Introduction
Esophageal cancer is a major health burden that
constitutes one of the major causes of cancer-related mortality
worldwide (1). The incidence of
esophageal cancer has increased considerably during the past
decades. A recent report from the GLOBOCAN project indicated that
~481,000 new cases and ~406,000 mortalities occurred globally
during 2008, while China was estimated to have 258,000 new cases
and ~210,000 mortalities, accounting for 53.6% of the new cases and
51.7% of the mortalities worldwide, respectively (2). Currently, the gold standard approach
to treating early stage esophageal cancer has been to perform an
esophagectomy, either alone or in combination with chemotherapy
and/or radiotherapy (3). Although
the survival of patients has been shown to improve, this approach
has been reported to have significant risks. With the exception of
the risks associated with esophagectomy in early-stage esophageal
cancer treatment, >65% of all esophageal cancers are incurable
at diagnosis (4). These two factors
may account for the poor five-year survival rate of esophageal
cancer, which has been shown to be <20%, remaining one of the
lowest for all cancers (5).
Therefore, a safer and more effective medical treatment strategy
for esophageal cancer is required.
For decades, hyperthermia has been recognized as an
effective approach for various cancers, and has been shown to play
a significant role in multimodal concepts for cancer treatment
(6). Hyperthermia has unique
advantages, and the biological effectiveness of heat in treating
cancer has been fully recognized for decades. However, in clinical
oncology, hyperthermia is currently regarded as the fourth line of
therapy and is mainly applied as an adjunct, ranked below surgery,
chemotherapy and radiotherapy (7).
The technical challenges that are associated with the currently
available hyperthermia modalities mainly include the difficulty of
uniform heating within the tumor region until the required
temperature is reached, without damaging the adjacent normal
tissues (8). The recent
breakthrough in magnetic-mediated hyperthermia (MMH) may bring new
alternatives for cancer locoregional hyperthermia by confining the
heat to within the tumor site (9).
Based on the mechanism of heating ferromagnetic
agents under an alternative magnetic field (AMF), marked progress
has been made in MMH research and in clinical oncology. For the
treatment of esophageal cancer, previous studies have shown that
magnetic stent hyperthermia (MSH) may be a safe and effective
strategy that is able to combine MMH with stent placement for
patients with inoperable esophageal cancer (10–14).
The medical nickel-titanium (Ni-Ti) stent has been demonstrated to
possess excellent inductive heating characteristics under an AMF
(11–14). In vitro investigations on
human esophageal squamous carcinoma ECA-109 cells demonstrated that
MSH has an inhibitory effect on cell viability and that such an
effect is dependent on the thermal dose (13).
With the explosive growth of nanotechnology,
magnetic nanothermotherapy, also termed magnetic fluid hyperthermia
(MFH) or magnetic nanoparticles (MNPs) hyperthermia (MNH), has been
making rapid progress (15). The
research output of MFH has been successfully applied in clinical
oncology (16). In 2010, MFH
received European regulatory approval as a primary treatment for
brain cancer (17). Although MFH
has been widely studied in the treatment of diseases, including
prostate cancer (18), glioblastoma
multiforme (19) and pancreatic
(20), breast (21), liver (22) and lung (23) cancer, no insight has been reached on
the therapeutic effect in esophageal cancer. In the present study,
MNPs were synthesized using the chemical co-precipitation procedure
and then the particles were modified by
3-aminopropyltriethoxysilane (APTES), a type of aminosilane. The
antitumor effect of the fabricated MNPs as mediators for MFH was
evaluated on a rabbit tumor model. Furthermore, the safety and
advantages of MSH and MFH on rabbit esophageal cancer treatment
were compared. The results may provide useful information for
further elucidating the therapeutic effect of magnetic hyperthermia
in esophageal cancer treatment. Deductions from the experimental
observations may have clinical significance for the future
application of magnetic hyperthermia to treat esophageal
cancer.
Materials and methods
MNPs, esophageal stenting, application of
AMF and temperature measurements
APTES-coated MNPs, with diameters of 8–10 nm, were
prepared using the co-precipitation of ferrous salts
(FeCl2•4H2O and
FeCl3•6H2O) by the addition of excess
ammonium hydroxide as described previously (24). The morphology of the MNPs was
observed using transmission electron microscopy (H-800; Brookhaven
Instruments Corp., Holtsville, NY, USA) and the images are shown in
Fig. 1. The superelastic nitinol
stents (Grinkin Advanced Materials Co., Ltd., Beijing, China),
which are made of Ni-Ti alloy, were of the same composition as the
clinical esophageal stent. The stents were specially designed for
the rabbit esophagus, with a length of 30 mm and a macroscopic
diameter of 8 mm. Two types of stents, which were the same in
diameter but with a difference in weight, were employed in the
present study. The lighter stent (type A) was ~0.15 g, while the
heavier stent (type B) was 0.29 g (Fig.
