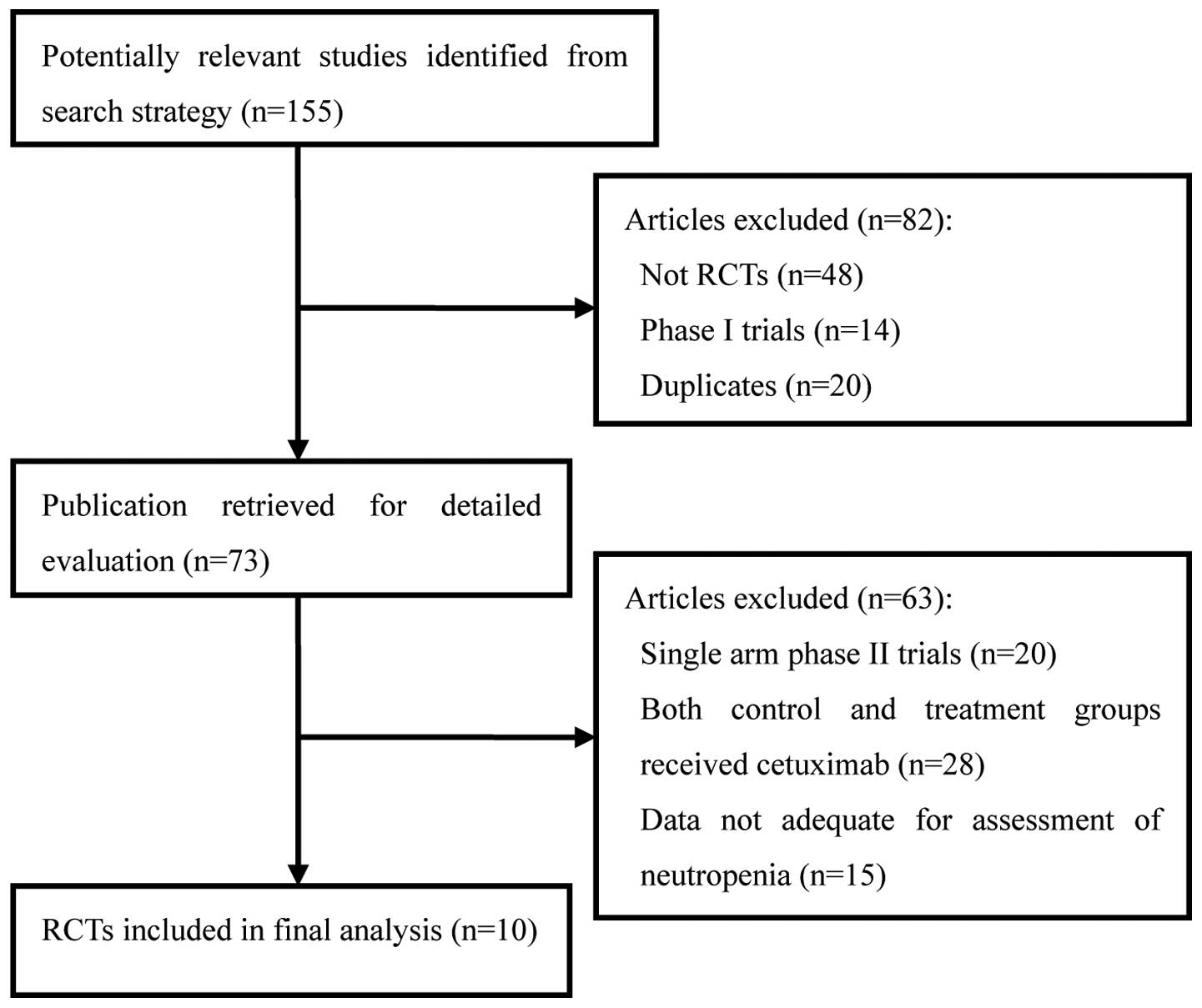
A total of 155 clinical studies relevant to
cetuximab were identified by the literature search. Review
articles, case reports, meta-analyses, observational studies
(n=48), phase I studies (n=14), single-arm phase II studies (n=20),
duplicates (n=20), studies in which the control and treatment
groups each received cetuximab (n=28) and those data not adequate
for assessment of severe neutropenia (n=15; Fig. 1) were excluded. Ultimately, 10 RCTs,
including five phase II and five phase III studies, were selected
for analysis, involving a total of 7,045 patients. The main
characteristics (type of study design, underlying malignancy of
included patients, concurrent treatment and number of patients) of
the 10 included RCTs are presented in Table I. Randomized treatment allocation
sequences were generated in all trials. Only one trial was
double-blinded and placebo-controlled (14), five of the trials were open-label
(15–19) and four trials were not specified
(20–23). All trials reported the number and
reason of withdrawals and drop-outs. None mentioned allocation
concealment. A total of seven trials were described as multicenter
trials and three did not mention their status (19,20,23).
