Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequent malignant tumors and its morbidity and mortality rates
have risen (1). The majority of
cancer-related mortalities, including those that are a result of
HCC, are not caused by the growth of the primary tumor, but by the
invasive spread of cancer cells to a secondary site (2). Tumor metastasis occurs by a series of
steps, including vessel formation, cell attachment, invasion and
cell proliferation (3). Tumor cells
must move through and degrade the surrounding tissue barriers to
escape the primary site and colonize secondary organs. Therefore,
the degradation of basement membranes and the extracellular matrix
(ECM) is a crucial step in tumor metastasis. This process requires
various cellular proteolytic enzymes, among which matrix
metalloproteinases (MMPs) are an important family of proteinases
that are responsible for the destruction of the ECM. Among the 20
MMPs that have been identified, MMP-2 (gelatinase-A) and MMP-9
(gelatinase-B) are able to efficiently degrade native collagen
types IV and V, fibronectin, entactin and elastin. Therefore, the
two proteases are considered crucial for cell invasion and the
overexpression of MMP-2/−9 is closely associated with a poor
prognosis in patients (4–7).
Taspine was screened for the first time from Radix
et Rhizoma leonticis, termed ‘Hong Mao Qi’ (HMQ) in Chinese,
using cell membrane chromatography in the laboratory of the School
of Medicine (Xi'an Jiaotong University, Xi'an, Shaanxi, China) and
is now known to exhibit a variety of biological properties,
including bacteriostasis, antibiosis and antiviral,
anti-inflammatory, anti-ulcer and anti-cancer effects (8,9). The
anticancer and anti-angiogenic properties of taspine have been
demonstrated and the compound may be an ideal candidate for a
chemotherapeutic agent (10).
Tas1611 is a ring-opened and biphenyl derivative of taspine with
increased activity and solubility. The present study analyzed the
tumor invasion cascade in the metastatic process and investigated
the effect of the tas1611 on the invasion of SMMC-7721 human liver
cancer cells and the enzymatic degradation of the extracellular
matrices in order to understand the mechanisms of its
anti-metastasis effect.
Materials and methods
Reagents
Tas1611 was provided by the Natural Drug Research
and Engineering Center of Xi'an Jiaotong University (Shaanxi,
China). A stock concentration of tas1611 (20 mM) was prepared using
dimethyl sulfoxide (DMSO) and stored at 4°C. The stock solution was
further diluted with serum-free RPMI-1640 medium immediately prior
to being used. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from Amresco (Cleveland, OH, USA). The
RPMI-1640 medium was purchased from Sigma-Aldrich (St. Louis, MO,
USA). All the antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA). The Total RNA kit was obtained from
Shanghai Fastagen Biotechnology Co., Ltd. (Shanghai, China) and the
Revert AID™ first strand cDNA synthesis kit was from Fermentas
(Hanover, Lithuania).
Cells
The SMMC-7721 human liver cell line was purchased
from the Shanghai Institute of Cell Biology of the Chinese Academy
of Sciences (Shanghai, China). The SMMC-7721 cells were cultured in
RPMI-1640 supplemented with 10% FBS and incubated at 37°C in a 5%
CO2 atmosphere.
Mice
BALB/c nude mice (4–6 weeks old) were supplied by
the Experimental Animal Center of Xi'an Jiaotong University. The
mice were housed and cared for under the standard conditions, with
a 12-12 h day/night cycle. Laboratory food and water were freely
available. All procedures were carried out in accordance with the
guidelines on the care and use of laboratory animals set out by the
Xi'an Jiaotong University Animal Ethics Committee (Xi'an,
China).
Cell Culture
The SMMC-7721 human liver cancer cell line was
cultured in RPMI-1640 medium (Gibco, Invitrogen Corp., Carlsbad,
CA, USA) supplemented with 10% (v/v) FBS in a humidified atmosphere
of 5% CO2 at 37°C.
Cell viability assay
The effect of tas1611 on the viability of the
SMMC-7721 cells was evaluated using the MTT assay (11). Briefly, the exponentially growing
cells were harvested and plated in 96-well plates at a
concentration of 2×104 cells/well. Following a 24-h
incubation period at 37°C, the cells were treated with various
concentrations of tas1611 (0, 0.4, 2, 10 and 50 M) for 48 h.