2).
The portable inductive heating device with 300 kHz
and an adjustable field intensity was provided by Shuangping
Instrument Technology, Co., Ltd. (Shenzhen, China). The field
generator consisted of an alternating current generator feeding the
coil inductor. The diameter of the coil was large enough to place
the rabbit inside (Fig. 3).
A thermal-couple temperature probe (model IT-18
Copper-Constantan; Physitemp, Clifton, NJ, USA) was used for the
temperature measurements. The probe fibers were connected to a
four-channel millivoltmeter (model XSOL-4; Beijing Kunlun Tianchen
Instrument Technology, Co., Ltd., Beijing, China) and the data were
collected every 6 sec using a PC with home-written software. Prior
to each experiment, calibration of the thermocouple was performed
at 0 and 100°C.
Inductive heating properties of
esophageal stent and MNPs under AMF
For heating the stent, the thermocouple probe was
fixed at the surface of the stent by inserting it to the mesh of
the device. Following this, the thermocouple loaded stent was
wrapped carefully by thermal insulation materials, including
asbestos fibers, and placed into a water jacket incubator. The
incubator was designed for temperature maintenance. The
double-layer jacket was connected with a water bath so that the
temperature inside the jacket was adjustable and could be
maintained at ~37°C. The jacket was made of glass so the device
itself did not induce heat when exposed under the AMF.
To investigate the inductive heating properties of
the MNPs, a series of MNP suspensions of various particle
concentrations were prepared by dispersing the MNPs using PBS. For
taking the measurements, 2 ml of each suspension was carefully
pipetted into an eppendorf tube. Prior to placing the tube inside
the water jacket incubator, the thermocouple probe was placed
inside the tube with the tip immersed under the suspension
interface.
Animal care and establishment of rabbit
esophageal cancer
The maintenance and care of all the experimental
animals that were used in this study was performed according to
guidelines of the Institutional Animal Care and Use Committee of
Tsinghua University (Beijing, China). Japanese white rabbits, each
weighing ~2 kg, were provided by the Beijing Center for Disease
Control and Prevention (Beijing, China). The rabbits were
maintained in a specific pathogen-free animal house under a 12-h
light and 12-h dark cycle and were fed a standard laboratory diet
and tap water ad libitum.
The frozen VX2 tumor cells were thawed in a 37°C
water bath and washed twice with PBS followed by centrifugation at
560 × g for 5 min. The viable cells were counted by trypan blue dye
and re-suspended at 1×106 cells/ml in PBS. A 1-ml viable
cell suspension was injected into the thigh subcutis of a host
rabbit to obtain the tumor node. The node was extracted when the
diameter reached 3–4 cm. Furthermore, the grossly necrotic tissues
in the tumor center and the surrounding myofascial tissue were
discarded. The viable VX2 tissue was finely minced into fragments
and ground in Dulbecco’s modified Eagle’s medium (DMEM). The
cellular suspension was filtered through a sterile 74-μm mesh
filter. The filtered cell suspension was re-suspended with DMEM at
1×108 cells/ml.
The rabbits were anesthetized with i.v. 2% sodium
pentobarbital into an ear vein. Under aseptic conditions, the
rabbits were placed in a supine position and the esophagus region
was shaved and prepared with iodophors and 75% alcohol. A 4–5-cm
midline vertical incision was made along the trachea to expose the
esophagus. A 0.1-ml VX2 tumor cell suspension, as mentioned
previously, which contained 1×107 viable cells was
injected from the esophageal tunica adventitia into the submucosa
of the cervical esophagus. The tumor was measured using a digital
caliper. The tumor volumes (V) were determined by applying the
following formula: V = a + b2, where a and b represented
the maximal and minimal tumor diameters, respectively.
Safety evaluation of MSH on the rabbit
esophagus
Three levels of temperature (43, 46 and 50°C) were
adopted for the safety evaluation of MSH on the rabbit esophagus.
For each temperature, the rabbits underwent a 10- or 30-min MSH
treatment. Therefore, six groups of rabbits were involved in this
analysis (n=10). Following each treatment, the rabbits were
routinely maintained for one week and then sacrificed for the
histological analysis.
Experimental groups for MSH and MFH
For the MSH treatment, the tumor-bearing rabbits
were divided into two groups, the control (rabbits with stent
implantation only) and MSH (n=6) groups. Three experimental groups,
including the control (untreated group), MF (rabbits with
esophageal tumor site infused with MNPs only) and MFH groups were
involved in the MFH treatment.
