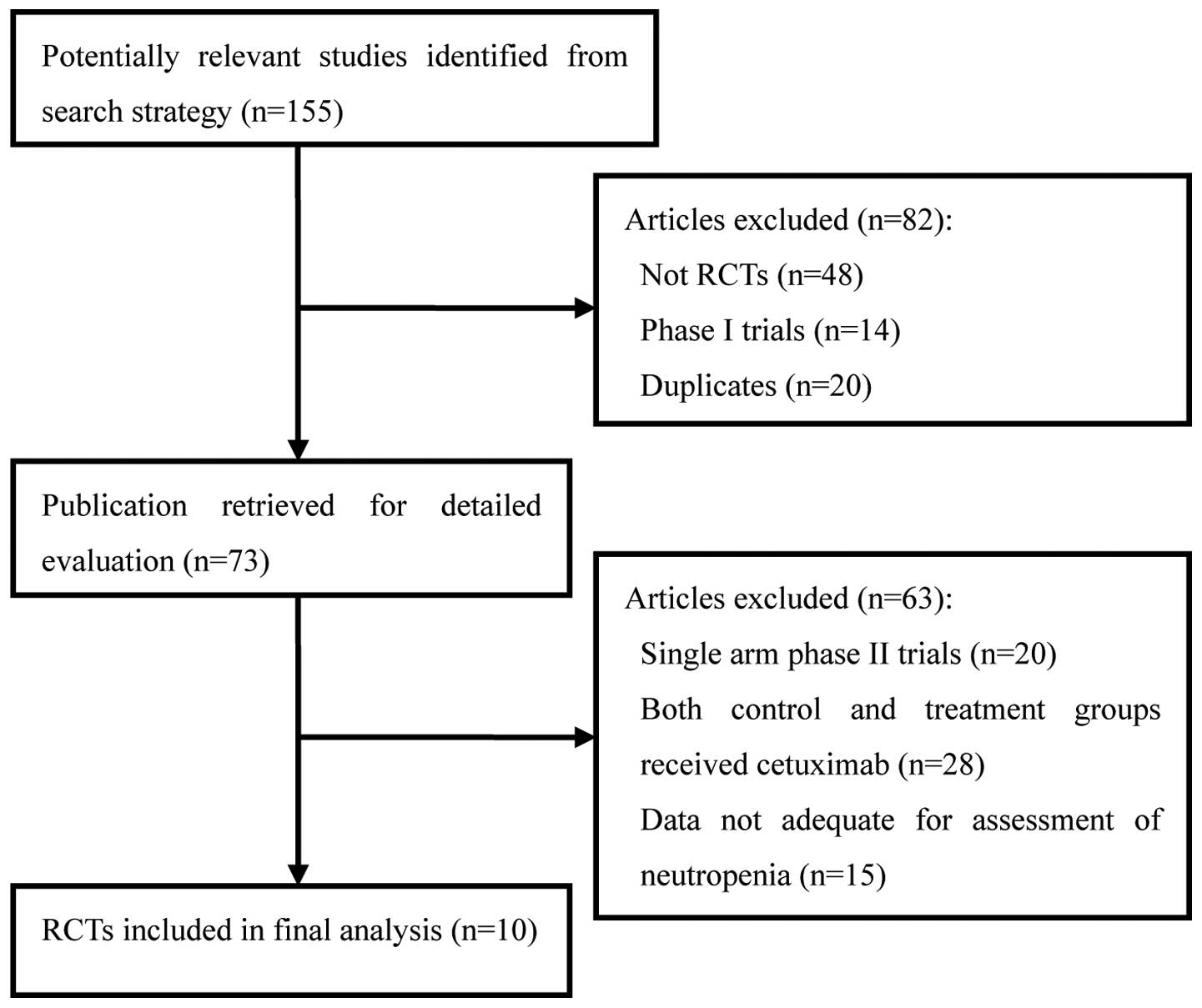
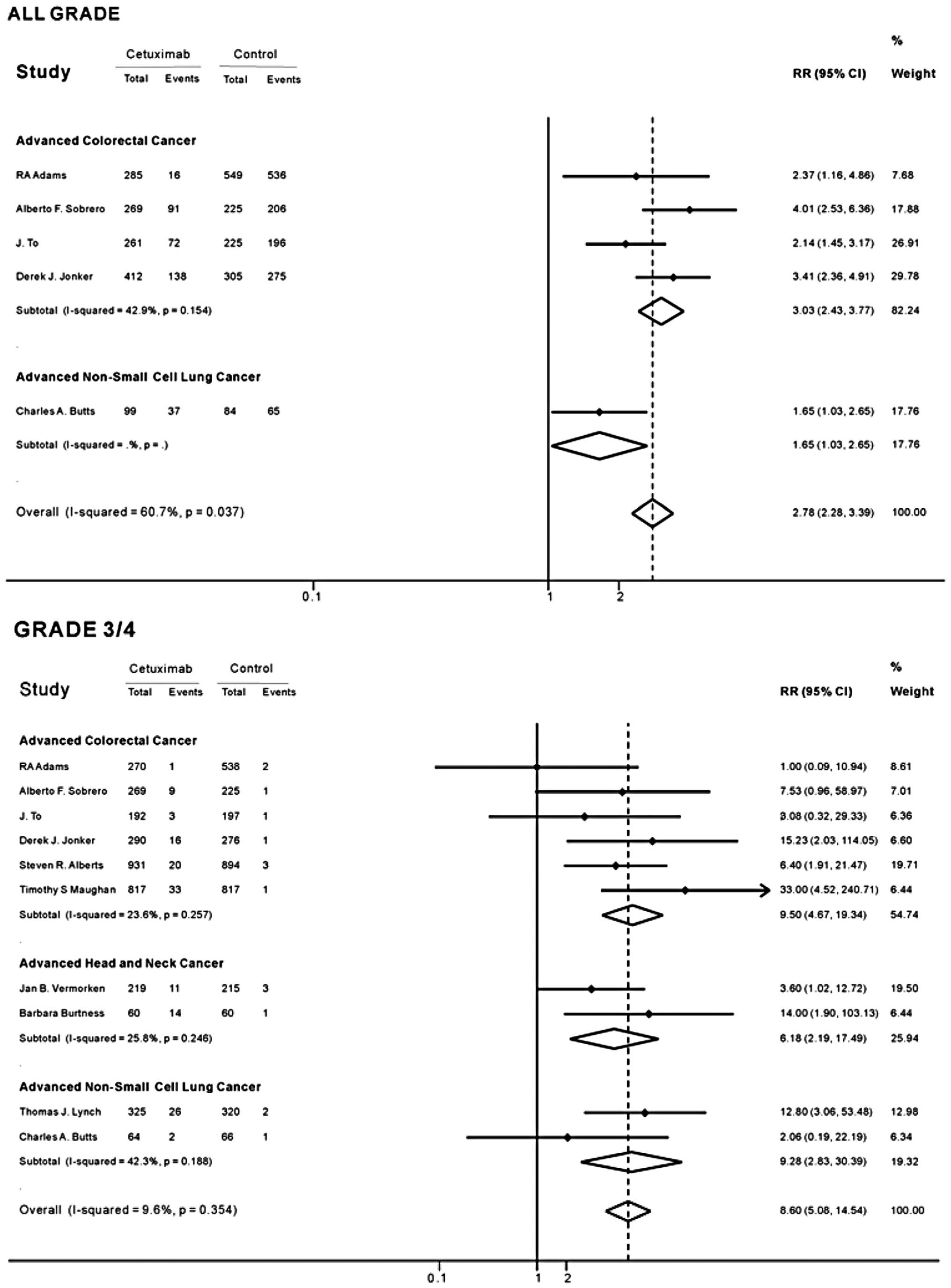
|
1
|
Milano G, Spano JP and Leyland-Jones B:
EGFR-targeting drugs in combination with cytotoxic agents: from
bench to bedside, a contrasted reality. Br J Cancer. 99:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heist RS and Christiani D: EGFR-targeted
therapies in lung cancer: predictors of response and toxicity.