Subsequently, 20 μl MTT (5 mg/ml) was added to each well and the
cells were incubated at 37°C for 4 h. Once the supernatant was
discarded, 150 μl DMSO was added to each well and the optical
density of the cells was determined using a microplate reader
(BioRad Instruments, Berkeley, CA, USA) at 490 nm and expressed as
absorbance values (12).
Invasion assay
The invasive potential of the SMMC-7721 cells was
assessed in 24-well chemotaxis chambers (Millipore, Billerica, MA,
USA) that were pre-coated with 100 μl Matrigel® (1
mg/ml; BD Biosciences, Bedford, MA, USA). The cells were suspended
in 200 μl serum-free medium, loaded into the upper chamber and
allowed to pass through a polyethylene terephthalate filter with
8-μm pores. The lower chamber was filled with complete medium. The
cells that failed to pass through the filters were removed by
scrubbing with cotton swabs after 24 h (invasion assay). The cells
on the undersurface were fixed in methanol and stained with 0.5%
Crystal Violet (Beijing Chemical works, Beijing, China), then
images were captured and the cells were quantified in 10 random
fields per membrane (13).
Zymogram analysis of MMP activity
Gelatin zymography was performed as described
previously (14). In brief, the
SMMC-7721 cells were cultured in RPMI-1640 supplemented with 10%
FBS until confluency. The cells were washed three times with PBS
and incubated in a serum-free medium or a medium that contained the
indicated concentration of tas1611 for 24 h. The media were
collected and centrifuged for 10 min at 4°C and 356 × g. The
supernatant was mixed with sodium dodecyl sulfate (SDS) sample
buffer without a reducing agent, incubated at room temperature for
15 min and loaded on 10% acrylamide gels containing 1% gelatin
(Sigma). Following the electrophoresis procedure, the gels were
washed with 2.5% Triton X-100 to remove the excess SDS and
incubated at 37°C for 20 h in 10 mM Tris-HCl (pH 7.5), containing
150 mm NaCl and 5 mM CaCl2. The gels were stained with
0.25% (w/v) coomassie blue G-250 and then destained in 20% methanol
containing 10% acetic acid. The areas of protease activity were
detected as clear bands against the blue gelatin background. The
experiments that were performed in the presence of 10 mM EDTA in
the incubation buffer resulted in the abolishment of all zones of
gelatin digestion, confirming that the enzymes were
metalloproteinases. The amount of protein that was loaded onto the
gels was within the linearity of the enzymatic activity. The
quantification and comparison of the gelatinolytic activity
(relative intensity of the lysis bands) of MMP-9 and MMP-2 were
performed by densitometry analysis using an image quantitative
analysis system (Image-Pro Plus; Media Cybernetics, Rockville, MD,
USA).
Western blot analysis
Subsequent to the cells being lysed, a standard
western blot analysis was performed to investigate the abilities of
MMP-2 and MMP-9. The SMMC-7721 cell lines that were treated with 0,
3.3 or 10 μM tas1611 for 48 h were extracted using a cell lysis
buffer on ice. The protein concentration was determined using the
BCA Protein Quantification kit (Joincare Biosciences, Zhuhai,
China) according to the manufacturer's instructions.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed in 10%
tricine gels loading 40 mg cell lysates per lane. Following the
electrophoresis procedure, the separated proteins were transferred
to nitrocellulose membranes and blocked using 5% skimmed milk in
Tris-buffered saline Tween-20 (TBST) buffer for 2 h. The membranes
were then incubated with primary antibodies (MMP-2, MMP-9 and GAPDH
polyclonal rabbit antibodies; 1:1,000 dilution) in 5% skimmed milk
overnight at 4°C with continuous agitation. Subsequently, the
membranes were incubated with secondary anti-rabbit antibodies
conjugated with horseradish peroxidase (1:20,000 dilution) for 2 h
at room temperature according to the manufacturer's instructions.