Stent deployment and MNP injection
The stents were deployed into the cervical esophagus
using a stent delivery device. In order to monitor the temperature
around the rabbit esophagus, the thermocouple probe was inserted
into the stent mesh. As the stent has a good shape memory, it is
easily deformed and inserted into the delivery device. Under X-ray,
the stent loaded delivery device was inserted perorally and
positioned precisely into the rabbit esophagus. The stent was then
released and expanded to its original shape to impose a suitable
force on the internal wall of the esophagus. A detailed
illustration on the stent delivery device may be referred to in our
previous publication (12).
Following the confirmation for the successful
establishment of the rabbit tumor model by administration of a
barium meal, the rabbits underwent an open surgical exposure of the
esophagus. The colloidal liquid of aminosilane-coated MNPs, which
dispersed with PBS, was administered to the tumor tissue by direct
injection. In order to achieve a homogenous distribution of the
MNPs within the tumor tissue, the intratumoral injection was
performed from four directions, all of which were through the
pinhole with a 27G syringe.
Histological examination
Following the sacrifice of the rabbits, the
esophagus, tumor and other tissues or organs were fixed in a 10%
formalin solution. The tissue samples were paraffin-embedded, cut
into 4-μm sections and further subjected to HE staining.
Statistical analysis
A one-way analysis of variance was used for the
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inductive heating properties of
esophageal stent and MNPs under AMF
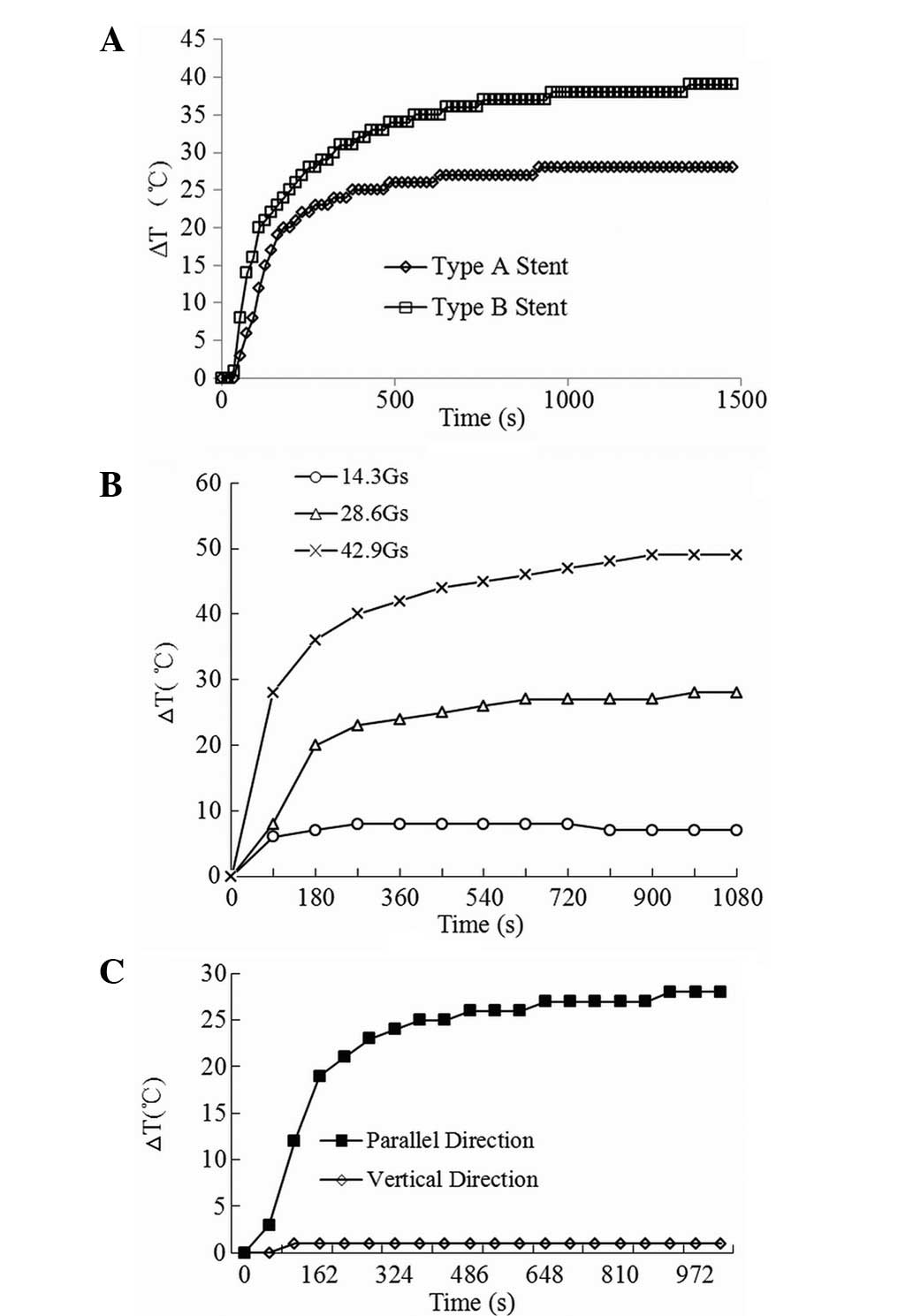
The inductive heating properties of the two types of
esophageal stents under AMF are shown in Fig. 4A. The stents were observed to
possess an excellent property for heat generation upon exposure to
the AMF. Rapid temperature increases, as indicated by the initial
slope of the curves, were observed. Within 200 sec, a high
equilibrium temperature was reached in the two stents, with the
higher equilibrium temperature occurring in the heavier stent.
