Introduction
The incidence of differentiated thyroid carcinoma
(DTC) has increased worldwide over the past two decades, with
papillary thyroid carcinoma (PTC) being markedly more common than
follicular thyroid carcinoma (FTC) (1,2). The
prognosis of DTC is generally favorable due to the indolent nature
of the disease and the efficacy of combined treatment comprising
surgery, radioactive iodine (RAI) and levothyroxine. However,
10–20% of patients with DTC develop distant metastases,
approximately half of which do not respond to traditional
therapies. In RAI-refractory DTC patients, there is no standard
therapy and the 10-year survival rate has decreased to 10%
(3,4).
The recent expansion of knowledge in molecular
oncology has facilitated the development of targeted agents for the
treatment of various types of advanced thyroid carcinoma (5). Of these agents, tyrosine kinase
inhibitors (TKIs) have emerged as novel cancer therapies with
promising results (6). Sorafenib is
an oral, small-molecule TKI, which targets vascular endothelial
growth factor receptors (VEGFRs), rearranged during transfection
(RET)/PTC proteins and BRAF-mediated events (7). Four phase II trials with sorafenib
have been conducted at a dose of 400 mg, twice daily, demonstrating
the clinical potential and acceptable safety of the agent (8–11). We
have also successfully performed two studies on sorafenib therapy
for pulmonary metastases from PTC and brain metastasis from FTC,
using a low-dose strategy (200 mg, twice daily) in which tolerance
to the drug and a potential therapeutic effect were demonstrated in
patients with RAI-refractory DTC (12,13).
To assess the objective response to molecular
targeted therapy, response evaluation criteria in solid tumors
(RECIST 1.0) is commonly used (14). However, a number of questions and
issues have arisen with regard to RECIST 1.0, leading to a revised
version (RECIST 1.1) (15–18). Recently, RECIST 1.1 has been
successfully used to evaluate responses to treatment in numerous
types of solid tumors, including advanced non-small cell lung
cancer and advanced gastric cancer, demonstrating superiority to
the original guidelines (19,20).
More recently, in a study by Marotta et al, this novel
system has also been utilized in the initial evaluation of tumor
responses to sorafenib treatment in advanced RAI-refractory DTC
(21). However, RECIST 1.1 has not
yet been compared with RECIST 1.0 in the evaluation of tumor
responses to molecular targeted therapy in patients with
RAI-refractory DTC. Moreover, in this novel system,
subcentimeter-sized lesions and blastic bone lesions are considered
to be non-measurable. In addition, the cavitation of lesions with
internal necrosis without a change in the size of the lesion, but
with a paradoxical increase in the tumor size, in response to
therapy due to hemorrhage or necrosis, is not able to be correctly
evaluated (22,23). Although it would be ideal to have
objective criteria to apply to non-measurable lesions, the very
nature of the disease makes it impossible to do so (18). Therefore, quantitative strategies
are required for the evaluation of the tumor response in patients
with non-measurable disease only.
Serum thyroglobulin (Tg), a specific biological
marker for DTC, is measured routinely and automatically in the
follow-up of patients with DTC, and serves as an indicator of the
efficacy of surgery and RAI therapy (24–26). A
decrease in serum Tg levels following sorafenib therapy at various
doses has been observed in patients with RAI-refractory DTC in a
number of studies, including a previous study by our group
(9,11,12).
However, in evaluating responses to molecular targeted therapy,
limited data with regard to the correlation between Tg levels and
the radiographic response are available, while data on the role of
serum Tg measurements are controversial (9,11).
Therefore, the present study was conducted to
investigate the association between RECIST 1.0 and 1.1, and the
correlation between serum Tg levels and the radiographic response
in sorafenib-treated patients with RAI-refractory DTC and
measurable disease. The feasibility of using Tg measurements in
assessing the tumor responses to sorafenib treatment in patients
with measurable disease and subjects with non-measurable disease
only was also explored.
Patients and methods
Patients
Patients with RAI-refractory DTC who demonstrated
evidence of disease progression within 12 months prior to the
initiation of treatment, despite the administration of sufficient
thyroid hormones to reduce the serum thyroid stimulating hormone
(TSH) levels to <0.1 mIU/l, were enrolled in the study. Other
eligibility criteria included an Eastern Cooperative Oncology Group
performance status of less than two, with preserved renal, hepatic
and bone marrow function. Premenopausal women were required to have
negative pregnancy test results, and all patients of child-bearing
age were required to use contraception. The open-label use of
sorafenib was administered at a dose of 200 mg orally, twice a day.
Screening evaluations, including medical history, demography,
review of prior treatment, physical examination and laboratory
evaluations, were completed within one week prior to sorafenib
treatment initiation.
Patients were observed at four-week intervals
following the initiation of treatment. At each visit, a history was
taken, a physical examination was performed and complete blood
count (CBC), chemistry panel and TSH, Tg and anti-Tg antibody
(TgAb) levels were measured. The patients were assessed for the
appearance of novel symptoms, the compliance with study medications
(pill count) and concomitant medications. The response was assessed
radiographically at 12-week intervals.
Approval of the protocol was received from the
ethics board of Shanghai Sixth People’s Hospital prior to the
initiation of the study. All subjects provided written informed
consent for participation in the study.