The blots were detected using an enhanced chemiluminescence (ECL)
reagent (Amersham Pharmacia Biotechnology, Piscataway, NJ, USA) and
analyzed using Quantity One 1D Analysis software (version 4.4;
BioRad) (15,16).
RNA extraction and quantitative PCR
MMP-2 and MMP-9 mRNA expression in the SMMC-7721
cells was evaluated using quantitative PCR with glyceraldehyde
3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The
SMMC-7721 cells were treated with tas1611 (0, 0.2 and 10 μM) for 48
h. The total RNA was isolated using the Total RNA Extraction kit
(Shanghai Fastagen Biotechnology Co., Ltd.) and reverse-transcribed
in a 20-μl reaction solution using the First Strand cDNA Synthesis
kit (Takara, Shiga, Japan). Each reaction was conducted in 96-well
plates with a final volume of 20 μl consisting of 10 μl SYBR Green
PCR Master Mix (Takara) and 1 μl of each 2-μM primer. The sequences
of the individual pairs of primers of MMP-2, MMP-9 and GAPDH are as
follows: MMP-2 forward, 5′-CTCATCGCAGATGCCTGGAA-3′ and reverse,
5′-CAGCCTAGCCAGTCGGATTTG-3′; MMP-9 forward, 5′-ACGCACGACGTCTTCCAG-3
and reverse, 5′-CCACCTGGTTCAACTCACTCC-3′; and GAPDH forward,
5′-AAGGCTGTGGGCAAGGTCATC-3′ and reverse,
5′-GCGTCAAAGGTGGAGGAGTGG-3′. Thermal cycling and fluorescence
detection were conducted on a Thermal Cycler Dice® Real
Time System (Takara), in accordance with the manufacturer's
instructions, at 94°C for 2 min, followed by 40 cycles of 94°C for
20 sec, 55°C for 20 sec and 72°C for 40 sec. Each reaction was
performed in triplicate (17).
Anti-tumor effect of tas1611 on SMMC-7721
cell lines xenografted in athymic mice
The SMMC-7721 cells (2×107 cells/ml) were
implanted into the right axilla of athymic mice (0.2 ml/mouse) to