Compared with the type B stent, the type A stent was softer and
more flexible. In order to impose less mechanical injury to the
rabbit esophagus by the stent, the in vivo experiment was
conducted using the type A stent.
The effect of the AMF strength on the heating
profile of the stent is shown in Fig.
4B. The field strength was directly correlated with the
inductive heating characteristics of the stent; the higher the
field strength, the higher temperature at equilibrium. Fig. 4C illustrates the effect of the
orientation of the stent axis on the inductive heating. It was
clearly revealed that compared with the stent being perpendicular
to the direction of the field direction, the stent that was
positioned parallel to the field direction induced a higher
temperature, thus generating more heat. All the observations
strongly indicated that the quality of the stent, the orientation
of the stent axis and the field parameter affect the heating
profile of the stent under AMF.
The heating profiles of the aminosilane-MNP
suspensions with the various MNP concentrations under an AMF of 300
kHz are shown in Fig. 5. A higher
field intensity and particle concentration resulted in a greater
increase in the temperature. The desired temperature was achieved
by appropriately choosing the MNP concentration or adjusting the
field intensity, which guaranteed the temperature requirements for
the hyperthermia cancer treatment.
Safety evaluation of MSH on the rabbit
esophagus
Fig. 6 shows the
in vivo temperature profile of the rabbits under MSH. By
carefully adjusting the field parameter, the desired temperatures
were achieved and maintained. It was also observed that during the
treatment, the rectal temperature was constant, indicating that the
MSH was a local treatment. Fig. 6A
also shows that there was a slight temperature difference (<2°C)
between the inner and outer esophageal walls of the rabbit. The
temperature profiles of the various esophagus segments were also
examined during the heating process. As shown in Fig. 6B, the inner-side of the esophagus,
which is attached to the spine, demonstrated a higher temperature
than the outer-side of the esophagus, which is attached to the
trachea.
The histological evaluation of the rabbit esophagus
under MSH of various thermal doses is shown in Fig. 7. Generally, MSH of 50°C was not
tolerable to the rabbits, as transmural necrosis occurred in all
the animals treated with this temperature. For 46°C hyperthermia,
necrosis may reach the submucosa layer due to a longer treatment
time (30 min), and for 43°C, the treatment was observed to be safe
to the rabbit esophagus, regardless of the treatment time. However,
our previous results have demonstrated that esophageal cells are
heat resistant (14). A temperature
of 43°C has little effect on the viability or necrosis of the
ECA-109 cells in the treatment time range of 5–30 min. Relatively,
46°C MSH for 10 min is safe to use on the esophageal wall of the
rabbits, as only a slight necrosis was observed in the mucosa
layer. Therefore, such a thermal dose was adopted for the antitumor
effect evaluation of MSH on the rabbit esophageal tumor model.
Effect of MSH on esophageal cancer in a
rabbit tumor model
Fig. 8 shows the
successful establishment of the rabbit esophageal tumor model and
implantation of the stent into the rabbit esophagus. Stent
migration was not observed in any rabbits during the observation
period. Fig. 9 demonstrates the
effect of MSH on the tumor volume following one week of the
treatment. Prior to the treatment, the tumor volume of the control
and treatment groups was 286.3±174.5 and 195.0±162.7 mm3
(P>0.05), respectively. One week after MSH, the tumor volume was
415±228.1 mm3 for the rabbits under treatment whilst
that of the control group reached 913.7±404 mm3
(P<0.05). MSH using the thermal dose of 46°C for 10 min was able
to effectively inhibit the tumor growth in the rabbit esophageal
tumor model.
