Introduction
Multiple myeloma (MM) or plasma cell myeloma is a
malignant disorder characterized by the accumulation of
differentiated B cells (plasma cells). The incompletely
differentiated plasma cells are characterized by deregulated
apoptosis (1). The treatment of MM
remains unsatisfactory and new agents that specifically target key
signaling pathways required for myeloma growth or survival are
urgently required.
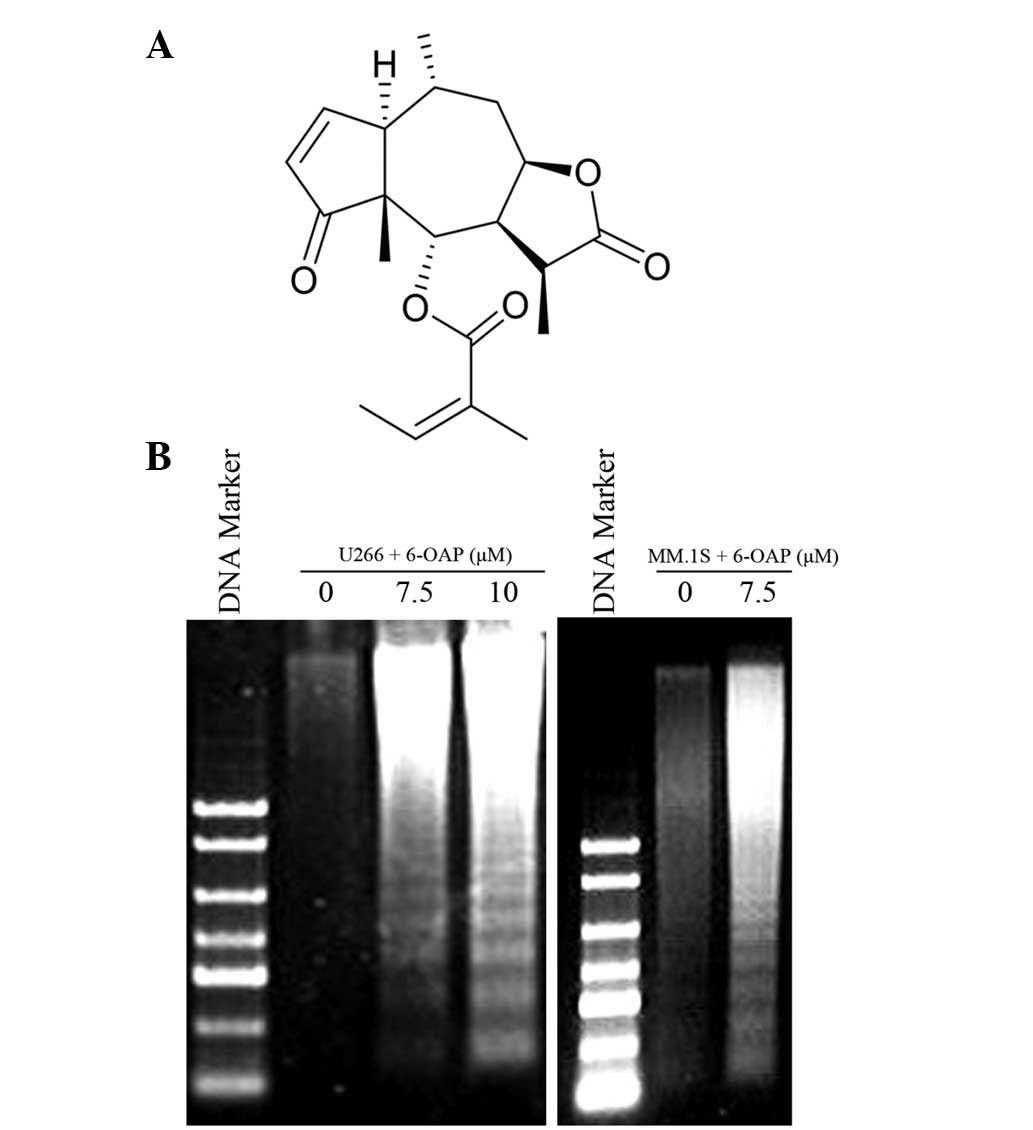
6-O-angeloylplenolin (6-OAP; Fig 1A) is a sesquiterpene lactone isolated
from Centipeda minima that has been studied in hematological
and solid forms of cancer and has been revealed to exhibit activity
without significant toxicity (2–4). The
results of our preliminary study demonstrated that 6-OAP inhibits
the proliferation of human MM cells by inducing the arrest of
mitosis and inhibiting specific key pathways (5). However, whether 6-OAP-induced mitosis
arrest and pathway inhibition are followed by apoptosis requires
further study. Therefore, the aim of the present study was to
investigate the apoptotic effect of 6-OAP against human myeloma
cells.