form a solid tumor. The athymic mice that developed solid tumors
were randomly divided into groups and were administered Tas1611
[100 mg/kg and 200 mg/kg in 0.5% sodium carboxymethyl cellulose
(CMC-Na); n=8] or vehicle alone (0.5% CMC-Na; n=8). The drugs were
administered once a day for two weeks when the tumor volumes were
noticeable. The tumors were measured using calipers every three
days and the tumor volume was calculated as follows: Tumor volume =
(length × width2)/2. The weight of the mice and the
tumor volume were recorded when the mice were sacrificed. Animal
care was in accordance with the institutional guidelines.
Statistical analysis
All the values are presented as the mean ± standard
error of the mean (SEM) and were analyzed for statistical
significance using an analysis of variance (ANOVA). The statistics
were determined with an ANOVA and P<0.05 was considered to
indicate a statistically significant difference.
Results
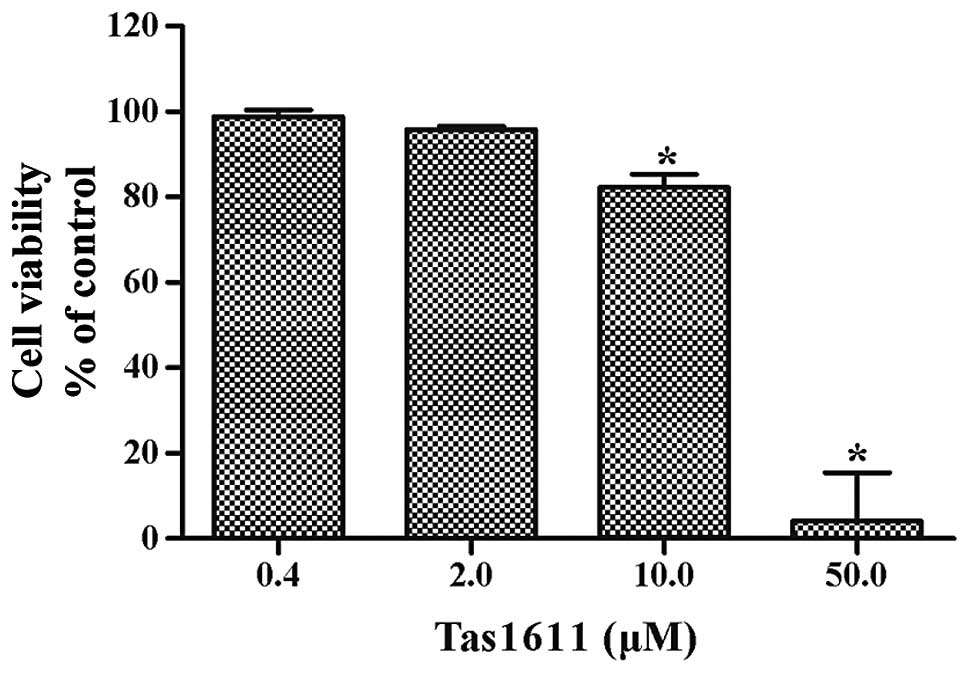
Tas1611 suppresses SMMC-7721 cell
growth
The effects of tas1611 on the viability of the
SMMC-7721 cells were examined using an MTT assay. Fig. 1 shows the dose-dependent inhibition
of viability by tas1611. The mean cell viability was 98.81±1.56,
95.83±0.69, 82.28±3.06 and 4.20±11.25% at 0.4, 2.0, 10.0 and 50.0
μM, respectively. The 50% growth inhibitory concentration (IC50) of
tas1611 on the SMMC-7721 cells was 12.03 M.
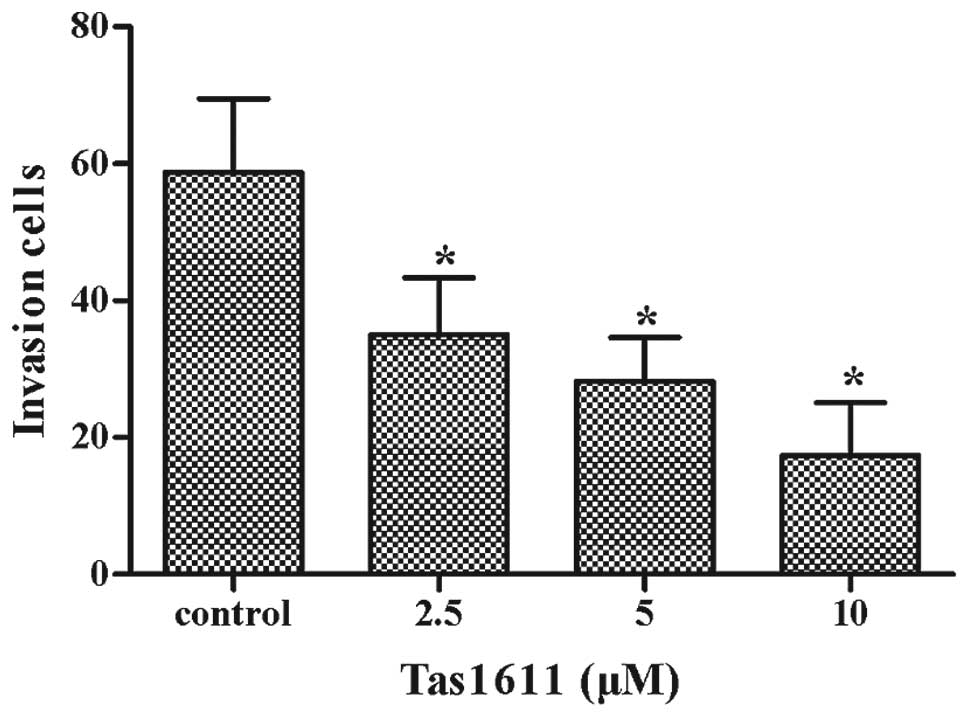
Tas1611 inhibits the invasive properties
of the of SMMC-7721 cells
Cell invasion was assessed using a Transwell insert
that contained polycarbonate membranes with 8-μm pores. The assay
was performed using a Transwell that was precoated with Matrigel.