Effect of MFH on esophageal cancer in a
rabbit tumor model
Fig. 10 shows the
temperature profile of the rabbits that were subjected to MFH. The
temperature of the rabbit rectum was kept constant during the
treatment, confirming the local treatment of MFH. MNPs also have
excellent inductive heating properties in vivo. The
temperature was able to reach 48°C within 5 min and was stably
maintained by carefully adjusting the field parameters. The
temperature dropped quickly subsequent to turning the power off,
indicating a rapid heat dissipation inside the rabbit. The time
course of subjecting the tumor volume to the various treatments is
shown in Fig. 11, and demonstrated
that the tumor volume of the rabbit in the control group steadily
increased with no evidence of regression. By contrast, the
injection of the MNPs within the tumor site had no therapeutic
effect to the esophageal cancer. However, MFH was able to greatly
inhibit the in vivo tumor growth (P<0.001). The
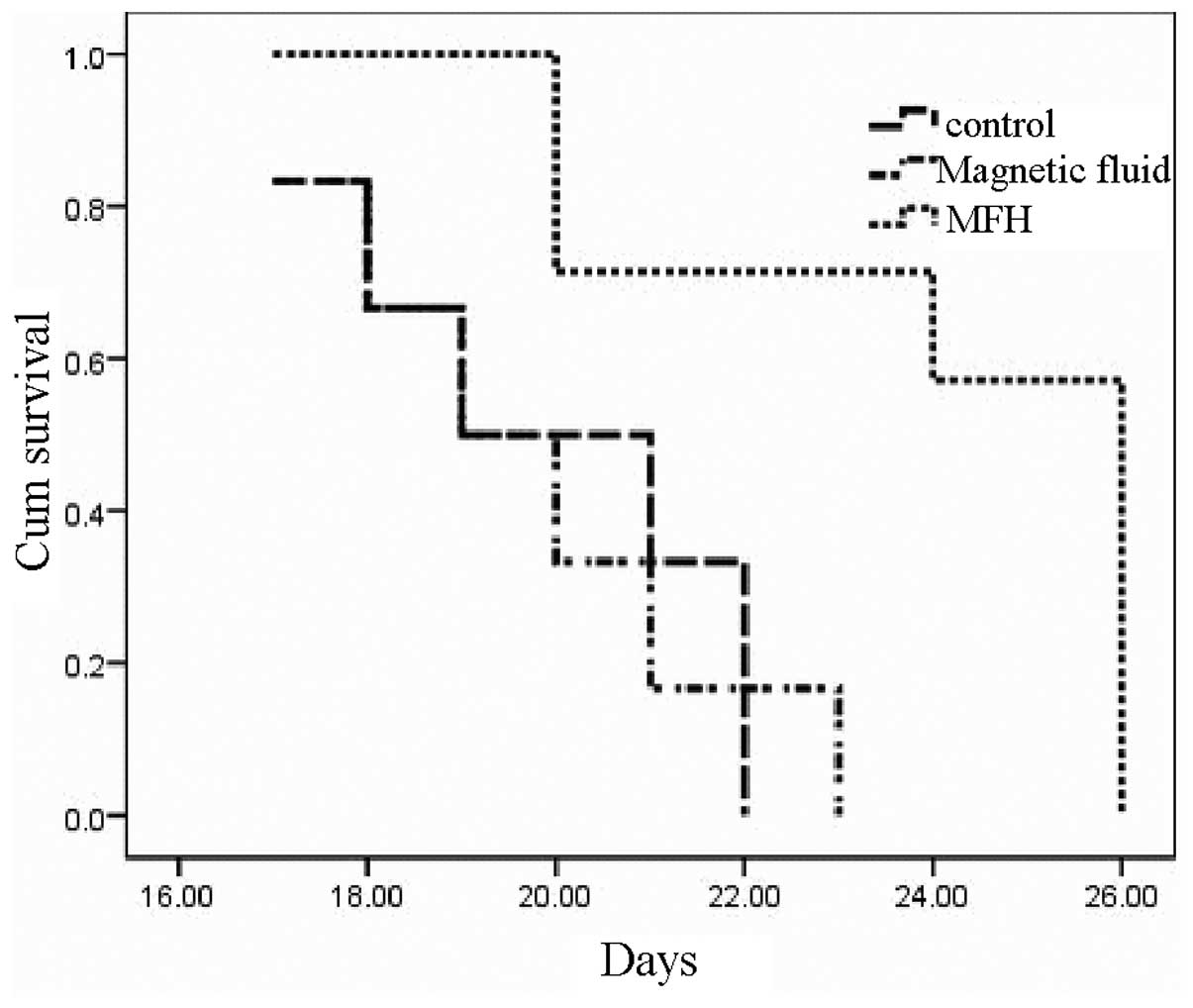
difference in survival among the three groups is shown in Fig. 12. MFH was able to significantly
increase the life span of the tumor-bearing rabbits over that of
the control and MNP injection groups. The histological evaluation
of the esophageal tumors that were subjected to the various
treatments is shown in Fig. 13.