The median follow-up time for four of the studies (18,19,21,22)
ranged from 6.8 to 31 months, while six studies did not state this
factor. Hypomagnesemia was assessed and recorded according to the
National Cancer Institute’s Common Toxicity Criteria, version 2 or
3 (12,13). The baseline Eastern Cooperative
Oncology Group (ECOG) performance status of all patients was
between 0 and 2. Patients were required to have adequate hepatic,
renal and hematological function. The underlying malignancies
observed consisted of colorectal cancer (six studies), non-small
cell lung cancer (two studies) and head and neck cancer (two
studies).
 | Table ICharacteristics of randomized
controlled clinical trials (RCTs) included in the
meta-analysis. |
Table I
Characteristics of randomized
controlled clinical trials (RCTs) included in the
meta-analysis.
| First author
(ref.) | Trial phase | Number of patients
enrolled | Number for
analysis | Underlying
malignancy | Concurrent
treatment | Jaded score | Cetuximab dose
(mg/m2 per week) |
|---|
| Tol (21) | III | 389 | 389 | Colorectal
cancer | Capecitabine,
oxaliplatin and bevacizumab | 3 | 250 |
| Lynch (15) | III | 676 | 645 | Non-small cell lung
cancer | Paclitaxel or
docetaxel | 3 | 250 |
| Jonker (19) | III | 572 | 566 | Colorectal
cancer | Fluoropyrimidine,
irinotecan and oxaliplatin | | 250 |
| Alberts (22) | III | 2686 | 1825 | Colorectal
cancer | Fluorouracil,
leucovorin and irinotecan | 4 | 250 |
| Maughan (23) | III | 1634 | 1634 | Colorectal
cancer | Oxaliplatin and
fuoropyrimidine | 3 | 250 |
| Adams (16) | III | 804 | 804 | Colorectal
cancer | Leucovorin,
oxaliplatin and iluorouracil or oxaliplatin and capecitabine | | 250 |