Laboratory studies and radiographic
assessments
Serum TSH, Tg and TgAb levels were measured using a
chemiluminescent immunoassay system (Immulite, Diagnostic Products
Corp., Los Angeles, CA, USA). RECIST 1.0 and 1.1 were used to
assess the tumor responses to sorafenib treatment.
The objective response to treatment at the baseline
and at each follow-up computed tomography (CT) examination,
according to the original RECIST 1.0 criteria, was assessed by a
study-designated radiologist (14).
Following completion of the study, tumor lesions were reviewed by
the radiologist for a second time, to generate a second set of CT
tumor measurements that met the RECIST 1.1 guidelines. Compared
with RECIST 1.0, there were certain changes according to RECIST
1.1: Pathological lymph nodes with a short axis ≥10 and <15 mm
were considered to be non-measurable lesions; and lytic bone
lesions or mixed lytic-blastic lesions with identifiable soft
tissue components that may be evaluated by cross-sectional imaging
techniques, such as CT or magnetic resonance imaging (MRI), and
cystic lesions considered to represent cystic metastases, were
considered as measurable lesions (provided that they met the
definition of measurability) (18).
In addition, the target lesions recorded in the original
measurements were reassessed if they met the criteria of RECIST
1.1. Lymph nodes with a short axis of <15 mm were excluded from
the target lesions, and when the number of target lesions exceeded
the limits according to RECIST 1.1 (up to five in total and up to
two per organ), smaller lesions were eliminated from the target
lesions. Furthermore, short-axis measurements were used for lymph
nodes, as opposed to long-axis measurements. Additionally, bone
lesions, which were either lytic or mixed lytic-blastic, with a
soft tissue component that met the criteria for measurability were
selected as target lesions. Moreover, the
fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT
clinical reports were also reviewed for the patients who underwent
such examinations during treatment, to determine whether any new
lesions were detected in the FDG-PET/CT scans that met the RECIST
1.1 criteria for progression.
Statistical analysis
All statistical analyses were performed using a
statistical software program (SPSS, version 11.0; SPSS, Inc.
Chicago, IL, USA). A paired Student’s t-test and a linear
correlation were used to assess the differences and the correlation
between RECIST 1.0 and 1.1, respectively. A rank correlation and
Wilcoxon signed rank sum test were used to assess the correlation
and the percentage changes between Tg levels and RECIST 1.1,
respectively. An independent samples t-test and a Wilcoxon rank sum
test were used to assess the changes in the Tg levels over time and
the concordance of Tg levels between patients with measurable
disease and non-measurable disease only, respectively. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients
Between August, 2009 and July, 2012, 23 consecutive
DTC patients, including 14 patients with RECIST-measurable disease
and nine patients with non-measurable disease only (14 females,
nine males; age range, 33–75 years; mean age, 54 years), who were
considered to have progressive metastases resistant to RAI
treatment, were enrolled in the study. None of these patients had
received chemotherapy or other kinase inhibitors prior to the
administration of sorafenib.
