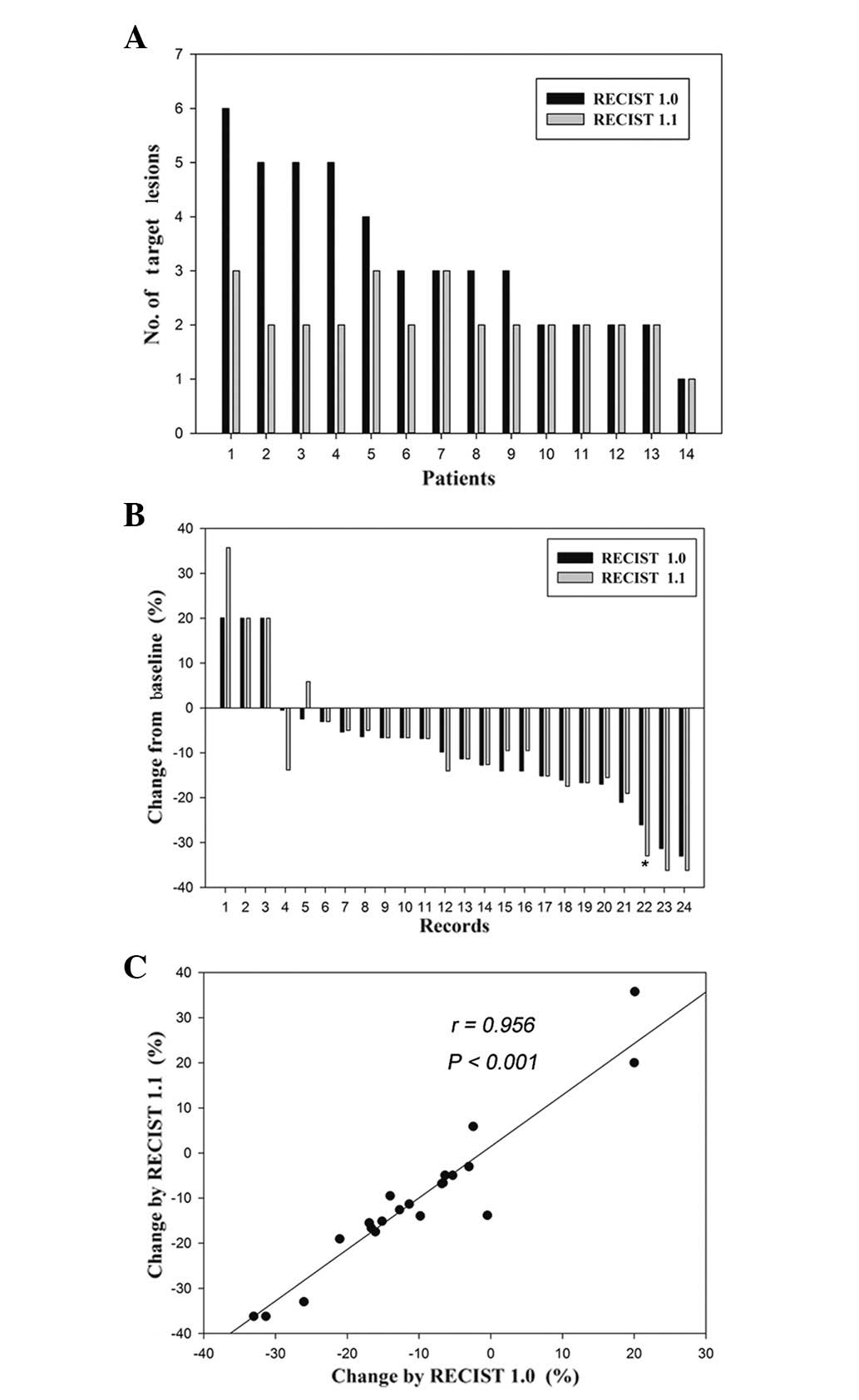
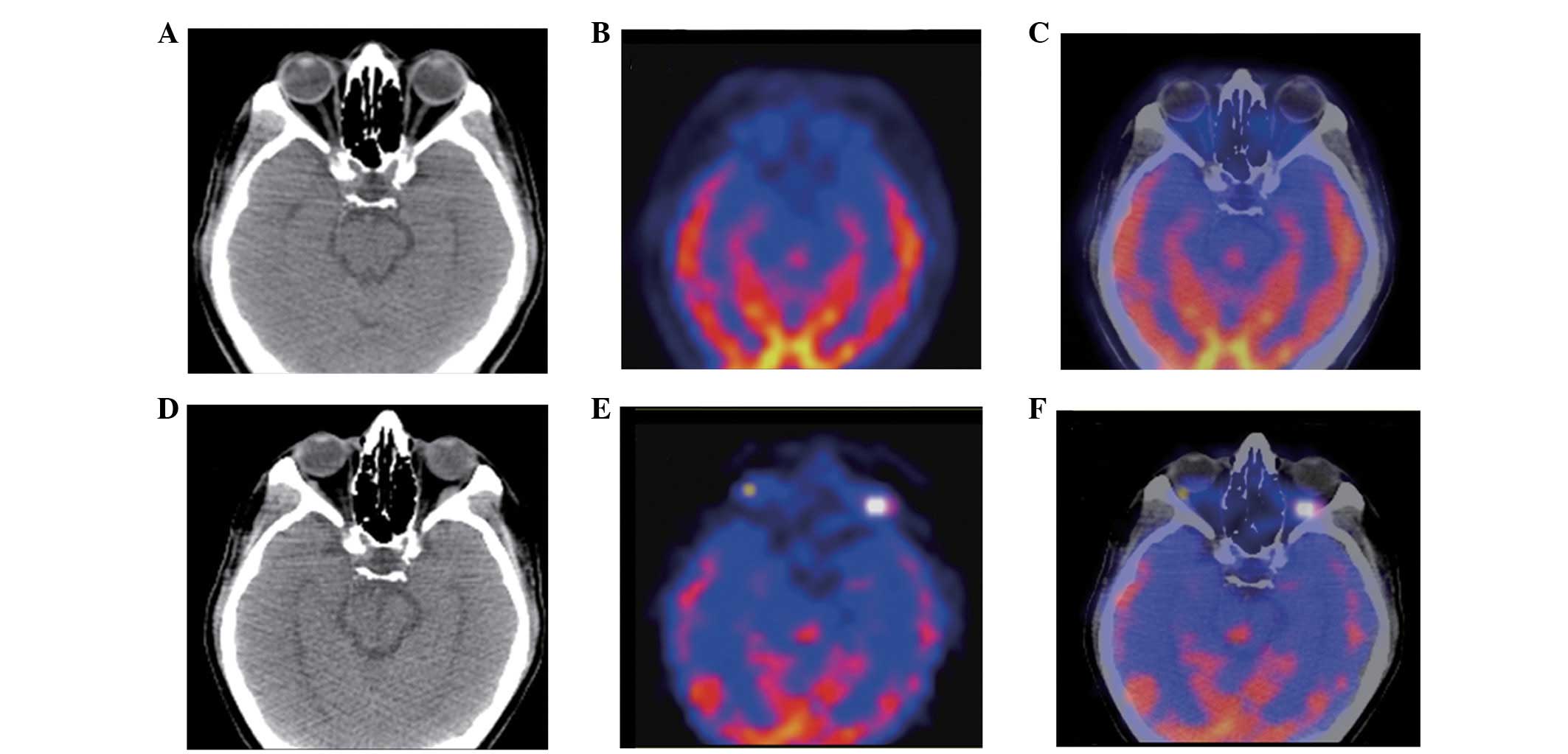
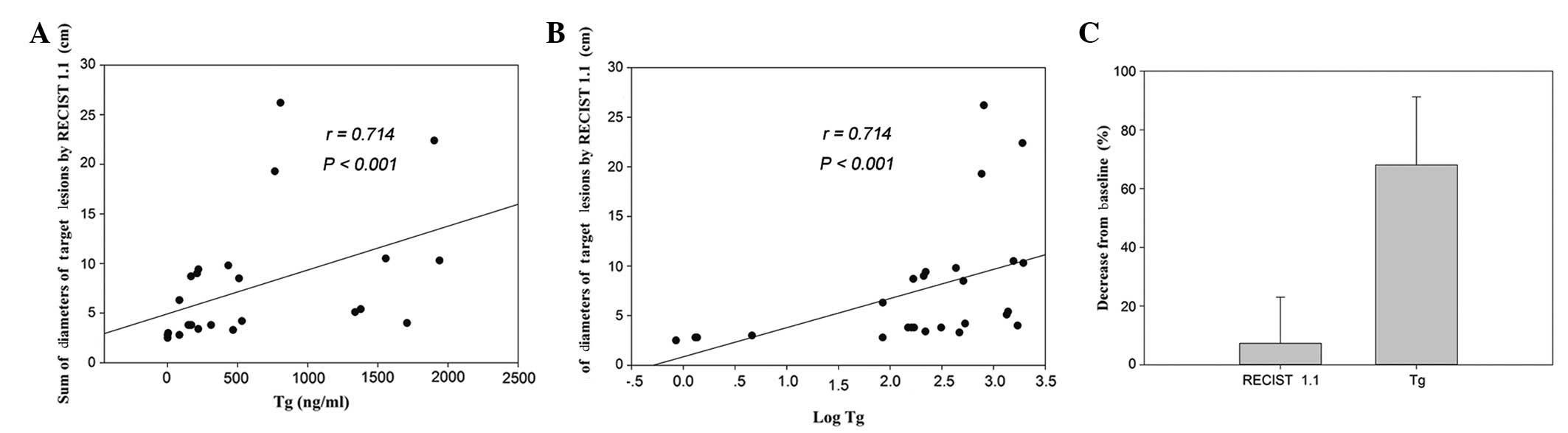
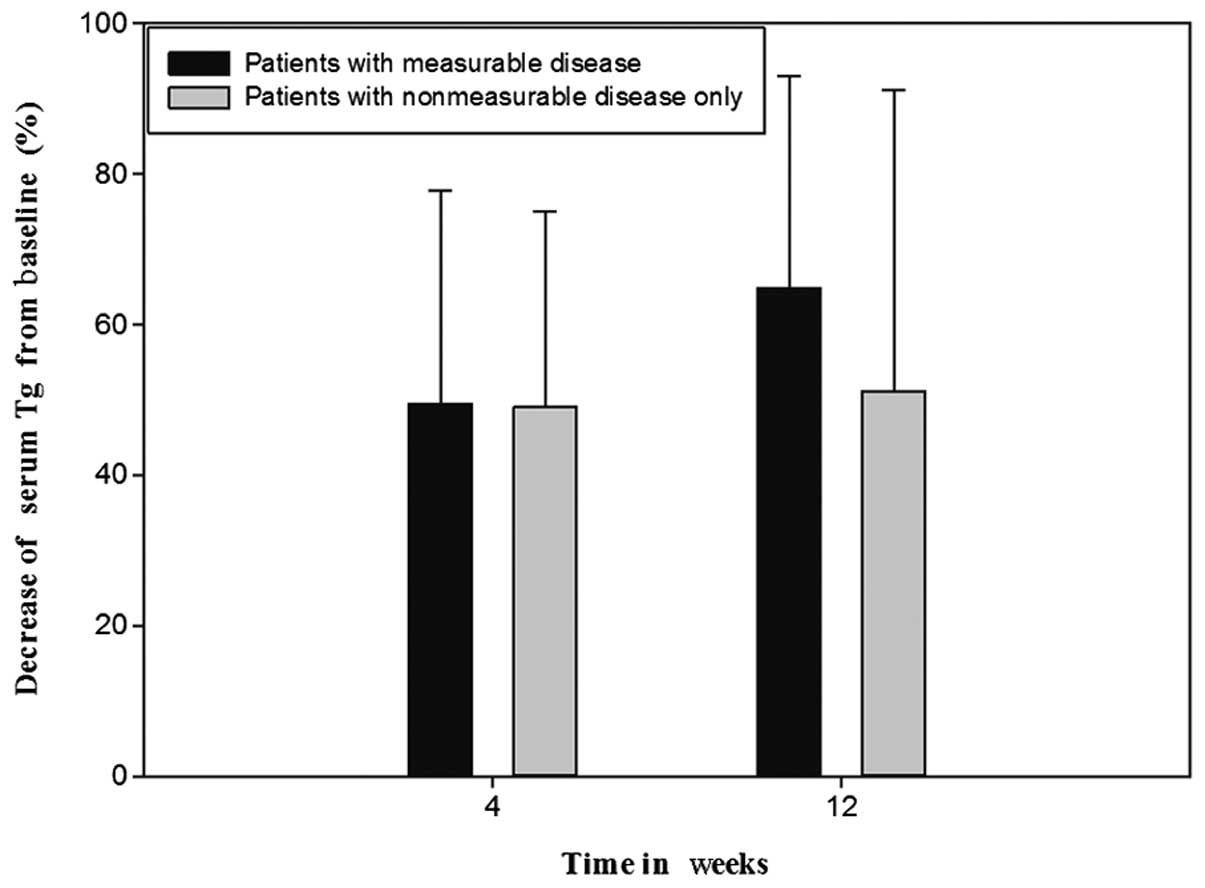
|
1.
|
Aschebrook-Kilfoy B, Ward MH, Sabra MM and
Devesa SS: Thyroid cancer incidence patterns in the United States
by histologic type, 1992–2006. Thyroid. 21:125–134. 2011.PubMed/NCBI
|
|
2.
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shaha AR, Shah JP and Loree TR: Patterns
of nodal and distant metastasis based on histologic varieties in
differentiated carcinoma of the thyroid. Am J Surg. 172:692–694.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Durante C, Haddy N, Baudin E, et al:
Long-term outcome of 444 patients with distant metastases from
papillary and follicular thyroid carcinoma: benefits and limits of
radioiodine therapy. J Clin Endocrinol Metab. 91:2892–2899. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Deshpande HA, Gettinger SN and Sosa JA:
Novel chemotherapy options for advanced thyroid tumors: small
molecules offer great hope. Curr Opin Oncol. 20:19–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhang J, Yang PL and Gray NS: Targeting
cancer with small molecule kinase inhibitors. Nat Rev Cancer.