| Vermorken (20) | III | 442 | 434 | Head and neck
cancer | Fluorouracil,
cisplatin or carboplatin | 4 | 250 |
| Sobrero (17) | III | 1298 | 1267 | Colorectal
cancer | Irinotecan | 3 | 250 |
| Butts (18) | II | 131 | 130 | Non-small cell lung
cancer | Cisplatin or
carboplatin | 3 | 250 |
| Burtness (14) | III | 117 | 116 | Head and neck
cancer | Cisplatin and
placebo | 5 | 125 |
Risk of hypomagnesemia for cetuximab
administration
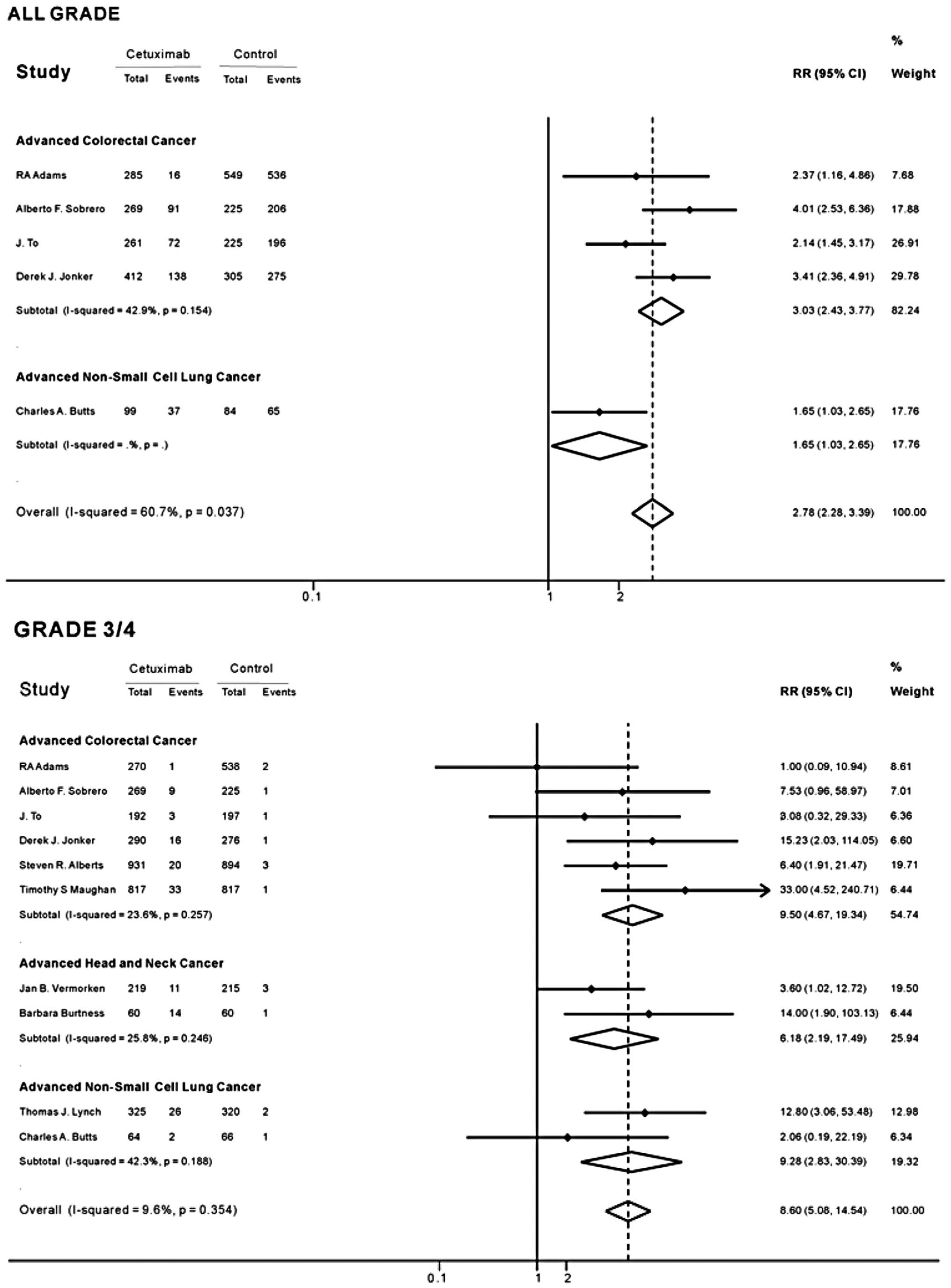
As no heterogeneity was found among the included
studies in the overall analysis (all-grades of hypomagnesemia
I2, 60.7%, P=0.037; grade 3/4 I2, 9.6%,
P=0.354), the fixed-effects model was used. The overall RR of grade
3/4 hypomagnesemia with cetuximab versus control was 8.60 (95% CI,
5.08–14.54; Fig. 2), indicating a
significantly higher incidence of grade 3/4 hypomagnesemia in the
cetuximab groups. The RR of the subgroup analysis suggested a
significant association between grade 3/4 hypomagnesemia and
cetuximab treatment among patients with non-small cell lung cancer
(RR, 9.28; 95% CI, 2.83–30.39; Fig.
2). The RR of grade 3/4 hypomagnesemia was lowest in patients
with head and neck cancer treated with cetuximab compared with
controls (RR, 6.18; 95% CI, 2.19–17.49), and highest in patients
with colorectal cancer (RR, 9.50; 95% CI, 4.67–19.34). Of all the
trials, five reported that the cetuximab groups had a higher
incidence of grade 3/4 hypomagnesemia compared with the control
groups.
Incidence of hypomagnesemia for cetuximab
administration
The overall incidence of grade 3/4 hypomagnesemia in
the patients receiving cetuximab was 3.9% (95% CI, 2.6–4.3).