The baseline characteristics of the patients entered
into the study are listed in Table
I. All patients exhibited lymph node metastases, while 22
presented with lung metastases, two with bone metastases and one
with brain metastases. In five of the patients with measurable
disease who underwent FDG-PET/CT, uptake of FDG prior to treatment
was observed. The average duration of therapy was 12 months (range,
3–25 months).
 | Table I.Baseline characteristics of
RAI-refractory DTC patients. |
Table I.
Baseline characteristics of
RAI-refractory DTC patients.
| Characteristics | No. of patients | % |
|---|
| Gender | | |
| Female | 14 | 60.87 |
| Male | 9 | 39.13 |
| Age, years | | |
| Mean | 54 | |
| Range | 33–75 | |
| Thyroid cancer
subtype | | |
| Papillary | 22 | 95.65 |
| Follicular | 1 | 4.35 |
| Site of
Metastasis | | |
| Lymph node | 23 | 100.00 |
| Lung | 22 | 95.65 |
| Bone | 2 | 8.70 |
| Brain | 1 | 4.35 |
| Measurability of
lesions | | |
| Measurable | 14 | 60.87 |
| Non-measurable | 9 | 39.13 |
| Prior FDG-PET/CT | | |
| FDG-PET/CT scan
completed | 5 | 21.74 |
| FDG
uptake-positive | 5 | 21.74 |
| Median duration of
therapy, months | 10 | |
| Average | 12 | |
| Range | 3–25 | |
Comparison between RECIST 1.0 and 1.1 in
patients with measurable disease
Of the 23 total patients, 14 patients with
measurable disease were enrolled to compare the radiographic
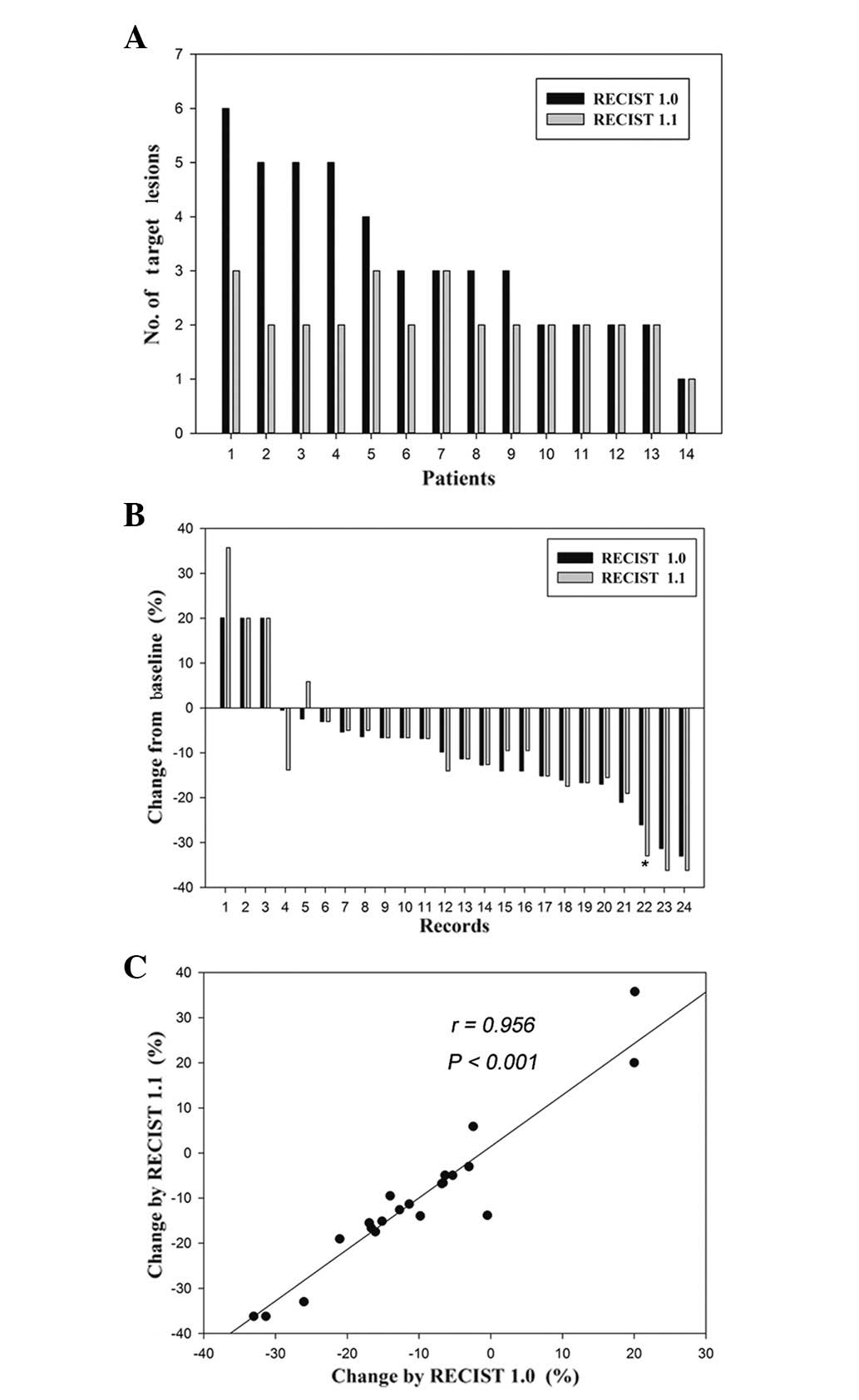
responses to sorafenib by RECIST 1.0 and 1.1. The target lesion
number, according to RECIST 1.1, was significantly lower than that
using RECIST 1.0 [Fig. 1A; paired
Student’s t-test; P=0.006; 95% CI, 0.40–1.89], with a decrease in
eight patients (57%) and no change in the remaining six patients
(43%). The number of target lesions was decreased as a result of
the reduction in the number of lesions required to assess the tumor
burden (from a maximum of 10 to a maximum of five in total, and
from five to two per organ) for five patients. This, in turn, was
as a result of the new definition of measurability of malignant
lymph nodes at the baseline (a lymph node is required to have a
short axis of 15 mm to be considered pathologically enlarged and
measurable) for one patient, and due to the new definition of
measurability of malignant lymph nodes at the baseline and the
reduction in the number of lesions required to assess the tumor
burden, for two patients. In one patient, the number of target
lesions did not change as a result of the reduction in the number
of pulmonary metastases, which was equivalent to the increase in
the number of bone lesions with a soft tissue component.
Of the 14 patients with measurable disease, a total
of 24 records of the percentage changes in the sum of the diameters
of the target lesions at all time points during therapy were
assessed by RECIST 1.0 and 1.1, respectively. Twenty-three (96%) of
the 24 records demonstrated a concordant radiographic response
(Fig. 1B; paired Student’s t-test;
P=0.868; 95% CI, −2.4130 to 2.0497). The one discordant percentage
change measurement exhibited a 26% decrease in stable disease (SD),
according to RECIST 1.0, and a 33% decrease in partial response
(PR), according to RECIST 1.1 (Fig.
1B). The percentage changes in the sum of the tumor diameters
of the target lesions at all time points according to RECIST 1.1
and 1.0 demonstrated a high correlation (Fig. 1C; linear correlation; r=0.956;
P<0.001).