The cells were treated with tas1611 or vehicle for 24 h, collected
and allowed to migrate through the Matrigel-coated Transwell. As
shown in Fig. 2, tas1611 inhibited
the invasive abilities of the SMMC-7721 cells in a
concentration-dependent manner. The mean cell invasion at 0, 2.5, 5
and 10 μM was 59±11, 35±8, 28±6 and 17±8, respectively.
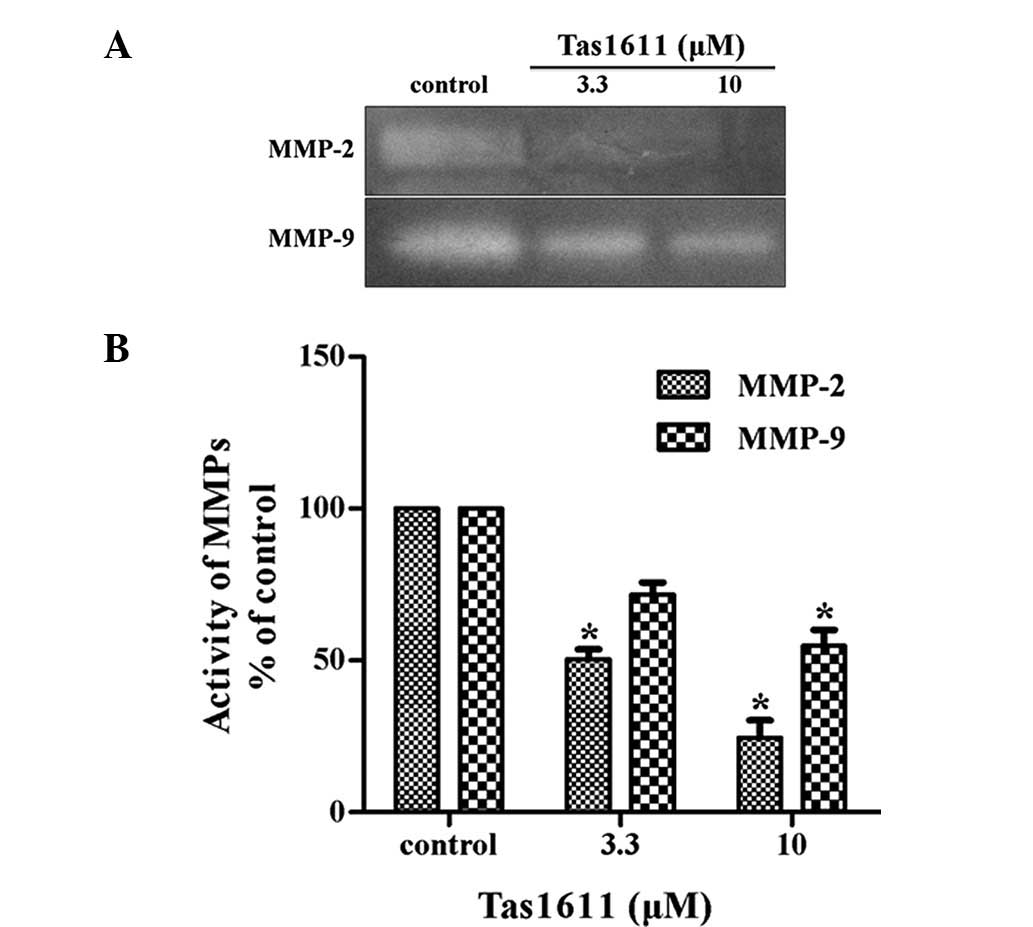
Tas1611 suppresses the activity of MMP-2
and MMP-9 in SMMC-7721 cells
The potential effect of tas1611 pre-treatment on
MMP-2 and MMP-9 secretion by the SMMC-7721 cells was examined using
gelatin zymography. MMP-2 and MMP-9 activity in the SMMC-7721 cells
was inhibited significantly by tas1611 pre-treatment (Fig. 3). The relative quantification of
MMP-2 activity (percentage of control) was 50.40±3.29 and
24.51±5.77 at 3.3 and 10 μM, respectively. Similarly, MMP-9
activity was 71.74±3.98 and 54.86±5.17 at the same doses. This
indicated that the inhibition of invasion by tas1611 was associated
with the changes in gelatinase secretion or activation.