MNPs aggregated and distributed within the tumor tissues in the MFH
and MF groups. The tumors in the MF group revealed no appearance of
necrosis. In the MFH group, the esophageal tumors displayed large
areas of necrosis, cell shrinkage and ruptured cell pieces. It was
confirmed that no esophagus perforation or tracheoesophageal
fistulae occurred in the rabbits in the MFH group, indicating that
MFH at 48°C for 30 min is safe for rabbits.
Discussion
The biological effectiveness of heat in treating
cancer has been fully recognized and a number of molecular
mechanisms have been elucidated. Since the 1970s, several aspects
of heat action have been examined in numerous pre-clinical studies
(8). However, an unequivocal
identification of the mechanisms leading to favorable clinical
results using hyperthermia has not yet been identified. The
technical limitations of the heat locoregional delivery and the
poor control of the thermal dosage are possible reasons that impede
the effective distribution of the therapeutic temperatures and
doses in the tumor site, thus restricting the successful
application or translation of the research output into clinical
oncology. MMH is able to couple the heat magnetically to the
mediators or agents only within the tumor site. Generally, as the
magnetic mediators are conformably distributed within the tumor
site, a homogenous temperature field may be realized. The concept
of MMH was first proposed by Gilchrist et al in the 1950s,
where it was demonstrated that magnetic particles were able to be
deposited selectively at the tumor site and heat the tumor tissue
specifically when exposed to an AMF (25). Following years of exploration, the
research output on MMH has been successfully applied in clinical
oncology with inspiring results, thus providing an alternative
procedure for cancer treatment.
As the mediator of MMH, the magnetic agents play a
critical role in the hyperthermia treatment. The results of the
present study confirmed that the stent and MNPs were able to
rapidly reach the desired hyperthermia temperature and that the
temperature was stably maintained under the proper field
parameters. Briefly, the magnetic field induces current to flow in
the stent and the resistance of the stent impedes the current flow,
thereby producing heat. For the micro- or nano-scaled agents, the
mechanisms may be more complicated. However, it has been generally
acknowledged that Brownian movement and Neel relaxation mainly
account for the inductive heating (26). Although there is a difference
between the heating mechanisms, the results of the present study
show that the two agents possess excellent inductive heating
properties under AMF. The rapid temperature rise is favorable
during hyperthermia treatment with regard to thermotolerance
(27). More thermotolerance, which
may reduce the treatment efficiency, is induced if the temperature
rises slowly.
The present study also revealed that the field
intensity of the AMF and the quality of the agents were positively
correlated with the inductive heating properties. A higher
temperature may be achieved with a higher field intensity, heavier
stent and higher contents of MNPs within the magnetic suspensions.
This observation indicates that during the treatment, the
hyperthermic temperature may be controlled by a proper choice of
agents or careful adjustment of the field intensity. It was also
noteworthy that the orientation of the stents affected the heating
profile, and a parallel position of the stent to the field
direction produced the highest temperature. However, there was no
directional dependence of AMF to the inductive heating property of
the nano-scaled or micro-scaled agents. Such a phenomenon may be
explained from the difference in the heating mechanisms of the
agents.
The in vivo heating profile of MSH
demonstrated an existing inhomogeneous heat distribution in the
esophagus. As shown in Fig. 6, the
deep-seated esophagus segment was able to hold more heat than the
superficial segment. This is easy to explain from the viewpoint of
in vivo heat transfer. The present results also demonstrated
that the rabbit esophagus was not thermally insulated in the radial
direction, as only a slight temperature difference was observed
between the temperatures of the inner and outer esophagus segments.
This observation is inconsistent with results observed in pigs. In
a previous study, a significant difference was observed between the
temperatures of the inner and outer esophageal walls of the pig,
although an extremely high temperature (>50°C) was reached at
the inner esophageal wall, the maximal temperature of the outer
wall did not exceed 40°C (14). The
thermal conductivity of an organ or a tissue is of vital
significance during hyperthermia treatment. A low thermal
conductivity may result in a poor heat transfer performance, which
is unfavorable for MSH, as the therapeutic effect may be
compromised by the thermal insulation of the esophageal wall.
Therefore, a thorough understanding of the temperature distribution
and heat transfer performance with regard to the esophagus is a
priority for the clinical application of MSH.
During either MSH or MFH in the present study, the
rectal temperatures of all the rabbits were kept constant during
the treatment. Therefore, local hyperthermia was confirmed.
Compared with MFH, MSH was able to heat the esophagus segment that
was implanted with the stent, as well as the tumor site. As shown
in Fig. 7, transmural necrosis was
observed when using the thermal dose with a higher temperature or
longer treatment time. Safety considerations, particularly heat
resistance of the healthy esophagus should thus be carefully
evaluated. It should be noted that properties of the human
esophagus may not be deduced from observations of the rabbit
esophagus, as there may be a difference in the heat resistance and
heat transfer performance between humans and rabbits. A similar
study was conducted on pigs, with results showing that the pig
esophagus was able to endure a higher temperature treatment without
mucous hyperemia or tissue edema (14). Although humans and pigs share
similar physiological indexes in a number of aspects, it is
impossible or inconvenient to grow a tumor in the pig’s esophagus.