Patients with differing tumors may be at varying risks of grade 3/4
hypomagnesemia due to differences in tumor malignancy and the
associated treatments. The present study explored whether having a
specific type of cancer was associated with a higher risk of severe
neutropenia compared with other cancers. As shown in Table II, the risk of grade 3/4
hypomagnesemia varied according to the tumor type. The highest
incidence of grade 3/4 hypomagnesemia was observed in patients with
non-small cell lung cancer (9.0%; 95% CI, 5.0–15.4), while the
lowest incidence was observed in patients with colorectal cancer
(2.9%; 95% CI, 1.7–3.1).
 | Table IIIncidence of grade 3/4 hypomagnesemia
with cetuximab among patients with various tumor types. |
Table II
Incidence of grade 3/4 hypomagnesemia
with cetuximab among patients with various tumor types.
| Tumor type | Number of
studies | Cetuximab | Control | Incidence (95%
CI) |
|---|
| Overall | 10 | 135 (3437) | 16 (3608) | 0.039
(0.026–0.043) |
| Colorectal
cancer | 6 | 82 (2769) | 9 (2947) | 0.029
(0.017–0.031) |
| Non-small cell lung
cancer | 2 | 25 (279) | 4 (275) | 0.090
(0.050–0.154) |
| Head and neck
cancer | 2 | 28 (389) | 3 (386) | 0.07 (0.015–0.1
77) |
Publication bias
No publication bias was detected for the primary
variable of the present study (RR of grade 3/4 hypomagnesemia) by
Begg’s and Egger’s tests (P=0.38; P=0.29, respectively).
Discussion
Hypomagnesemia may result in cardiac arrhythmia,
coronary artery vasospasm and sudden cardiac death. Adequate
management of hypomagnesemia is important for the numerous patients
who receive cetuximab-based therapy. However, the symptoms of
hypomagnesemia may be fairly non-specific, including irritability,
paresthesia and severe fatigue, which may easily be attributed to
the underlying tumor or to previous chemotherapy regimens (24). Hypomagnesemia is often ignored in
studies, and serum magnesium levels should be monitored better when
cetuximab-based therapy is performed for advanced cancer. In RCTs
discussing the association of hypomagnesemia and cetuximab, an
individual RCT is not powerful enough to detect a significant
correlation; therefore the contribution of cetuximab to the
development of hypomagnesemia is difficult to assess. The present
study combined 10 RCTs to overcome this limitation. The result
demonstrated a high incidence of grade 3/4 hypomagnesemia (3.9%;
95% CI, 2.6–4.3) associated with cetuximab treatment for advanced
cancer. Cetuximab treatment had a higher risk of grade 3/4
hypomagnesemia compared with the control (RR, 8.60; 95% CI,
5.08–14.54). The present study also showed that the risk of grade
3/4 hypomagnesemia with cetuximab may vary with the tumor type.
Patients with advanced colorectal cancer had the highest incidence
of grade 3/4 hypomagnesemia.
The mechanisms behind this toxicity have not been
well defined. Numerous studies on hereditary renal
Mg2+-wasting syndromes and inborn errors of the
Mg2+ balance demonstrated that several new proteins were
involved in transepithelial Mg2+ transport in the distal
convoluted tubule, including the Mg2+-permeable channel
TRPM6 (transient receptor potential cation channel, subfamily M,
member 6) and TRPM7 (25–27). Groenestege et al(28) revealed that in vitro
cetuximab preincubation abolished the stimulatory effect of EGF on
TRPM6 activity. Moreover, EGFR is highly expressed in the kidney,
particularly in the ascending limb of the loop of Henle, where 70%
of filtered Mg2+ is reabsorbed. Cetuximab, as an EGFR
blockade, may affect Mg2+ transport (29). However, this effect has not been
described with other small molecule anti-EGFR agents, such as
gefitinib and erlotinib. Thus, a pure anti-EGFR effect does not
adequately explain this toxicity. Recent data from panitumumab
clinical trials have also reported hypomagnesemia toxicity in
patients with metastatic colorectal cancer (30). This suggests that hypomagnesemia
toxicity is a monoclonal antibody anti-EGFR-specific
phenomenon.
There are several limitations in the present study
analysis that require consideration. Firstly, the meta-analysis
results are affected by clinical heterogeneity. The trials have
varying patient clinical profiles, concurrent chemotherapies,
lengths of follow-up and lengths of treatment; thus, differences
among trials are inevitable, and there is always some
heterogeneity, even within individual trials. However,
heterogeneity does not necessarily preclude pooling of the results
since individual patients are only directly compared with other
patients within the same trial and not across trials (31,32).
Given the uncertainty resulting from this clinical heterogeneity,
subgroup analyses were performed in the present meta-analysis.