Materials and methods
Reagents
6-OAP with a purity ≤99.5% was extracted from
Centipeda minima (L.) as described previously (4). The 6-OAP was then dissolved in DMSO
(Sigma-Aldrich, St. Louis, MO, USA) to produce a stock solution of
10−2 M, which was stored at −20°C.
Cell culture
MM.1S, U266 and RPMI 8226 human MM cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in RPMI-1640 medium supplemented with
10% (for U266) or 15% (for RPMI 8226 and MM.1S) fetal bovine serum
(Hyclone Laboratories, Inc., Logan, UT, USA) and incubated in a
humidified atmosphere with 5% CO2 at 37°C.
Patient samples
CD138+ cells from a single patient with
MM were isolated with informed consent from bone marrow (BM)
mononuclear cells using positive immunomagnetic column separation
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of
the CD138+ cells was >97% as determined by flow
cytometry. This study was approved by the ethics committee of
Shenzhen Graduate School, Tsinghua University, Shenzhen, China.
DNA fragmentation
The MM cells were collected and lysed in 0.5 ml
lysis buffer containing 10 mM Tris (pH 8.0), 10 mM EDTA and 0.05%
Triton X-100. The lysate was centrifuged, RNase (0.2 mg/ml) was
added and the lysate was incubated for 30 min at 37°C. Proteinase K
(0.1 mg/ml) and sodium dodecyl sulfate (SDS; final concentration
1%) were added, followed by incubation at 50°C for 16 h. DNA was
extracted with phenol/chloroform and then chloroform, prior to
being precipitated with ethanol and sodium acetate and
electrophoresed on 1.5% agarose gels, and then visualized with
ethidium bromide (EB) staining.
Flow cytometric assays for Annexin-V
(AV)
Cell apoptosis was evaluated by AV detection using
an AV-FITC kit (BD Biosciences, Franklin Lakes, NJ, USA), according
to the manufacturer’s instructions.
Western blot
Cell pellets were lysed in RIPA buffer containing 50
mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1%
NP-40, 1 mM DTT, 1 mM NaF, 1 mM sodium vanadate and a protease
inhibitor cocktail (Sigma-Aldrich). Protein extracts were
quantitated, loaded on 8–12% SDS-polyacrylamide gels,
electrophoresed and then transferred to a nitrocellulose membrane
(Whatman plc, Maidstone, Kent). The membrane was incubated with
primary antibody, washed and incubated with horseradish
peroxidase-conjugated secondary antibody. Detection was performed
using a chemiluminescent western detection kit (Cell Signaling
Technology, Inc., Danvers, MA, USA). The antibodies used were
anti-caspase-3, anti-poly (ADP-ribose) polymerase (PARP; Cell
Signaling Technology, Inc.) and anti-β-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
All experiments were repeated at least three times
and the data are presented as the mean ± SD unless noted otherwise.
P<0.05 was considered to indicate a statistically significant
difference.