The best response assessed by RECIST 1.1 had an
objective PR of 14% (2/14), SD of 64% (9/14) and progressive
disease (PD) of 22% (3/14), which were similar to those recorded
according to RECIST 1.0 (PR, 7%; SD, 71%; and PD, 22%). The results
remained the same in 13 patients (93%), while a difference was only
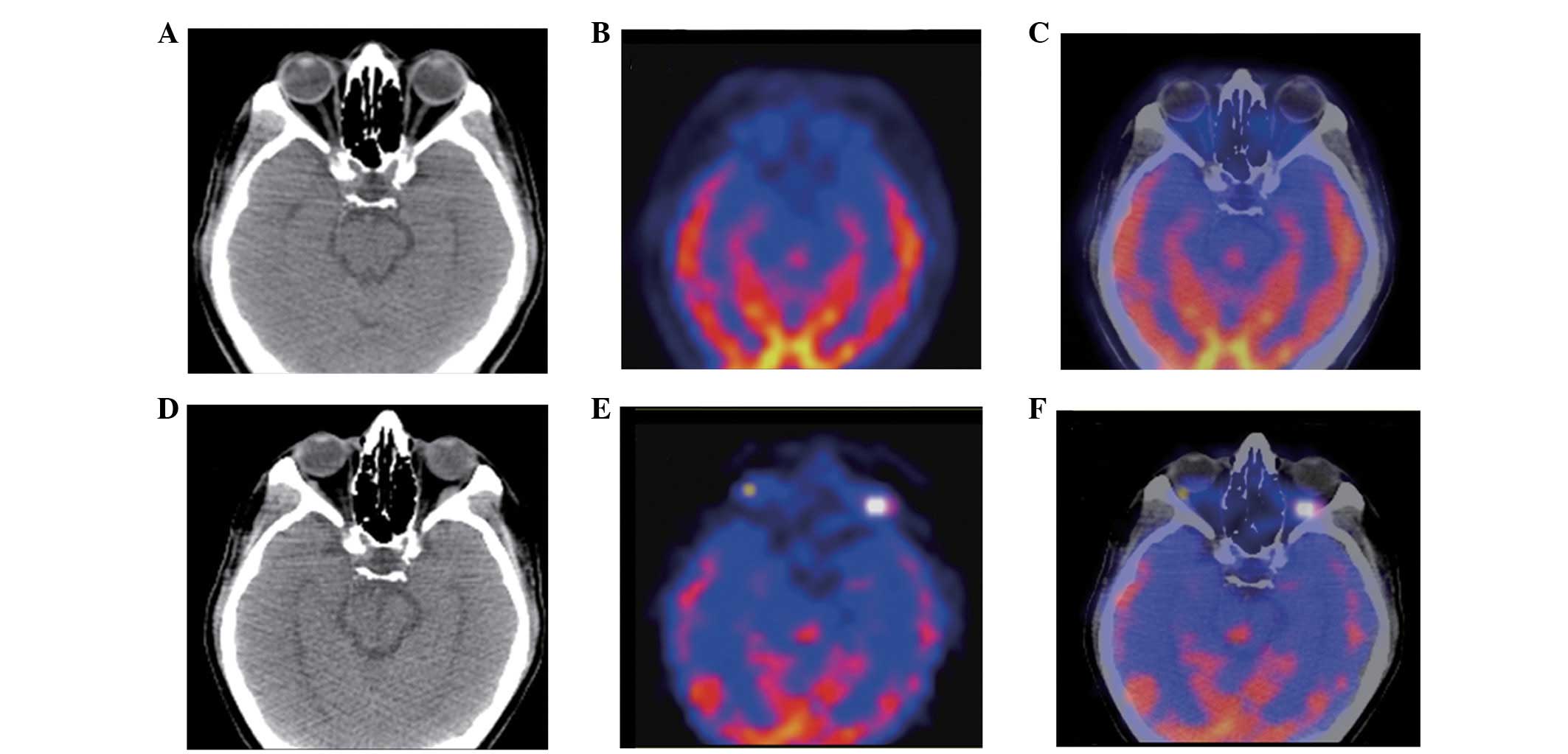
observed in one patient (7%), as mentioned previously. Of the five
patients who underwent FDG-PET/CT scanning at the baseline, PD was
identified in one patient with a new lateral rectus lesion, which
was revealed by the follow-up FDG-PET/CT study (Fig. 2).