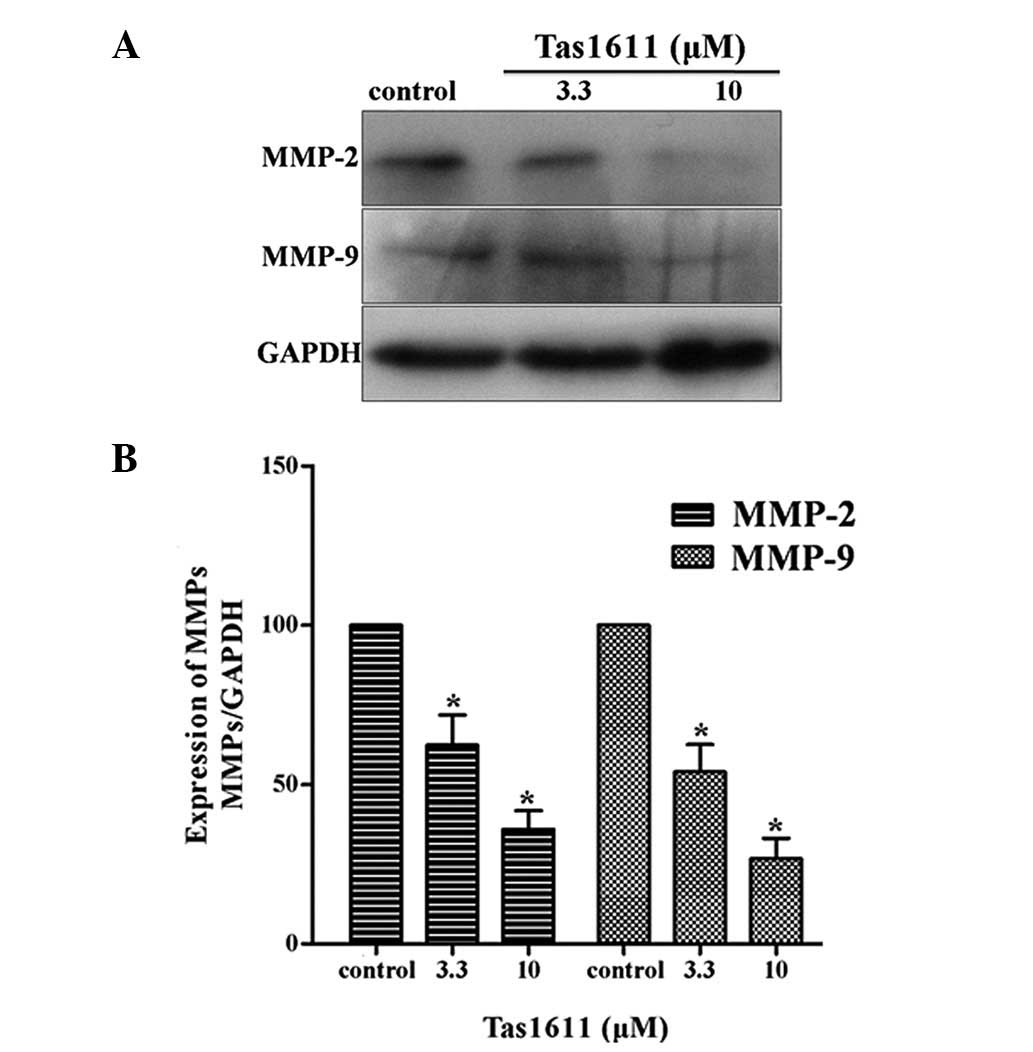
Tas1611 downregulates the protein
expression levels of MMP-2 and MMP-9 in SMMC-7721 cells
Western blot anlaysis was performed to examine the
protein expression of MMP-2 and MMP-9 in the SMMC-7721 cells, as
shown in Fig. 4. The relative
quantification of MMP-2 expression was 62.34±9.42 and 36.02±5.80
and the relative quantification of MMP-9 expression at the same
dose of Tas1611 was 54.00±8.51 and 26.71±6.41, respectively.