Secondly, the meta-analysis only included 10 studies out of 155
identified in the literature search. In this regard, only those
trials conducted with a rigorous methodology were selected in order
to provide solid conclusions. Meta-analyses often include small
numbers of studies and heterogeneity is therefore a necessary
consequence. Higgins et al evaluated Cochrane reviews and
identified that 67% included five studies and that 20% included ten
studies (33). A lower threshold
for the number of studies to be included in a meta-analysis has not
yet been established. Finally, not all articles had data available
on all grades of hypomagnesemia.
In conclusion, the present study showed that
cetuximab is associated with a significant risk of hypomagnesemia
in patients with advanced cancer who were receiving concurrent
chemotherapy. This risk varies with the tumor type. Early
monitoring of hypomagnesemia is important when cetuximab-based
therapy is performed. Patients undergoing cetuximab administration
with grade 3/4 of hypomagnesemia should receive appropriate and
aggressive replacement therapy due to the high risk of cardiac
arrhythmias and sudden mortality.
References
|
1
|
Milano G, Spano JP and Leyland-Jones B:
EGFR-targeting drugs in combination with cytotoxic agents: from
bench to bedside, a contrasted reality. Br J Cancer. 99:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heist RS and Christiani D: EGFR-targeted
therapies in lung cancer: predictors of response and toxicity.
Pharmacogenomics. 10:59–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brandes AA, Franceschi E, Tosoni A, Hegi
ME and Stupp R: Epidermal growth factor receptor inhibitors in
neuro-oncology: hopes and disappointments. Clin Cancer Res.
14:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reynolds NA and Wagstaff AJ: Cetuximab: in
the treatment of metastatic colorectal cancer. Drugs. 64:109–121.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, Chau I and Van Cutsem E: Cetuximab monotherapy and cetuximab
plus irinotecan in irinotecan-refractory metastatic colorectal
cancer. N Engl J Med. 351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ocvirk J, Brodowicz T, Wrba F, Ciuleanu
TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U,
Faluhelyi Z, Yalcin S, Papamichael D, Wenczl M, Mrsic-Krmpotic Z,
Shacham-Shmueli E, Vrbanec D, Esser R, Scheithauer W and Zielinski
CC: Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal
cancer: CECOG trial. World J Gastroenterol. 16:3133–3143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen P, Wang L, Liu B, Zhang HZ, Liu HC
and Zou Z: EGFR-targeted therapies combined with chemotherapy for
treating advanced non-small-cell lung cancer: a meta-analysis. Eur
J Clin Pharmacol. 67:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blay JY, Chauvin F, Le Cesne A, Anglaret
B, Bouhour D, Lasset C, Freyer G, Philip T and Biron P: Early
lymphopenia after cytotoxic chemotherapy as a risk factor for
febrile neutropenia. J Clin Oncol. 14:636–643. 1996.PubMed/NCBI
|
|
9
|
Crawford J, Dale DC and Lyman GH:
Chemotherapy-induced neutropenia: risks, consequences, and new
directions for its management. Cancer. 100:228–237. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
12
|
Okayama N, Nishioka M, Hazama S, Sakai K,
Suehiro Y, Maekawa M, Sakamoto J, Iwamoto S, Kato T, Mishima H, Oka
M and Hinoda Y: The importance of evaluation of DNA
amplific-ability in KRAS mutation testing with dideoxy sequencing
using formalin-fixed and paraffin-embedded colorectal cancer
tissues. Jpn J Clin Oncol. 41:165–171. 2011. View Article : Google Scholar
|
|
13
|
Wu L, Parton A, Lu L, Adams M, Schafer P
and Bartlett JB: Lenalidomide enhances antibody-dependent cellular
cytotoxicity of solid tumor cells in vitro: influence of host
immune and tumor markers. Cancer Immunol Immunother. 60:61–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burtness B, Goldwasser MA, Flood W, Mattar
B and Forastiere AA; Eastern Cooperative Onclogy Group: Phase III
randomized trial of cisplatin plus placebo compared with cisplatin
plus cetuximab in metastatic/recurrent head and neck cancer: an
Eastern Cooperative Oncology Group study. J Clin Oncol.
23:8646–8654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynch TJ, Patel T, Dreisbach L, McCleod M,
Heim WJ, Hermann RC, Paschold E, Iannotti NO, Dakhil S, Gorton S,
Pautret V, Weber MR and Woytowitz D: Cetuximab and first-line
taxane/carboplatin chemotherapy in advanced non-small-cell lung
cancer: results of the randomized multicenter phase III trial
BMS099. J Clin Oncol. 28:911–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams RA, Meade AM, Madi A, Fisher D, Kay
E, Kenny S, Kaplan RS and Maughan TS: Toxicity associated with
combination oxaliplatin plus fluoropyrimidine with or without
cetuximab in the MRC COIN trial experience. Br J Cancer.