Pharmacogenomics. 10:59–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brandes AA, Franceschi E, Tosoni A, Hegi
ME and Stupp R: Epidermal growth factor receptor inhibitors in
neuro-oncology: hopes and disappointments. Clin Cancer Res.
14:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reynolds NA and Wagstaff AJ: Cetuximab: in
the treatment of metastatic colorectal cancer. Drugs. 64:109–121.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, Chau I and Van Cutsem E: Cetuximab monotherapy and cetuximab
plus irinotecan in irinotecan-refractory metastatic colorectal
cancer. N Engl J Med. 351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ocvirk J, Brodowicz T, Wrba F, Ciuleanu
TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U,
Faluhelyi Z, Yalcin S, Papamichael D, Wenczl M, Mrsic-Krmpotic Z,
Shacham-Shmueli E, Vrbanec D, Esser R, Scheithauer W and Zielinski
CC: Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal
cancer: CECOG trial. World J Gastroenterol. 16:3133–3143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen P, Wang L, Liu B, Zhang HZ, Liu HC
and Zou Z: EGFR-targeted therapies combined with chemotherapy for
treating advanced non-small-cell lung cancer: a meta-analysis. Eur
J Clin Pharmacol. 67:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blay JY, Chauvin F, Le Cesne A, Anglaret
B, Bouhour D, Lasset C, Freyer G, Philip T and Biron P: Early
lymphopenia after cytotoxic chemotherapy as a risk factor for
febrile neutropenia. J Clin Oncol. 14:636–643. 1996.PubMed/NCBI
|
|
9
|
Crawford J, Dale DC and Lyman GH:
Chemotherapy-induced neutropenia: risks, consequences, and new
directions for its management. Cancer. 100:228–237. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
12
|
Okayama N, Nishioka M, Hazama S, Sakai K,
Suehiro Y, Maekawa M, Sakamoto J, Iwamoto S, Kato T, Mishima H, Oka
M and Hinoda Y: The importance of evaluation of DNA
amplific-ability in KRAS mutation testing with dideoxy sequencing
using formalin-fixed and paraffin-embedded colorectal cancer
tissues. Jpn J Clin Oncol. 41:165–171. 2011. View Article : Google Scholar
|
|
13
|
Wu L, Parton A, Lu L, Adams M, Schafer P
and Bartlett JB: Lenalidomide enhances antibody-dependent cellular
cytotoxicity of solid tumor cells in vitro: influence of host
immune and tumor markers. Cancer Immunol Immunother. 60:61–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burtness B, Goldwasser MA, Flood W, Mattar
B and Forastiere AA; Eastern Cooperative Onclogy Group: Phase III
randomized trial of cisplatin plus placebo compared with cisplatin
plus cetuximab in metastatic/recurrent head and neck cancer: an
Eastern Cooperative Oncology Group study. J Clin Oncol.
23:8646–8654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynch TJ, Patel T, Dreisbach L, McCleod M,
Heim WJ, Hermann RC, Paschold E, Iannotti NO, Dakhil S, Gorton S,
Pautret V, Weber MR and Woytowitz D: Cetuximab and first-line
taxane/carboplatin chemotherapy in advanced non-small-cell lung
cancer: results of the randomized multicenter phase III trial
BMS099. J Clin Oncol. 28:911–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams RA, Meade AM, Madi A, Fisher D, Kay
E, Kenny S, Kaplan RS and Maughan TS: Toxicity associated with
combination oxaliplatin plus fluoropyrimidine with or without
cetuximab in the MRC COIN trial experience. Br J Cancer.