Tas1611 was observed to inhibit MMP-2 and MMP-9 protein expression
in a dose-dependent manner compared with the control.
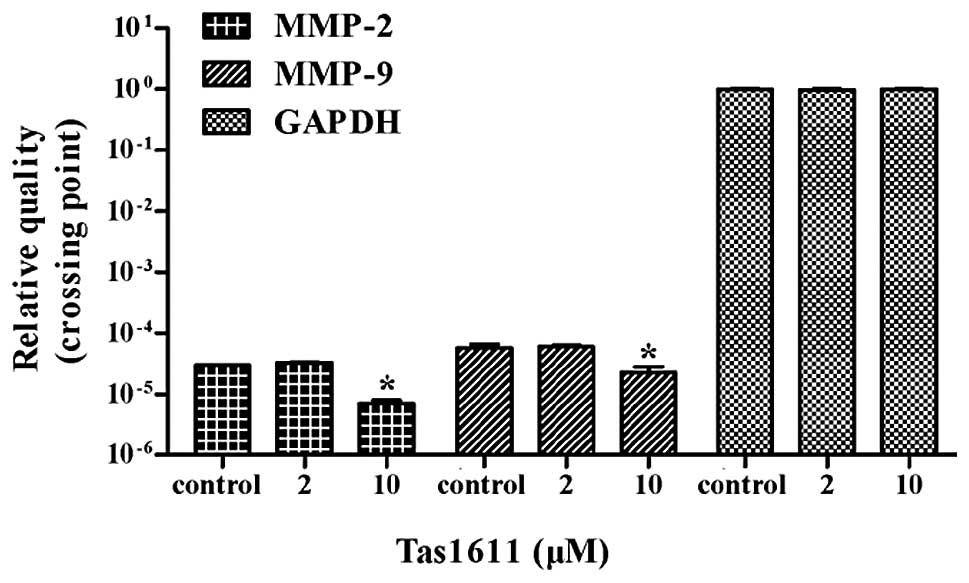
Tas1611 downregulates the mRNA expression
of MMP-2 and MMP-9 in SMMC-7721 cells
Quantitative PCR was performed to evaluate whether
tas1611 was able to effect the synthesis of the MMP-2 and MMP-9
transcripts. The mRNA levels of MMP-2 and MMP-9 were both decreased
by 66.6% at 10 μM (P<0.05). No significant difference was
observed at 2 μM in MMP-2 or MMP-9 mRNA level (Fig. 5).
Tas1611 inhibits tumor growth in an
athymic mouse tumor model
The anti-tumor properties of tas1611 were evaluated
using human tumor models that were xenografted in athymic mice.
Tas1611 significantly inhibited tumor growth in the SMMC-7721
xenografted athymic mice in a dose-dependent manner and there was
no substantial change in the body weight of the athymic mice during
the experiment. Compared with the control group, the
tas1611-treated group exhibited significantly inhibited tumor
growth, with rates of 13.51 and 48.95%, respectively.
Discussion
Invasion plays a critical role in tumor metastasis,
which is the final stage of tumor progression (18). The evidence for MMPs, including
MMP-2 and MMP-9, as active contributors to cancer progression
arises from animal studies. Relatively benign cancer cells acquire
malignant properties when MMP expression is upregulated.
Conversely, highly malignant cells become less aggressive when MMP
expression or activity is reduced (19).
The present study investigated the effect of the
taspine derivative, tas1611, on the viability and invasion of
SMMC-7721 liver cancer cells. The effect of tas1611 on the invasive
properties of the SMMC-7721 liver cancer cells was investigated
using a Matrigel chamber invasion assay. The results revealed that
tas1611 demonstrated a marked inhibition of invasion in a
concentration-dependent manner. The zymogram analysis of MMP
activity showed that MMP-2 and MMP-9 activity was inhibited by
tas1611 significantly. Tas1611 also downregulated the expression of
MMP-2 and MMP-9 mRNA and protein levels. The results suggest that
the anti-invasive action of tas1611 is partly mediated by
diminishing the ability of cancer cells to degrade the components
of the ECM by modulating the expression and activity of MMP-2 and
MMP-9.