Correlation between Tg levels and the
radiographic response in patients with measurable disease
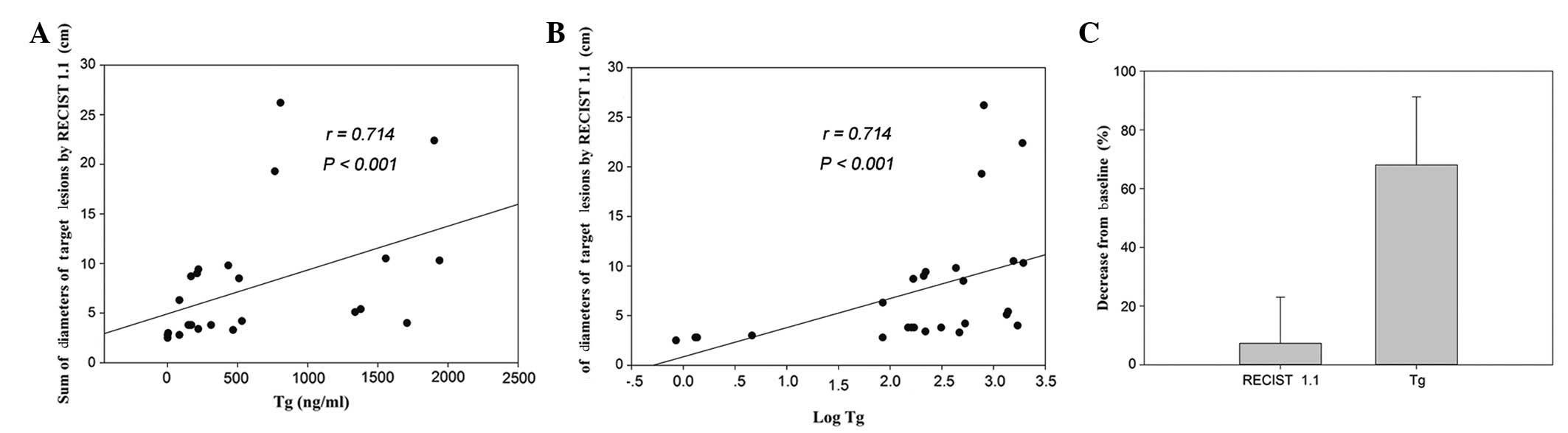
Following the exclusion of five serum TgAb-positive
patients, nine patients with measurable disease and TSH-suppressed
Tg (at all time points during therapy) were enrolled to explore the
correlation between Tg levels and the tumor size (as demonstrated
radiographically). The levels of Tg, as well as the log of the Tg
levels, were correlated with the sum of the diameters of the target
lesions, as assessed by RECIST 1.1, with the same correlation
coefficient (Fig. 3A and B; rank
correlation; rs=0.714; P<0.001). Furthermore, the percentage
change in Tg levels (mean, 68%; standard deviation, 23%) was
significantly greater than that of the radiographic response (mean,
7%; standard deviation, 16%; Fig.
3C; Wilcoxon signed rank sum test; P<0.001). However, the
percentage change in Tg concentration was not correlated with the
change in the sum of the tumor diameters of the target lesions
(rank correlation; P=0.663).
Concordance of changes in serum Tg levels
between patients with measurable and non-measurable disease
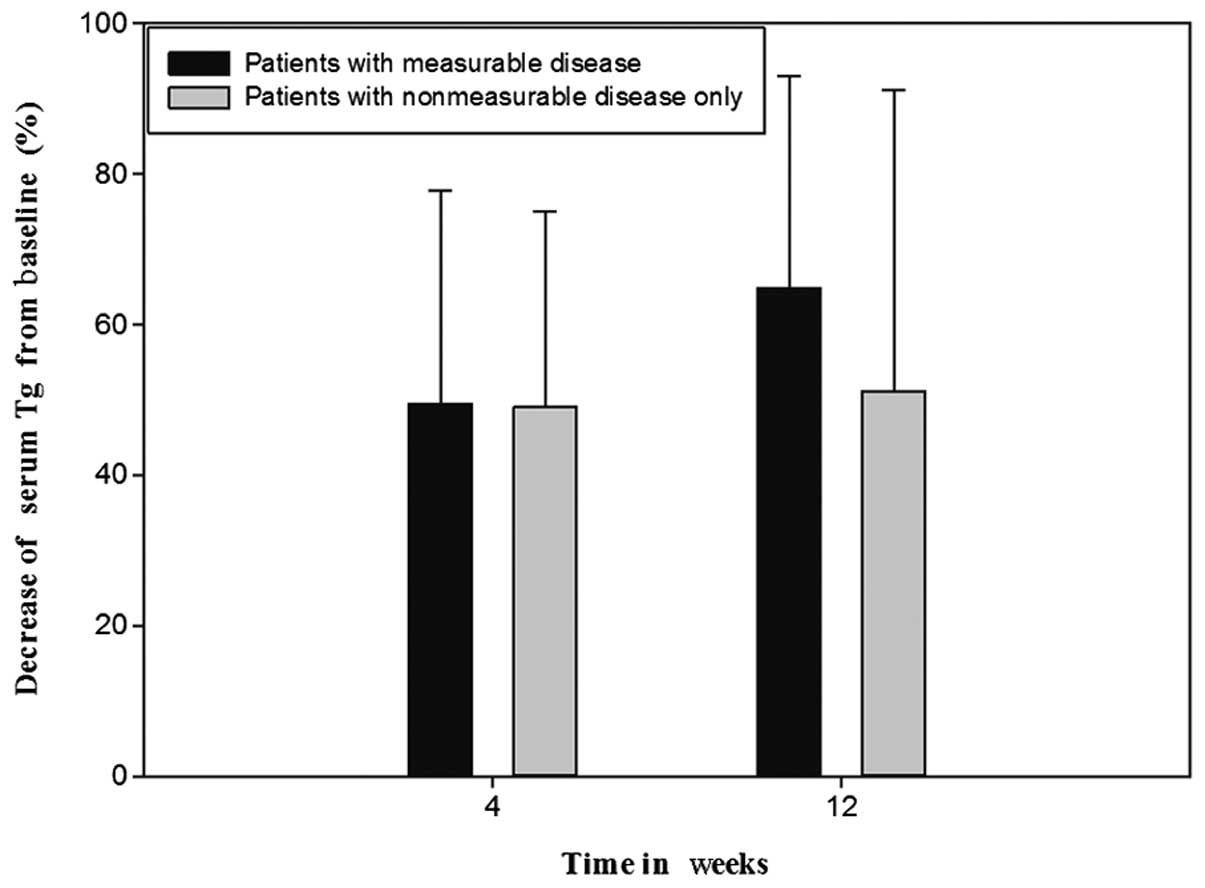
Following the exclusion of five TgAb-positive
patients from the 23-patient total, the serum Tg levels
demonstrated no significant difference at the baseline between nine
patients with measurable disease and nine patients with
non-measurable disease only, which could not be quantitatively
assessed by RECIST (Wilcoxon rank sum test; P=0.085). In addition,
no significant difference in the serum Tg levels was demonstrated
between these two groups of nine patients at 4 weeks (independent
samples t-test; P=0.055) and 12 weeks (Wilcoxon rank sum test;
P=0.122) following the initiation of treatment. Furthermore, there
was no significant difference in the percentage change in serum Tg
levels between patients with measurable disease (mean, 50%; SD,
28%) and non-measurable disease only (mean, 49%; SD, 26%) at 4
weeks from the baseline (Fig. 4;
independent samples t-test; P=0.969; 95% CI, 26.67–27.69). The
percentage change in serum Tg levels in patients with measurable
disease (mean, 65%; SD, 28%) was also statistically consistent with
that of patients with non-measurable disease only (mean, 52%; SD,
40%) at 12 weeks from the baseline (Fig. 4; Wilcoxon rank sum test;
P=0.453).
Discussion
In cancer therapy, a reliable assessment of the
responses to treatment is essential, as the response parameters
often represent surrogate markers for improved survival. For this
reason, RECIST was developed and has become the main evaluation
system used in current oncological investigations. However, a
number of questions and issues have arisen with regard to the
system, and continuous updating of RECIST is required (15–18,22,23).