100:251–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C,
Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H,
Luppi G, Kisker O, Zubel A, Langer C, Kopit J and Burris HA III:
EPIC: phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butts CA, Bodkin D, Middleman EL, Englund
CW, Ellison D, Alam Y, Kreisman H, Graze P, Maher J, Ross HJ, Ellis
PM, McNulty W, Kaplan E, Pautret V, Weber MR and Shepherd FA:
Randomized phase II study of gemcitabine plus cisplatin or
carboplatin [corrected], with or without cetuximab, as first-line
therapy for patients with advanced or metastatic non small-cell
lung cancer. J Clin Oncol. 25:5777–5784. 2007.
|
|
19
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ,
Tebbutt NC, van Hazel G, Wierzbicki R, Langer C and Moore MJ:
Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C,
Schueler A, Amellal N and Hitt R: Platinum-based chemotherapy plus
cetuximab in head and neck cancer. N Engl J Med. 359:1116–1127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tol J, Koopman M, Rodenburg CJ, Cats A,
Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, Mol L, Antonini NF and
Punt CJ: A randomised phase III study on capecitabine, oxaliplatin
and bevacizumab with or without cetuximab in first-line advanced
colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer
Group (DCCG). An interim analysis of toxicity Ann Oncol.
19:734–738. 2008.PubMed/NCBI
|
|
22
|
Alberts SR, Sargent DJ, Nair S, Mahoney
MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S,
Kahlenberg MS, Shields AF, Quesenberry JT, Webb TA, Farr GH Jr,
Pockaj BA, Grothey A and Goldberg RM: Effect of oxaliplatin,
fluorouracil, and leucovorin with or without cetuximab on survival
among patients with resected stage III colon cancer: a randomized
trial. JAMA. 307:1383–1393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J,
Kennedy MJ, Claes B, Lambrechts D, Kaplan R and Cheadle JP; MRC
COIN Trial Investigators: Addition of cetuximab to
oxaliplatin-based first-line combination chemotherapy for treatment
of advanced colorectal cancer: results of the randomised phase 3
MRC COIN trial. Lancet. 377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schrag D, Chung KY, Flombaum C and Saltz
L: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer
Inst. 97:1221–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Touyz RM: Transient receptor potential
melastatin 6 and 7 channels, magnesium transport, and vascular
biology: implications in hypertension. Am J Physiol Heart Circ
Physiol. 294:H1103–H1118. 2008. View Article : Google Scholar
|
|
26
|
Walder RY, Landau D, Meyer P, Shalev H,
Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi
R and Sheffield VC: Mutation of TRPM6 causes familial
hypomagnesemia with secondary hypocalcemia. Nat Genet. 31:171–174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schlingmann KP, Weber S, Peters M, Niemann
Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E,
Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW
and Konrad M: Hypomagnesemia with secondary hypocalcemia is caused
by mutations in TRPM6, a new member of the TRPM gene family. Nat
Genet. 31:166–170. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Groenestege WM, Thébault S, van der Wijst
J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van
Cutsem E, Hoenderop JG, Knoers NV and Bindels RJ: Impaired
basolateral sorting of pro-EGF causes isolated recessive renal
hypomagnesemia. J Clin Invest. 117:2260–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vincenzi B, Santini D and Tonini G:
Biological interaction between anti-epidermal growth factor
receptor agent cetuximab and magnesium. Expert Opin Pharmacother.
9:1267–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petrelli F, Borgonovo K, Cabiddu M,
Ghilardi M and Barni S: Risk of anti-EGFR monoclonal
antibody-related hypomagnesemia: systematic review and pooled
analysis of randomized studies. Expert Opin Drug Saf. 11(Suppl 1):
S9–S19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lau J, Ioannidis JP and Schmid CH: Summing
up evidence: one answer is not always enough. Lancet. 351:123–127.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson SG: Why sources of heterogeneity
in meta-analysis should be investigated. BMJ. 309:1351–1355.
1994.PubMed/NCBI
|
|
33
|
Higgins J, Thompson S, Deeks J and Altman
D: Statistical heterogeneity in systematic reviews of clinical
trials: a critical appraisal of guidelines and practice. J Health
Serv Res Policy. 7:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|