Results
6-OAP induces apoptosis in MM cells
The levels of apoptosis were analyzed using the DNA
fragmentation assay in dexamethasone-sensitive (MM.1S) and
dexamethasone-resistant (U266) myeloma cell lines treated with
6-OAP. As demonstrated in Fig. 1B,
marked DNA ladders were observed in MM.1S and U266 cells treated
with 6-OAP, indicative of apoptosis detection.
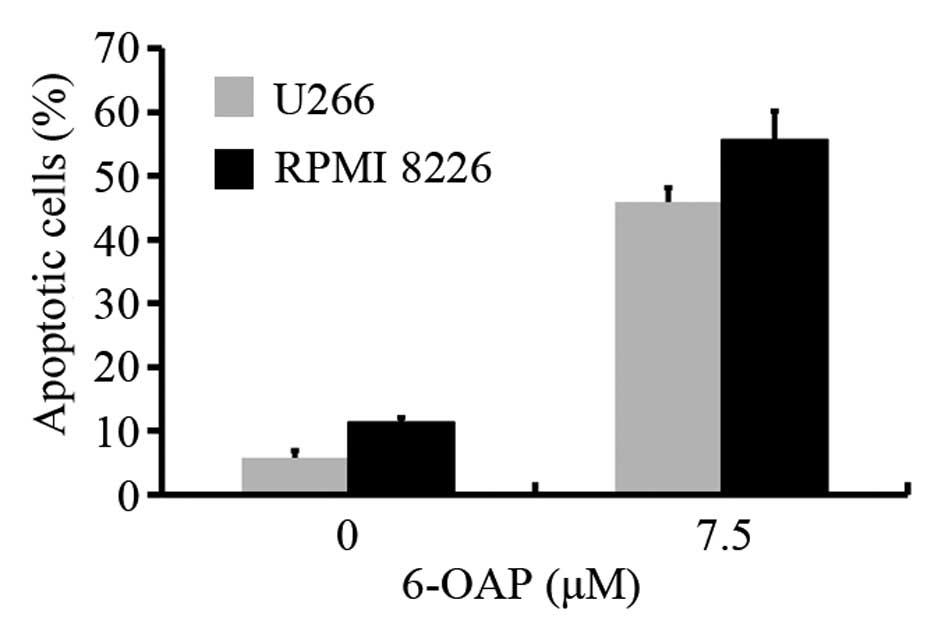
In addition, AV staining was conducted to assess
apoptosis in U266 and chemotherapy-sensitive RPMI 8226 cell lines
treated with 6-OAP. Using flow cytometry, 7.5 μM 6-OAP was
identified to induce apoptosis at a ratio of 28 and 46% in U266 and
RPMI 8226 cells, respectively (Fig.
2). These results indicate that 6-OAP induces apoptosis in MM
cells.
6-OAP-induced apoptosis in MM cells is
caspase-dependent
The apoptotic pathways that ultimately lead to the
activation of effector caspases (casp-3, -2 and -7) and the
cleavage of PARP have been characterized in MM (6). Therefore, a western blot analysis was
used to detect the activation of the casp-3 effector caspase and
its substrate, PARP, in the MM cells. 6-OAP was demonstrated to
induce a significant dose-dependent decrease in pro-casp-3 and the
cleavage of its substrate, PARP, in the three cell lines,
indicating the activation of casp-3 (Fig. 3A). 6-OAP also markedly induced the
cleavage of PARP in a time-dependent manner in the U266 and MM.1S
cells (Fig. 3B). In addition, the
expression of pro-casp-3 and the cleavage of PARP was investigated
in CD138+ primary cells isolated from a single MM
patient (Fig. 3C). The results of
the western blot analysis demonstrated that 6-OAP significantly
induces the activation of casp-3. These observations indicate that
6-OAP induces caspase-dependent apoptosis in MM cells.
Discussion
The natural agent, 6-OAP, was initially demonstrated
to exhibit anti-bacterial and anti-protozoal activities (7–9).