The in vivo effect on the growth of the
SMMC-7721 cells that were xenografted in athymic mice was evaluated
to test the efficacy of tas1611 on tumor inhibition. Compared with
the control, the growth of the SMMC-7721 xenografts in the athymic
mouse groups, which were treated with tas1611 at two different
doses, were significantly inhibited. The final volume and weight of
the xenografts were markedly reduced. This demonstrates that
tas1611 plays a role in tumor inhibition.
Taken together, the results of the present study
demonstrate that tas1611 was able to inhibit liver cancer cell
growth and invasion. The compound was also able to reduce tumor
growth in nude mice with xenografted SMMC-7721 cells. The mechanism
underlying the invasion effect was attributed to the downregulation
of MMP-2 and MMP-9 protein and mRNA levels. The data suggest that
tas1611 is a potential candidate for an intervention against
metastatic liver tumors, which otherwise lead to a higher mortality
rate.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81001447, 81230079 and
81227802) and the Shaanxi Young Star of Science and Technology
Program (grant no. 2012KJXX-06).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yodkeeree S, Chaiwangyen W, Garbisa S and
Limtrakul P: Curcumin, demethoxycurcumin and bisdemethoxycurcumin
differentially inhibit cancer cell invasion through the
down-regulation of MMPs and uPA. J Nutr Biochem. 20:87–95. 2009.
View Article : Google Scholar
|
|
3
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XX, Fu Z, Zhang Z, et al:
Microcystin-LR promotes melanoma cell invasion and enhances matrix
metalloproteinase-2/−9 expression mediated by NF-κB activation.
Environ Sci Technol. 46:11319–11326. 2012.PubMed/NCBI
|
|
5
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: one size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, He L, Meng L, Luo W and Xu X:
Suppression of tumor-induced angiogenesis by taspine isolated from
Radix et Rhizoma Leonticis and its mechanism of action in vitro.
Cancer Lett. 262:103–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan Y, Wang N, Liu C, Chen Y, Zheng L and
He L: A novel taspine derivative, HMQ1611, suppresses adhesion,
migration and invasion of ZR-75-30 human breast cancer cells.
Breast Cancer. Aug 9–2012.(Epub ahead of print).
|
|
10
|
Zhang Y, Zheng L, Zhang J, Dai B, Wang N,
Chen Y and He L: Anti-tumor activity of taspine by modulating EGFR
signaling pathway of Erk1/2 and Akt in vitro and in vivo. Planta
Med. 77:1774–1781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, He L, Meng L and Luo W: Taspine
isolated from Radix et Rhizoma Leonticis inhibits proliferation and
migration of endothelial cells as well as chicken chorioallantoic
membrane neovascularisation. Vascul Pharmacol. 48:129–137. 2008.
View Article : Google Scholar
|
|
12
|
Zhou Y, Luo W, Zheng L, Li M and Zhang Y:
Construction of recombinant FGFR1 containing full-length gene and
its potential application. Plasmid. 64:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park MK, Jo SH, Lee HJ, et al: Novel
suppressive effects of cardamonin on the activity and expression of
transglutaminase-2 lead to blocking the migration and invasion of
cancer cells. Life Sci. 92:154–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bourd-Boittin K, Fridman R, Fanchon S,
Septier D, Goldberg M and Menashi S: Matrix metalloproteinase
inhibition impairs the processing, formation and mineralization of
dental tissues during mouse molar development. Exp Cell Res.
304:493–505. 2005. View Article : Google Scholar
|
|
15
|
Zhang YM, Dai BL, Zheng L, et al: A novel
angiogenesis inhibitor impairs lovo cell survival via targeting
against human VEGFR and its signaling pathway of phosphorylation.
Cell Death Dis. 3:e4062012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhang J, Dai B, Wang N and He L:
Anti-proliferative and apoptotic effects of the novel taspine
derivative tas41 in the Caco-2 cell line. Environ Toxicol
Pharmacol. 31:406–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong D, Czerwenka K, Sedlak J, et al:
Association of in vitro invasiveness and gene expression of
estrogen receptor, progesterone receptor, pS2 and plasminogen
activator inhibitor-1 in human breast cancer cell lines. Breast
Cancer Res Treat. 56:91–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|