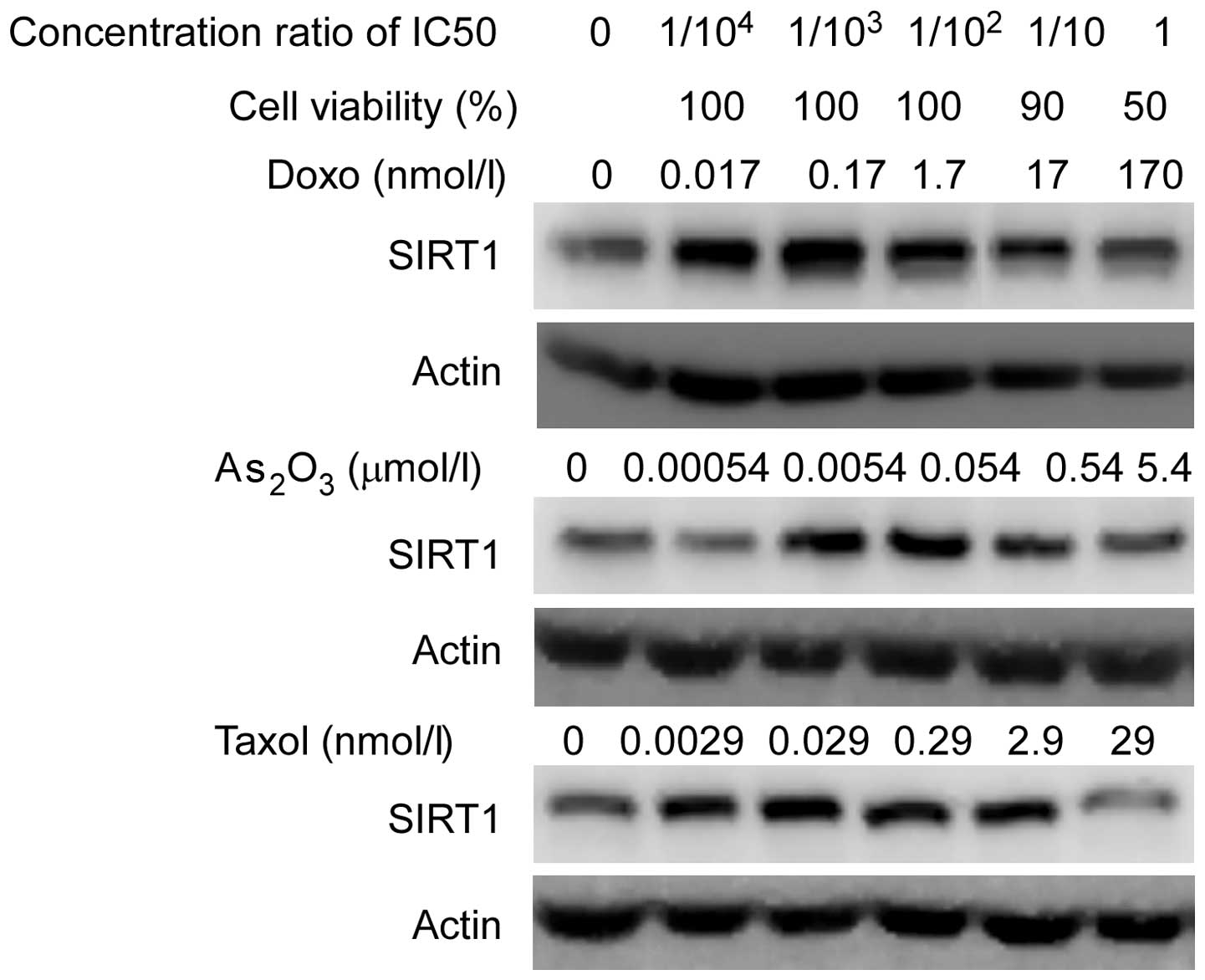
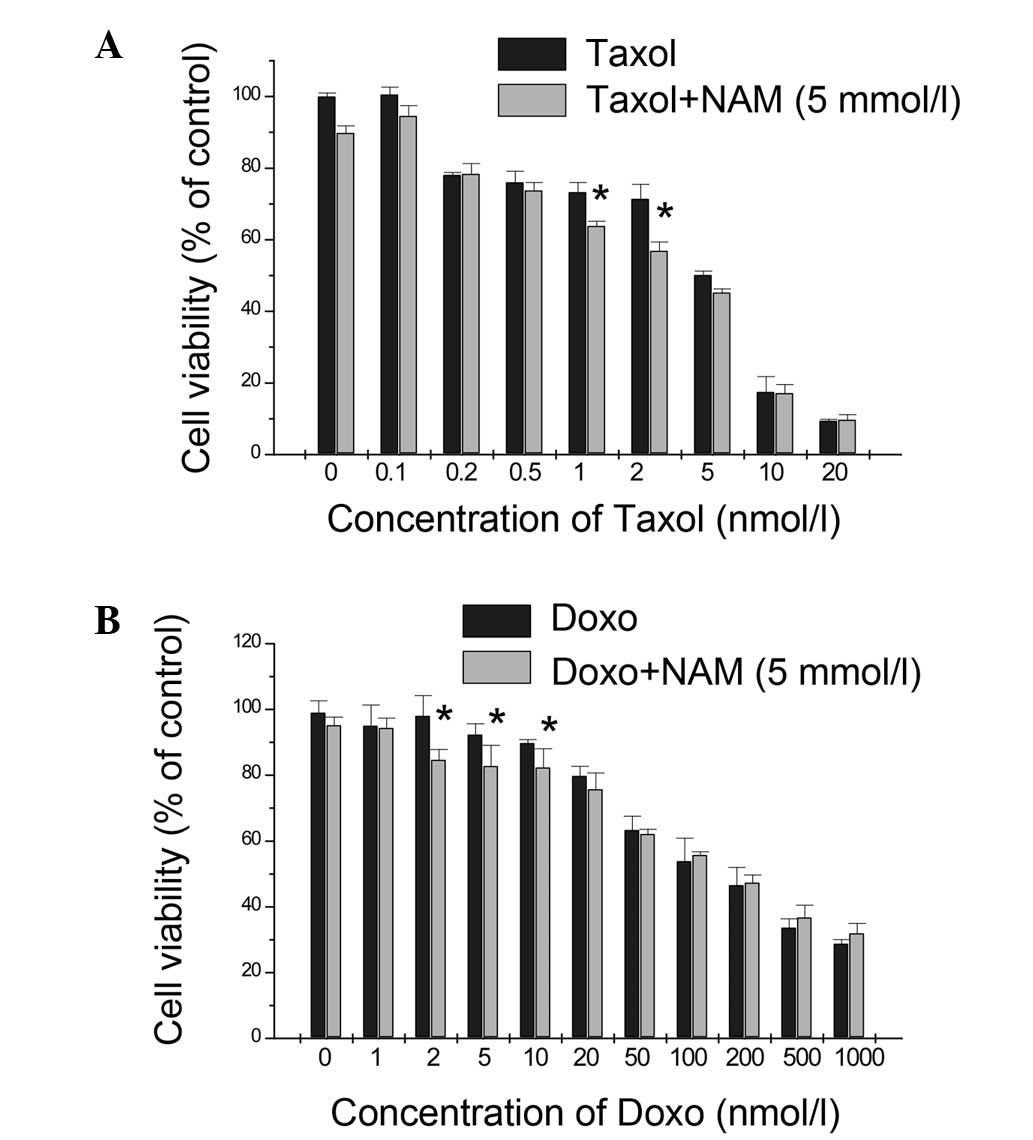
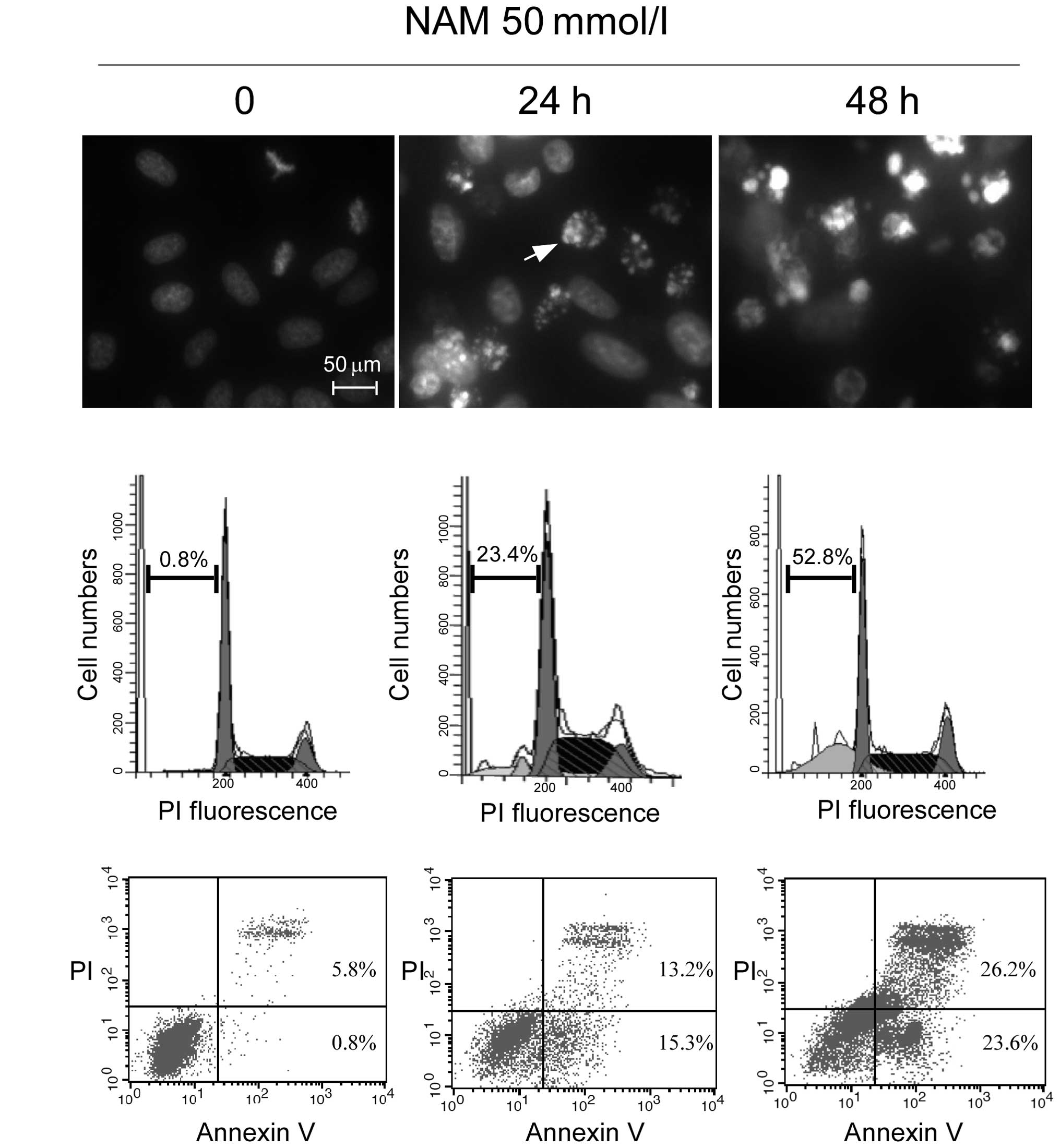
|
1.
|
Imai S, Armstrong CM, Kaeberlein M and
Guarente L: Transcriptional silencing and longevity protein Sir2 is
an NAD-dependent histone deacetylase. Nature. 403:795–800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jang KY, Kim KS, Hwang SH, et al:
Expression and prognostic significance of SIRT1 in ovarian
epithelial tumours. Pathology. 41:366–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Cha EJ, Noh SJ, Kwon KS, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Stünkel W, Peh BK, Tan YC, et al: Function
of the SIRT1 protein deacetylase in cancer. Biotechnol J.
2:1360–1368. 2007.
|
|
6.
|
Chen J, Zhang B, Wong N, et al: Sirtuin 1
is upregulated in a subset of hepatocellular carcinomas where it is
essential for telomere maintenance and tumor cell growth. Cancer
Res. 71:4138–4149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lin SJ, Defossez PA and Guarente L:
Requirement of NAD and SIR2 for life-span extension by calorie
restriction in Saccharomyces cerevisiae. Science.
289:2126–2128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Liu T, Liu PY and Marshall GM: The
critical role of the class iii histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wojcik M, Mac-Marcjanek K and Wozniak LA:
Physiological and pathophysiological functions of SIRT1. Mini Rev
Med Chem. 9:386–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Milne JC, Lambert PD, Schenk S, et al:
Small molecule activators of SIRT1 as therapeutics for the
treatment of type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rodgers JT, Lerin C, Haas W, et al:
Nutrient control of glucose homeostasis through a complex of
PGC-1alpha and SIRT1. Nature. 434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
mir-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Luo J, Nikolaev AY, Imai S, et al:
Negative control of p53 by Sir2alpha promotes cell survival under
stress. Cell. 107:137–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Motta MC, Divecha N, Lemieux M, et al:
Mammalian SIRT1 represses forkhead transcription factors. Cell.