100:251–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C,
Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H,
Luppi G, Kisker O, Zubel A, Langer C, Kopit J and Burris HA III:
EPIC: phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butts CA, Bodkin D, Middleman EL, Englund
CW, Ellison D, Alam Y, Kreisman H, Graze P, Maher J, Ross HJ, Ellis
PM, McNulty W, Kaplan E, Pautret V, Weber MR and Shepherd FA:
Randomized phase II study of gemcitabine plus cisplatin or
carboplatin [corrected], with or without cetuximab, as first-line
therapy for patients with advanced or metastatic non small-cell
lung cancer. J Clin Oncol. 25:5777–5784. 2007.
|
|
19
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ,
Tebbutt NC, van Hazel G, Wierzbicki R, Langer C and Moore MJ:
Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C,
Schueler A, Amellal N and Hitt R: Platinum-based chemotherapy plus
cetuximab in head and neck cancer. N Engl J Med. 359:1116–1127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tol J, Koopman M, Rodenburg CJ, Cats A,
Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF and
Punt CJ: A randomised phase III study on capecitabine, oxaliplatin
and bevacizumab with or without cetuximab in first-line advanced
colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer
Group (DCCG). An interim analysis of toxicity Ann Oncol.
19:734–738. 2008.PubMed/NCBI
|
|
22
|
Alberts SR, Sargent DJ, Nair S, Mahoney
MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S,
Kahlenberg MS, Shields AF, Quesenberry JT, Webb TA, Farr GH Jr,
Pockaj BA, Grothey A and Goldberg RM: Effect of oxaliplatin,
fluorouracil, and leucovorin with or without cetuximab on survival
among patients with resected stage III colon cancer: a randomized
trial. JAMA. 307:1383–1393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J,
Kennedy MJ, Claes B, Lambrechts D, Kaplan R and Cheadle JP; MRC
COIN Trial Investigators: Addition of cetuximab to
oxaliplatin-based first-line combination chemotherapy for treatment
of advanced colorectal cancer: results of the randomised phase 3
MRC COIN trial. Lancet. 377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schrag D, Chung KY, Flombaum C and Saltz
L: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer
Inst. 97:1221–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Touyz RM: Transient receptor potential
melastatin 6 and 7 channels, magnesium transport, and vascular
biology: implications in hypertension. Am J Physiol Heart Circ
Physiol. 294:H1103–H1118. 2008. View Article : Google Scholar
|
|
26
|
Walder RY, Landau D, Meyer P, Shalev H,
Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi
R and Sheffield VC: Mutation of TRPM6 causes familial
hypomagnesemia with secondary hypocalcemia. Nat Genet. 31:171–174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlingmann KP, Weber S, Peters M, Niemann
Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E,
Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW
and Konrad M: Hypomagnesemia with secondary hypocalcemia is caused
by mutations in TRPM6, a new member of the TRPM gene family. Nat
Genet. 31:166–170. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Groenestege WM, Thébault S, van der Wijst
J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van
Cutsem E, Hoenderop JG, Knoers NV and Bindels RJ: Impaired
basolateral sorting of pro-EGF causes isolated recessive renal
hypomagnesemia. J Clin Invest. 117:2260–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vincenzi B, Santini D and Tonini G:
Biological interaction between anti-epidermal growth factor
receptor agent cetuximab and magnesium. Expert Opin Pharmacother.
9:1267–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petrelli F, Borgonovo K, Cabiddu M,
Ghilardi M and Barni S: Risk of anti-EGFR monoclonal
antibody-related hypomagnesemia: systematic review and pooled
analysis of randomized studies. Expert Opin Drug Saf. 11(Suppl 1):
S9–S19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lau J, Ioannidis JP and Schmid CH: Summing
up evidence: one answer is not always enough. Lancet. 351:123–127.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson SG: Why sources of heterogeneity
in meta-analysis should be investigated. BMJ. 309:1351–1355.
1994.PubMed/NCBI
|
|
33
|
Higgins J, Thompson S, Deeks J and Altman
D: Statistical heterogeneity in systematic reviews of clinical
trials: a critical appraisal of guidelines and practice. J Health
Serv Res Policy. 7:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|