In the present study, despite the significantly
decreased number of target lesions assessed by RECIST 1.1, a high
concordance was demonstrated between RECIST 1.1 and 1.0 in the
assessment of the tumor response, indicating an almost complete
agreement between the two versions. The tumor response assessed by
RECIST 1.1 and 1.0 was discordant in only one record, as a result
of the reduction in the number of target lesions required to assess
the tumor burden. This suggested that if the value of the tumor
response assessed by RECIST approached the critical value, a
reduction in the number of target lesions may have resulted in a
different tumor response being observed between RECIST 1.0 and 1.1,
particularly in patients with small target lesions at the baseline.
A reduction in the maximum number of target lesions occurred in
approximately half of the patients (57%) when RECIST 1.1 was used,
implying a substantial decrease in the time and effort demanded
from the radiologists with this version of RECIST. An additional
reason for the decrease in the number of target lesions was the new
definition of measurability of malignant lymph nodes, which
affected three patients via a reduction in the target lesion number
and an increase in the number of non-measurable lesions. Notably,
the number of target lesions in one patient did not change as a
result of the reduction in pulmonary metastases, which was
equivalent to the increase in bone lesions with a soft tissue
component. This implied that the new definition of measurability of
lytic bone lesions or mixed lytic-blastic lesions with identifiable
soft tissue components resulted in an increase in the target lesion
number and influenced the eligibility of this system for clinical
trials.
In the present study, one patient demonstrated PD
with negative FDG-PET/CT at the baseline and positive findings 24
weeks after the initiation of treatment with sorafenib, implying
that it is occasionally acceptable to incorporate the use of
whole-body FDG-PET scanning to complement the CT examination in the
assessment of progression, as identified by RECIST 1.1. Although
there were only five patients with measurable disease who underwent
FDG-PET/CT examination in the study, all five exhibited positive
FDG uptake, which was similar to the results demonstrated by other
studies (8,21). These results confirmed the highly
malignant nature of RAI-refractory DTC. Moreover, as demonstrated
by Marotta et al, an FDG-PET assessment at the baseline may
predict the radiological response, and an early FDG-PET follow-up
scan may be useful for clinicians, as it may allow for the
identification of patients who are unlikely to exhibit a
morphological response (21).
Larger and randomized studies are required to confirm the efficacy
of FDG-PET/CT in the management of RAI-refractory DTC.