A rabbit esophageal tumor model may be the only choice to conduct
an in vivo investigation for MSH in the current situation.
Although there may be limitations for the tumor model, the results
of the present study have shown that MSH has a positive therapeutic
effect under the appropriate thermal dose.
MFH is able to induce heat that is confined within
the tumor site. Therefore, more specific heating may be achieved
compared with MSH. The results of the present study indicate that
MFH at a thermal dose of 48°C for 30 min may effectively inhibit
the tumor growth and significantly prolong the life-span of the
tumor-bearing rabbits without any harm to the nearby tissue or
organs. The present study systematically conducted the effect of
various thermal doses on the proliferation and apoptosis of the
human esophageal ECA-109 cancer line (13). The observation indicated that the
hyperthermia treatment of 48°C for 20–30 min may be the optimal
thermal dose. In recognition that a higher temperature and longer
treatment time may impose damage to the healthy esophagus if MSH
was applied, MFH may be considered safer and more effective than
MSH. However, MSH is based on a well-established approach of an
endoscopic placement of the stent and therefore other patient
procedures are not required, with the exception of AMF exposure.
For MFH, the infusion of a colloidal suspension of MNPs within the
tumor site is required. In the present study, the MNPs were infused
under direct view by an open surgical exposure of the esophagus. In
order to achieve a mini-invasive or non-invasive surgery when
applied in clinical oncology, a special infusion device should be
developed for the administration of the MNPs perorally. Therefore,
MSH may be more convenient for clinical translation. Furthermore,
hyperthermia is usually applied as an adjunct to an established
treatment modality and aims at improving the results of the
conventional treatment strategies within the framework of
multimodal treatment concepts. Therefore, an improved therapeutic
effect may be achieved by the combination of MSH and radiotherapy
or chemotherapy. Akiyama et al performed pioneering clinical
work on the feasibility and effectiveness of the multimodal
treatment of MSH combined with simultaneous chemotherapy in 13
patients and radiochemotherapy in five patients (11). The results indicated that MSH proved
effective in eight of the nine patients who were administered the
treatment at least three times. The clinical results indicated that
MSH is able to improve the effectiveness of combination therapy and
suppress local tumor growth. Therefore, it is suggested that while
MFH may be applied as a monotreatment for esophageal cancer, MSH
may be more suitable for multimodal treatment by combining
hyperthermia with other cancer treatment approaches.
MSH and MFH promise to be local and effective
hyperthermia treatments for esophageal cancer. MFH is able to
induce heat within the tumor site only, in order to achieve more
specific heating, and therefore, may be applied as a monotreatment
for esophageal cancer. The homogenous local infusion of colloid
MNPs with an ideal inductive heating property is required for MFH.
MSH is able to combine the advantages of stent endoscopic placement
with local heating. The clinical stent possesses an excellent
inductive heating property under an AMF, and therefore, there is no
requirement for the development or administration of other devices
for MSH. However, a thorough understanding of the heat resistance
and heat transfer performance of the human esophagus is of vital
importance to facilitate the logical transition of the technique
from the bench to the bedside.
Acknowledgements
This study was supported in part by the National
Science Foundation (nos. 81172182 and 81041040) and the China
Postdoc Science Foundation (no. 20080430045).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo P and Li K: Trends in esophageal
cancer mortality in China during 1987–2009: age, period and birth
cohort analyzes. Cancer Epidemiol. 36:99–105. 2012.
|
|
3
|
Corti L, Skarlatos J, Boso C, et al:
Outcome of patients receiving photodynamic therapy for early
esophageal cancer. Int J Radiat Oncol Biol Phys. 47:419–24. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tew WP, Kelsen DP and Ilson DH: Targeted
therapies for esophageal cancer. Oncologist. 10:590–601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCann P, Stafinski T, Wong C and Menon D:
The safety and effectiveness of endoscopic and non-endoscopic
approaches to the management of early esophageal cancer: A
systematic review. Cancer Treat Rev. 37:11–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mi Y, Liu X, Zhao J, Ding J and Feng SS:
Multimodality treatment of cancer with herceptin conjugated,
thermomagnetic iron oxides and docetaxel loaded nanoparticle of
biodegradable polymers. Biomaterials. 33:7519–7529. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrandt B, Wust P, Ahlers O, et al:
The cellular and molecular basis of hyperthermia. Crit Rev Oncol
Hemato. 43:33–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares PI, Ferreira IM, Igreja RA, Novo CM
and Borges JP: Application of hyperthermia for cancer treatment:
recent patents review. Recent Pat Anticancer Drug Discov. 7:64–73.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laurent S, Dutz S, Häfeli UO and Mahmoudi
M: Magnetic fluid hyperthermia: focus on superparamagnetic iron
oxide nanoparticles. Adv Colloid Interface Sci. 166:8–23.
