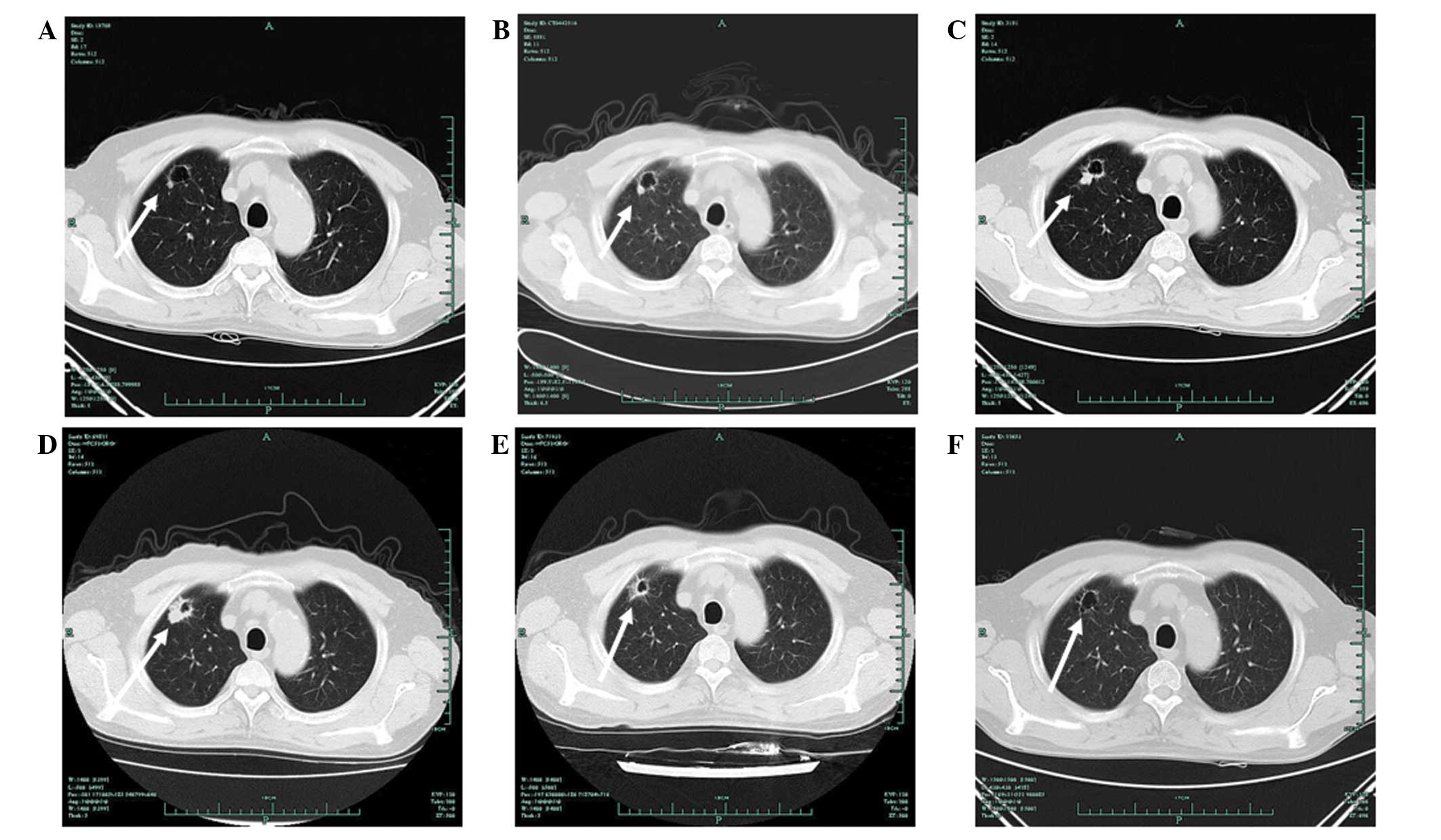
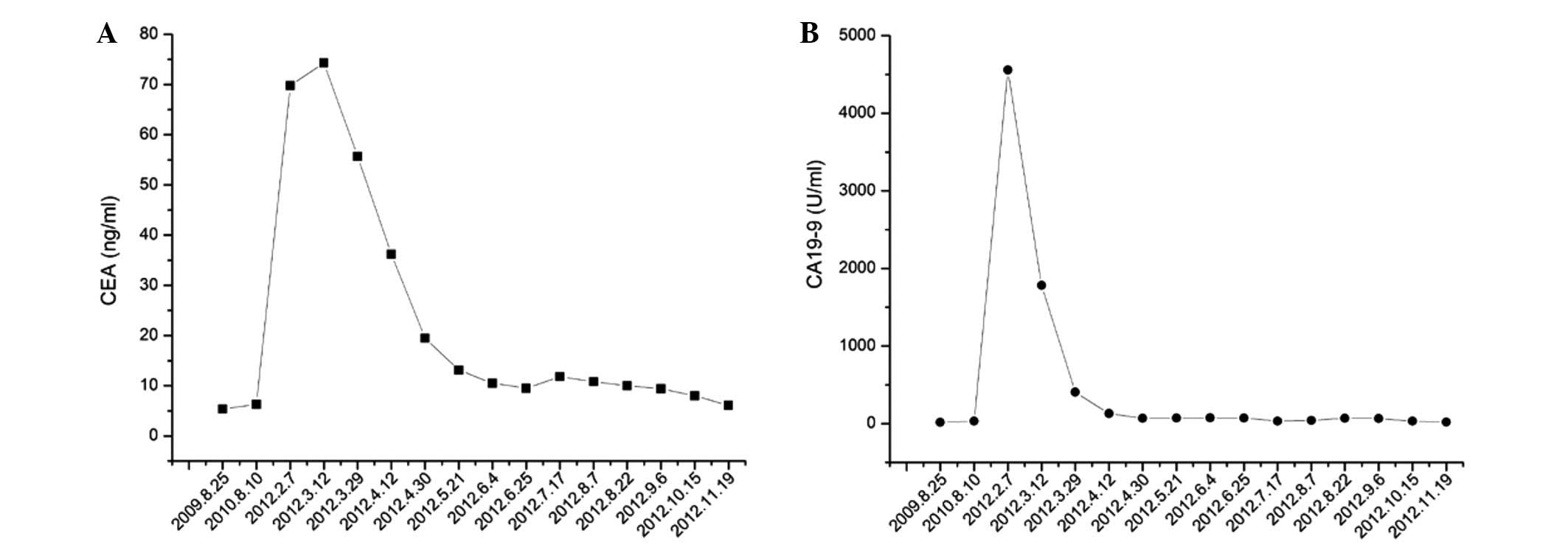