Despite the significant revisions made in RECIST
1.1, numerous issues remain to be resolved in the assessment of
tumor responses in clinical practice. Subcentimeter-sized lesions,
such as the miliary pulmonary metastases in the majority of
patients with RAI-refractory DTC, are considered to be
non-measurable by RECIST 1.1 criteria, resulting in difficulties in
the quantitation of the tumor burden and response (18). Furthermore, as has been identified
by our group and others previously, treatment with TKIs may result
in the cavitation of lesions with internal necrosis without a
change in lesion size, which is a challenge for radiologists who
aim to obtain the measurement that best represents the tumor burden
(13,22). In addition, it also important to
understand that radiological lesion size results may vary due to a
number of factors, including scan quality, timing of contrast
administration and the identity of the interpreting radiologist
(18,27). This leads to a requirement for newer
methods for precisely ascertaining the tumor response, which are
not solely based on the diameter, in patients receiving targeted
therapy.
As a specific tumor marker for DTC, the level of
serum Tg, during thyroid hormone treatment and following TSH
stimulation, is correlated with the quantity of neoplastic thyroid
tissue (28,29). As was demonstrated by the present
study, the level of Tg and the log of the level of Tg were
correlated with the sum of the diameters of the target lesions, as
assessed by RECIST 1.1, with the same correlation coefficient at
all time points, including the baseline and time points during the
treatment. Additionally, it has been demonstrated that baseline Tg
levels and Tg responses to treatment may be useful for predicting
the morphological response and clinical outcome (21). However, a correlation between the
change in serum Tg levels and the radiographic response was not
observed in the present study, which was possibly due to the small
sample size, as well as the definition of the objective response
based on RECIST (9). In addition,
it has been proposed that the tumor burden may be more sensitive
and reproducible when measured by the tumor volume, rather than the
sum of the diameters of the target lesions. Therefore, response
assessments based on tumor volumes may have a positive impact on
patient management and clinical trials (30).
Hoftijzer et al (10) demonstrated that the median time of
the nadir of Tg levels was 3 months, while a rapid decrease in the
serum Tg levels of 50% within 4 weeks, followed by a continued
decrease in such levels (with a mean decrease of 65%) within 12
weeks of the initiation of treatment, were observed in the present
study. Furthermore, the percentage change in Tg levels was
significantly greater than that in the radiographic response. These
results demonstrated a more marked tumor response to targeted
therapy when Tg was used as an evaluation criterion compared with
RECIST. This may be explained by the cytostatic effect of novel
anticancer agents, which may not have reduced the tumor size
significantly.
Until recently, no other quantitative criteria for
assessing tumor responses to sorafenib therapy in patients with
non-measurable disease only were available; the phase II (8–11) and
ongoing phase III DECISION trials (31) were conducted in patients with
measurable disease. In the present study, patients with measurable
disease and non-measurable disease only were enrolled to evaluate
the effectiveness of sorafenib treatment. Patients with
non-measurable disease only were analyzed as an individual group
for the first time. The levels of serum Tg between patients with
measurable target lesions and patients with non-measurable disease
only demonstrated no statistically significant difference at the
baseline or at 4 or 12 weeks following the initiation of treatment.
Furthermore, the percentage change in serum Tg levels from the
baseline for patients with measurable disease was consistent with
that for patients with non-measurable disease only at 4 and 12
weeks. These results suggested that such treatment in patients with
non-measurable disease only exhibited a similar efficacy in
patients with measurable disease. Additionally, these results
demonstrated that all patients suffered from the same disease and
that it was only our measurement convention that made them
different. As a correlation between Tg and the sum of the diameters
of the target lesions in patients with measurable disease was
demonstrated, the level of Tg may potentially be used to assess the
treatment response in patients with measurable disease and
non-measurable disease only.
However, certain issues remain to be resolved with
regard to the measurement of serum Tg. Tumor lysis during treatment
with sorafenib may lead to elevated Tg levels, which may be due to
either the tumor lysis itself or increased Tg synthesis (10). Furthermore, it has been demonstrated
that the secretion of Tg is likely to be affected by alterations in
cell signaling caused by sorafenib (8). Therefore, changes in the serum Tg
level in RAI-refractory DTC treated with sorafenib require cautious
interpretation. In addition, we acknowledge that the present study
possessed certain limitations, including its retrospective nature,
the small sample size and the short follow-up time.
In patients with RAI-refractory DTC, RECIST 1.1
demonstrated high levels of concordance with RECIST 1.0 in the
assessment of responses to sorafenib therapy, with the advantage of
simplified procedures and the complementary use of FDG-PET. The
level of serum Tg significantly correlated with the sum of the
diameters of target lesions, and the Tg response was significantly
greater than the radiographic response. In addition, the percentage
change in Tg levels was consistent between patients with measurable
disease and subjects with non-measurable disease only. In
accordance with RECIST 1.1, Tg measurements are of value in
assessing the tumor response to sorafenib therapy in patients with
RAI-refractory DTC, particularly in those with non-measurable
disease only, for which no quantitative criteria exist.
Acknowledgements
This study was sponsored by the
National Natural Science Foundation of China (grant no. 81271609)
and the Shanghai Rising-Star Program (grant no. 12QH1401600).
References
|
1.
|
Aschebrook-Kilfoy B, Ward MH, Sabra MM and
Devesa SS: Thyroid cancer incidence patterns in the United States
by histologic type, 1992–2006. Thyroid. 21:125–134. 2011.PubMed/NCBI
|
|
2.