2011.PubMed/NCBI
|
|
10
|
Khot VM, Salunkhe AB, Thorat ND,
Ningthoujam RS and Pawar SH: Induction heating studies of dextran
coated MgFe2O4 nanoparticles for magnetic
hyperthermia. Dalton Trans. 42:1249–1258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akiyama S, Kawasaki S, Kodera Y, Hibi K,
Kato S, Ito K and Nakao A: A new method of thermo-chemotherapy
using a stent for patients with esophageal cancer. Surg Today.
36:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou JM, Li N, Xia QS, et al: Hyperthermia
by a nitinol stent in an alternating magnetic field: safety and
feasibility in rabbit esophageal cancer. Prog Nat Sci.
19:1713–1719. 2009. View Article : Google Scholar
|
|
13
|
Liu JY, Zhao LY, Wang YY, Li DY, Tao D, Li
LY and Tang JT: Magnetic stent hyperthermia for esophageal cancer:
An in vitro investigation in the ECA-109 cell line. Oncol Rep.
27:791–797. 2012.PubMed/NCBI
|
|
14
|
Liu JY, Li DY, Chen HH, et al: Evaluation
on the feasibility and safety of magnetic stent hyperthermia for
esophageal cancer. IFMBE Proc. 39:1632–1635. 2013. View Article : Google Scholar
|
|
15
|
Zhao LY, Tang JT and Feng SS:
Nanothermotherapy by high performance magnetic nanoparticles.
Nanomedicine (Lond). 5:1305–1308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiesen B and Jordan A: Clinical
applications of magnetic nanoparticles for hyperthermia. Int J
Hyperthermia. 24:467–74. 2008. View Article : Google Scholar
|
|
17
|
Schütz CA, Juillerat-Jeanneret L, Mueller
H, et al: Therapeutic nanoparticles in clinics and under clinical
evaluation. Nanomedicine (Lond). 8:449–467. 2013.PubMed/NCBI
|
|
18
|
Johannsen M, Thiesen B, Wust P and Jordan
A: Magnetic nanoparticle hyperthermia for prostate cancer. Int J
Hyperthermia. 26:790–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Landeghem FK, Maier-Hauff K, Jordan A,
et al: Post-mortem studies in glioblastoma patients treated with
thermotherapy using magnetic nanoparticles. Biomaterials. 30:52–57.
2009.PubMed/NCBI
|
|
20
|
Wang L, Dong J, Ouyang W, Wang X and Tang
J: Anticancer effect and feasibility study of hyperthermia
treatment of pancreatic cancer using magnetic nanoparticles. Oncol
Rep. 27:719–726. 2012.PubMed/NCBI
|
|
21
|
Yoshida M, Sato M, Yamamoto Y, et al:
Tumor local chemohyperthermia using docetaxel-embedded
magnetoliposomes: Interaction of chemotherapy and hyperthermia. J
Gastroenterol Hepatol. 27:406–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du LH, Zhou JM, Wang XW, et al: Effect of
local hyperthermia induced by nanometer magnetic fluid of the
rabbit VX2 liver tumor model. Prog Nat Sci. 19:1705–1712. 2009.
View Article : Google Scholar
|
|
23
|
Lee H, Kim S, Choi BH, et al: Hyperthermia
improves therapeutic efficacy of doxorubicin carried by mesoporous
silica nanocontainers in human lung cancer cells. Int J
Hyperthermia. 27:698–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Yang B, Dai X, Wang X, Gao F,
Zhang X and Tang J: Glutaraldehyde mediated conjugation of
amino-coated magnetic nanoparticles with albumin protein for
nanothermotherapy. J Nanosci Nanotechnol. 10:7117–7120. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gilchrist RK, Medal R, Shorey WD,
Hanselman RC, Rarrott JC and Taylor CB: Selective inductive heating
of lymph nodes. Annals Srug. 146:596–606. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XF, Tang JT and Shi LQ: Induction
heating of magnetic fluids for hyperthermia treatment. IEEE Trans
Magn. 46:1043–1051. 2010. View Article : Google Scholar
|
|
27
|
Pallepati P and Averill-Bates DA: Mild
thermotolerance induced at 40°C protects HeLa cells against
activation of death receptor-mediated apoptosis by hydrogen
peroxide. Free Radic Biol Med. 50:667–679. 2011.
|