116:551–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
McBurney MW, Yang X, Jardine K, et al: The
absence of Sir2alpha protein has no effect on global gene silencing
in mouse embryonic stem cells. Mol Cancer Res. 1:402–409.
2003.PubMed/NCBI
|
|
16.
|
Solomon JM, Pasupuleti R, Xu L, et al:
Inhibition of SIRT1 catalytic activity increases p53 acetylation
but does not alter cell survival following DNA damage. Mol Cell
Biol. 26:28–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sauve AA and Schramm VL: Sir2 regulation
by nicotinamide results from switching between base exchange and
deacetylation chemistry. Biochemistry. 42:9249–9256. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jackson MD, Schmidt MT, Oppenheimer NJ and
Denu JM: Mechanism of nicotinamide inhibition and
transglycosidation by Sir2 histone/protein deacetylases. J Biol
Chem. 278:50985–50998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Borra MT, Langer MR, Slama JT and Denu JM:
Substrate specificity and kinetic mechanism of the sir2 family of
NAD+-dependent histone/protein deacetylases. Biochemistry.
43:9877–9887. 2004.
|
|
20.
|
Bitterman KJ, Anderson RM, Cohen HY, et
al: Inhibition of silencing and accelerated aging by nicotinamide,
a putative negative regulator of yeast sir2 and human SIRT1. J Biol
Chem. 277:45099–45107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
22.
|
Lallemand-Breitenbach V, Zhu J, Chen Z and
de Thé H: Curing APL through PML/RARA degradation by As2O3. Trends
Mol Med. 18:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhang XW, Yan XJ, Zhou ZR, et al: Arsenic
trioxide controls the fate of the PML-RARalpha oncoprotein by
directly binding PML. Science. 328:240–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen G, Wang K, Yang BY, et al:
Synergistic antitumor activity of oridonin and arsenic trioxide on
hepatocellular carcinoma cells. Int J Oncol. 40:139–147.
2012.PubMed/NCBI
|
|
25.
|
Evens AM, Tallman MS and Gartenhaus RB:
The potential of arsenic trioxide in the treatment of malignant
disease: past, present, and future. Leuk Res. 28:891–900. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Arcamone F, Cassinelli G, Fantini G, et
al: Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic
from S. Peucetius var. Caesius. Biotechnol Bioeng.
11:1101–1110. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gewirtz DA: A critical evaluation of the
mechanisms of action proposed for the antitumor effects of the
anthracycline antibiotics adriamycin and daunorubicin. Biochem
Pharmacol. 57:727–741. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gewirtz DA: Growth arrest and cell death
in the breast tumor cell in response to ionizing radiation and
chemotherapeutic agents which induce DNA damage. Breast Cancer Res
Treat. 62:223–235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Roninson IB, Broude EV and Chang BD: If
not apoptosis, then what? Treatment-induced senescence and mitotic
catastrophe in tumor cells. Drug Resist Updat. 4:303–313. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chu F, Chou PM, Zheng X, et al: Control of
multidrug resistance gene mdr1 and cancer resistance to
chemotherapy by the longevity gene sirt1. Cancer Res.
65:10183–10187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Robinson SP, Howe FA, Stubbs M and
Griffiths JR: Effects of nicotinamide and carbogen on tumour
oxygenation, blood flow, energetics and blood glucose levels. Br J
Cancer. 82:2007–2014. 2000.PubMed/NCBI
|
|
35.
|
Hirst DG, Joiner B and Hirst VK: Blood
flow modification by nicotinamide and metoclopramide in mouse
tumours growing in different sites. Br J Cancer. 67:1–6. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Ford J, Jiang M and Milner J:
Cancer-specific functions of SIRT1 enable human epithelial cancer
cell growth and survival. Cancer Res. 65:10457–10463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zhao X, Allison D, Condon B, et al: The
2.5 Å crystal structure of the SIRT1 catalytic domain bound to
nicotinamide adenine dinucleotide (NAD+) and an indole (EX527
analogue) reveals a novel mechanism of histone deacetylase
inhibition. J Med Chem. 56:963–969. 2013.
|