However, more recently, studies have demonstrated an anti-tumor
activity for 6-OAP in solid tumors and hematological malignancies
(2–4). Our previous observations found that
6-OAP induces the arrest of mitosis in MM cells by the activation
of the spindle assembly checkpoint and the accumulation of cyclin
B1. In addition, 6-OAP was identified to inhibit the Jak2/Stat3 and
Akt signaling pathways, thereby blocking the facilitation of the BM
microenvironment on the MM cells. 6-OAP has also been found to
induce marked inhibition of NF-κB in MM cells (5). However, to date, no studies have
determined whether the inhibitory effects of 6-OAP on the cell
cycle and certain signal pathways ultimately result in apoptosis.
Therefore, in the present study, the effect of 6-OAP on apoptosis
in MM cells was analyzed.
Apoptosis is an active process that ultimately leads
to the activation of endonucleases and the cleavage of DNA into
fragments of 180–200 bp. The extrinsic and intrinsic apoptotic
pathways that ultimately lead to activation of effector caspases
(casp-3, -2 and -7) have also been characterized (10,11).
The present study demonstrated that 6-OAP-treated MM cells
exhibited evident DNA fragments of 180–200 bp (Fig. 1B), indicating that 6-OAP induces
apoptosis in MM cells. The activation of the effector caspases was
also analyzed and 6-OAP was found to induce casp-3 activation,
followed by PARP cleavage in various MM cell lines (Fig. 3), indicating that 6-OAP induces
caspase-dependent apoptosis in MM cells. In conclusion, 6-OAP
induces growth inhibition in human MM cells using a number of
different mechanisms, including the arrest of mitosis and the
inhibition of certain signaling pathways. These different
mechanisms ultimately lead to caspase-dependent apoptotic cell
death.
Acknowledgements
This study was supported, in part, by
the National Natural Science Foundation of China (No. 81101835) and
the Foundation of Zhejiang Provincial Education Department (No.
Y200804683).
References
|
1.
|
Chauhan D and Anderson KC: Apoptosis in
multiple myeloma: therapeutic implications. Apoptosis. 6:47–55.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Changlong L, Hezhen W, Yongping H, Yanfang
Y, Yanwen L and Jianwen L: 6-O-Angeloylenolin induces apoptosis
through a mitochondrial/caspase and NF-kappaB pathway in human
leukemia HL60 cells. Biomed Pharmacother. 62:401–409. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Su M, Chung HY and Li Y:
6-O-Angeloylenolin induced cell-cycle arrest and apoptosis in human
nasopharyngeal cancer cells. Chem Biol Interact. 189:167–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ding LF, Liu Y, Liang HX, Liu DP, Zhou GB
and Cheng YX: Two new terpene glucosides and antitumor agents from
Centipeda minima. J Asian Nat Prod Res. 11:732–736. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Liu Y, Chen XQ, Liang HX, Zhang FX, Zhang
B, Jin J, Chen YL, Cheng YX and Zhou GB: Small compound
6-o-angeloylplenolin induces mitotic arrest and exhibits
therapeutic potentials in multiple myeloma. PLoS One. 6:e219302011.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hideshima T, Richardson P, Chauhan D,
Palombella VJ, Elliott PJ, Adams J and Anderson KC: The proteasome
inhibitor PS-341 inhibits growth, induces apoptosis and overcomes
drug resistance in human multiple myeloma cells. Cancer Res.
61:3071–3076. 2001.PubMed/NCBI
|
|
7.
|
Taylor RS and Towers GH: Antibacterial
constituents of the Nepalese medicinal herb, Centipeda
minima. Phytochemistry. 47:631–634. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Francois G, Passreiter CM, Woerdenbag HJ
and Van LM: Antiplasmodial activities and cytotoxic effects of
aqueous extracts and sesquiterpene lactones from Neurolaena
lobata. Planta Med. 62:126–129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Schwikkard S and van Heerden FR:
Antimalarial activity of plant metabolites. Nat Prod Rep.
19:675–692. 2002.PubMed/NCBI
|
|
10.
|
Nicholson DW: Caspase structure,
proteolytic substrates and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|