9:28–39. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Gupta-Abramson V, Troxel AB, Nellore A, et
al: Phase II trial of sorafenib in advanced thyroid cancer. J Clin
Oncol. 26:4714–4719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kloos RT, Ringel MD, Knopp MV, et al:
Phase II trial of sorafenib in metastatic thyroid cancer. J Clin
Oncol. 27:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hoftijzer H, Heemstra KA, Morreau H, et
al: Beneficial effects of sorafenib on tumor progression, but not
on radioiodine uptake, in patients with differentiated thyroid
carcinoma. Eur J Endocrinol. 161:923–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cabanillas ME, Waguespack SG, Bronstein Y,
et al: Treatment with tyrosine kinase inhibitors for patients with
differentiated thyroid cancer: the M. D. Anderson experience. J
Clin Endocrinol Metab. 95:2588–2595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen L, Shen Y, Luo Q, Yu Y, Lu H and Zhu
R: Response to sorafenib at a low dose in patients with
radioiodine-refractory pulmonary metastases from papillary thyroid
carcinoma. Thyroid. 21:119–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shen Y, Ruan M, Luo Q, et al: Brain
metastasis from follicular thyroid carcinoma: treatment with
sorafenib. Thyroid. 22:856–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
15.
|
Gehan EA and Tefft MC: Will there be
resistance to the RECIST (Response Evaluation Criteria in Solid
Tumors)? J Natl Cancer Inst. 92:179–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tuma RS: Sometimes size doesn’t matter:
reevaluating RECIST and tumor response rate endpoints. J Natl
Cancer Inst. 98:1272–1274. 2006.PubMed/NCBI
|
|
17.
|
Ratain MJ and Eckhardt SG: Phase II
studies of modern drugs directed against new targets: if you are
fazed, too, then resist RECIST. J Clin Oncol. 22:4442–4445. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
19.
|
Fuse N, Nagahisa-Oku E, Doi T, et al:
Effect of RECIST revision on classification of target lesions and
overall response in advanced gastric cancer patients. Gastric
Cancer. Aug 22–2012.(Epub ahead of print).
|
|
20.
|
Nishino M, Jackman DM, Hatabu H, et al:
New Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines for advanced non-small cell lung cancer: comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
View Article : Google Scholar
|
|
21.
|
Marotta V, Ramundo V, Camera L, et al:
Sorafenib in advanced iodine-refractory differentiated thyroid
cancer: efficacy, safety and exploratory analysis of role of serum
thyroglobulin and FDG-PET. Clin Endocrinol (Oxf). 78:760–767. 2012.
View Article : Google Scholar
|
|
22.
|
Sun S and Schiller JH: Angiogenesis
inhibitors in the treatment of lung cancer. Crit Rev Oncol Hematol.
62:93–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Schlumberger M, Berg G, Cohen O, et al:
Follow-up of low-risk patients with differentiated thyroid
carcinoma: a European perspective. Eur J Endocrinol. 150:105–112.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Clark PM and Beckett G: Can we measure
serum thyroglobulin? Ann Clin Biochem. 39:196–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Spencer CA, Takeuchi M and Kazarosyan M:
Current status and performance goals for serum thyroglobulin
assays. Clin Chem. 42:164–173. 1996.PubMed/NCBI
|
|
27.
|
Erasmus JJ, Gladish GW, Broemeling L, et
al: Interobserver and intraobserver variability in measurement of
non-small-cell carcinoma lung lesions: implications for assessment
of tumor response. J Clin Oncol. 21:2574–2582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bachelot A, Cailleux AF, Klain M, et al:
Relationship between tumor burden and serum thyroglobulin level in
patients with papillary and follicular thyroid carcinoma. Thyroid.
12:707–711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Demers LM and Spencer CA: Laboratory
medicine practice guidelines: laboratory support for the diagnosis
and monitoring of thyroid disease. Clin Endocrinol (Oxf).
58:138–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mozley PD, Bendtsen C, Zhao B, et al:
Measurement of tumor volumes improves RECIST-based response
assessments in advanced lung cancer. Transl Oncol. 5:19–25.
2012.PubMed/NCBI
|
|
31.
|
Brose MS, Nutting CM, Sherman SI, et al:
Rationale and design of decision: a double-blind, randomized,
placebo-controlled phase III trial evaluating the efficacy and
safety of sorafenib in patients with locally advanced or metastatic
radioactive iodine (RAI)-refractory, differentiated thyroid cancer.
BMC Cancer. 11:3492011.
|