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shaha AR, Shah JP and Loree TR: Patterns
of nodal and distant metastasis based on histologic varieties in
differentiated carcinoma of the thyroid. Am J Surg. 172:692–694.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Durante C, Haddy N, Baudin E, et al:
Long-term outcome of 444 patients with distant metastases from
papillary and follicular thyroid carcinoma: benefits and limits of
radioiodine therapy. J Clin Endocrinol Metab. 91:2892–2899. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Deshpande HA, Gettinger SN and Sosa JA:
Novel chemotherapy options for advanced thyroid tumors: small
molecules offer great hope. Curr Opin Oncol. 20:19–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhang J, Yang PL and Gray NS: Targeting
cancer with small molecule kinase inhibitors. Nat Rev Cancer.
9:28–39. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gupta-Abramson V, Troxel AB, Nellore A, et
al: Phase II trial of sorafenib in advanced thyroid cancer. J Clin
Oncol. 26:4714–4719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kloos RT, Ringel MD, Knopp MV, et al:
Phase II trial of sorafenib in metastatic thyroid cancer. J Clin
Oncol. 27:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hoftijzer H, Heemstra KA, Morreau H, et
al: Beneficial effects of sorafenib on tumor progression, but not
on radioiodine uptake, in patients with differentiated thyroid
carcinoma. Eur J Endocrinol. 161:923–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cabanillas ME, Waguespack SG, Bronstein Y,
et al: Treatment with tyrosine kinase inhibitors for patients with
differentiated thyroid cancer: the M. D. Anderson experience. J
Clin Endocrinol Metab. 95:2588–2595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen L, Shen Y, Luo Q, Yu Y, Lu H and Zhu
R: Response to sorafenib at a low dose in patients with
radioiodine-refractory pulmonary metastases from papillary thyroid
carcinoma. Thyroid. 21:119–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shen Y, Ruan M, Luo Q, et al: Brain
metastasis from follicular thyroid carcinoma: treatment with
sorafenib. Thyroid. 22:856–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
15.
|
Gehan EA and Tefft MC: Will there be
resistance to the RECIST (Response Evaluation Criteria in Solid
Tumors)? J Natl Cancer Inst. 92:179–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tuma RS: Sometimes size doesn’t matter:
reevaluating RECIST and tumor response rate endpoints. J Natl
Cancer Inst. 98:1272–1274. 2006.PubMed/NCBI
|
|
17.
|
Ratain MJ and Eckhardt SG: Phase II
studies of modern drugs directed against new targets: if you are
fazed, too, then resist RECIST. J Clin Oncol. 22:4442–4445. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
19.
|
Fuse N, Nagahisa-Oku E, Doi T, et al:
Effect of RECIST revision on classification of target lesions and
overall response in advanced gastric cancer patients. Gastric
Cancer. Aug 22–2012.(Epub ahead of print).
|
|
20.
|
Nishino M, Jackman DM, Hatabu H, et al:
New Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines for advanced non-small cell lung cancer: comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
View Article : Google Scholar
|
|
21.
|
Marotta V, Ramundo V, Camera L, et al:
Sorafenib in advanced iodine-refractory differentiated thyroid
cancer: efficacy, safety and exploratory analysis of role of serum
thyroglobulin and FDG-PET. Clin Endocrinol (Oxf). 78:760–767. 2012.
View Article : Google Scholar
|
|
22.
|
Sun S and Schiller JH: Angiogenesis
inhibitors in the treatment of lung cancer. Crit Rev Oncol Hematol.
62:93–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Schlumberger M, Berg G, Cohen O, et al:
Follow-up of low-risk patients with differentiated thyroid
carcinoma: a European perspective. Eur J Endocrinol. 150:105–112.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Clark PM and Beckett G: Can we measure
serum thyroglobulin? Ann Clin Biochem. 39:196–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Spencer CA, Takeuchi M and Kazarosyan M:
Current status and performance goals for serum thyroglobulin
assays. Clin Chem. 42:164–173. 1996.PubMed/NCBI
|
|
27.
|
Erasmus JJ, Gladish GW, Broemeling L, et
al: Interobserver and intraobserver variability in measurement of
non-small-cell carcinoma lung lesions: implications for assessment
of tumor response. J Clin Oncol. 21:2574–2582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bachelot A, Cailleux AF, Klain M, et al:
Relationship between tumor burden and serum thyroglobulin level in
patients with papillary and follicular thyroid carcinoma. Thyroid.
12:707–711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Demers LM and Spencer CA: Laboratory
medicine practice guidelines: laboratory support for the diagnosis
and monitoring of thyroid disease. Clin Endocrinol (Oxf).
58:138–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mozley PD, Bendtsen C, Zhao B, et al:
Measurement of tumor volumes improves RECIST-based response
assessments in advanced lung cancer. Transl Oncol. 5:19–25.
2012.PubMed/NCBI
|
|
31.
|
Brose MS, Nutting CM, Sherman SI, et al:
Rationale and design of decision: a double-blind, randomized,
placebo-controlled phase III trial evaluating the efficacy and
safety of sorafenib in patients with locally advanced or metastatic
radioactive iodine (RAI)-refractory, differentiated thyroid cancer.
BMC Cancer. 11:3492011.
|