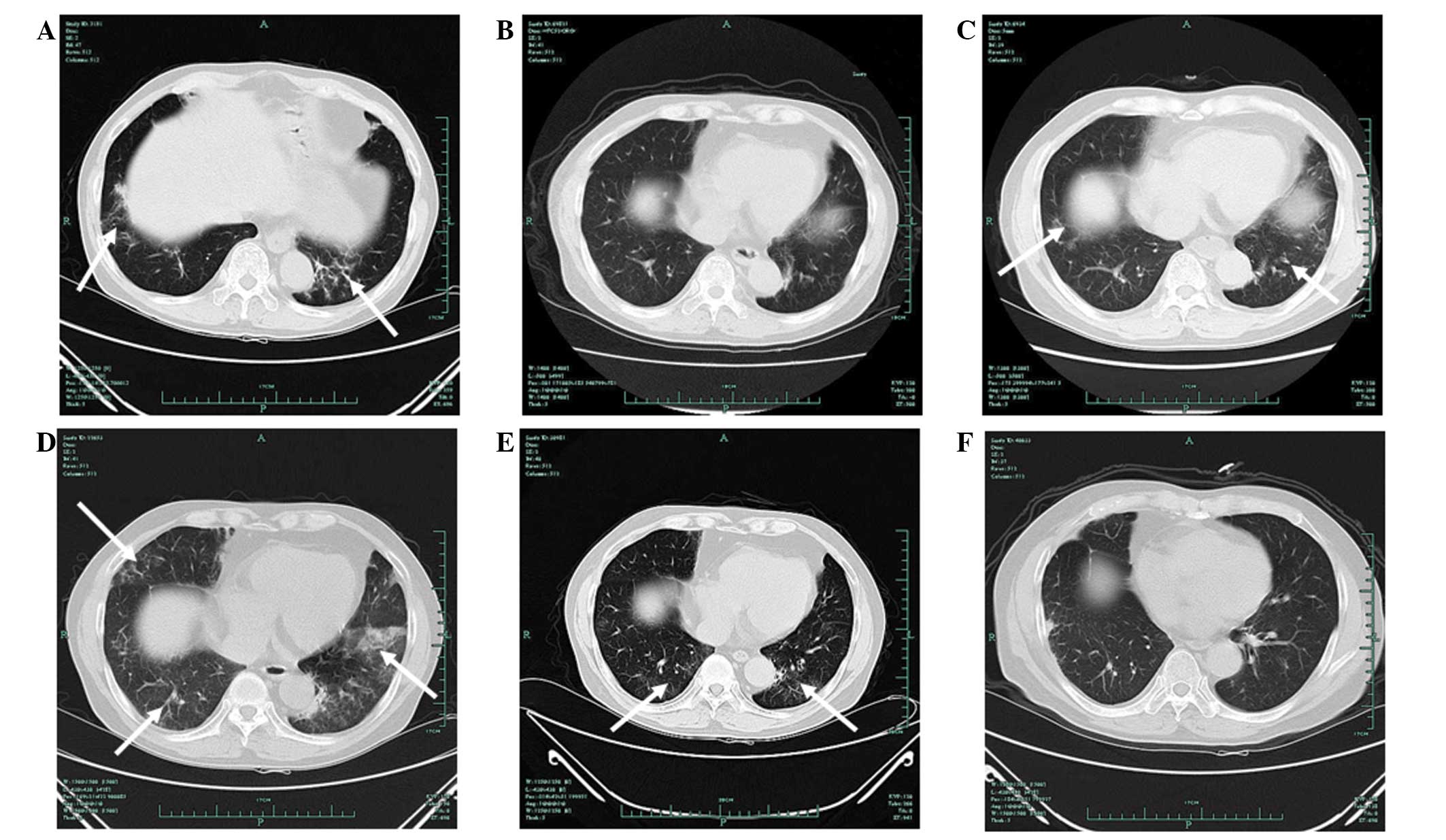
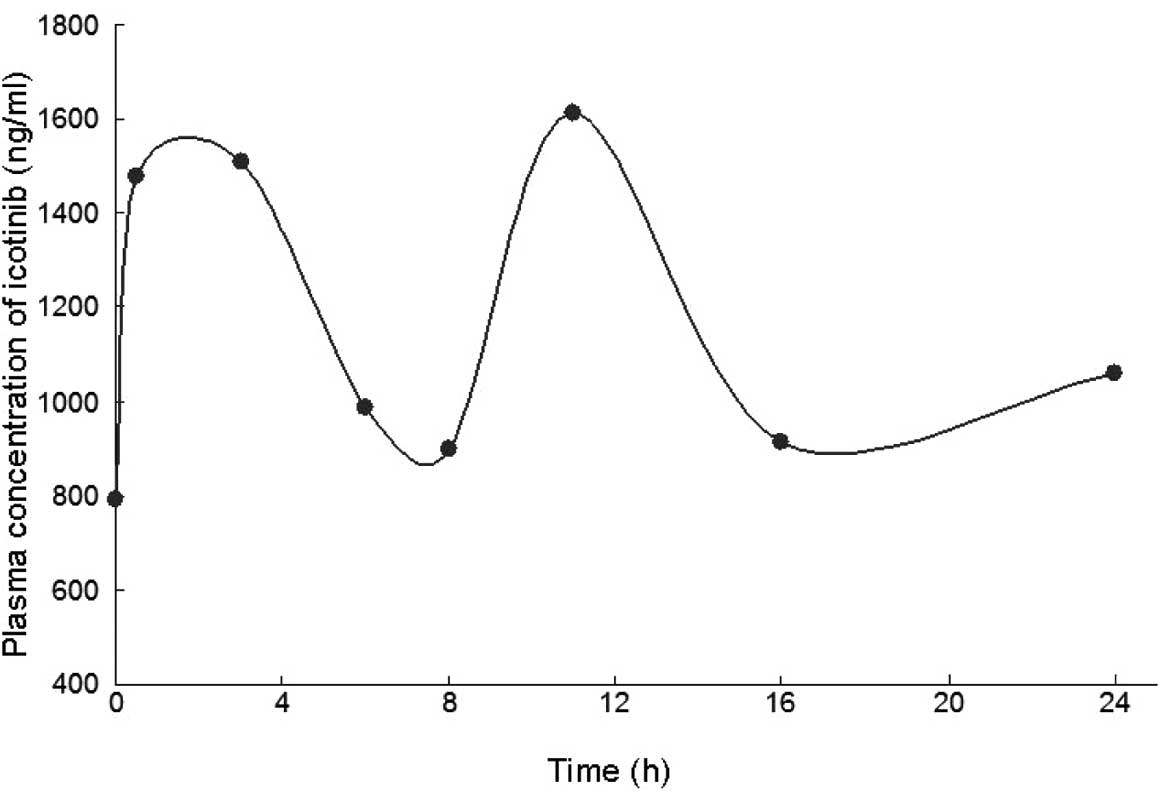
|
1
|
Engels EA, Pfeiffer RM, Fraumeni JF Jr, et
al: Spectrum of cancer risk among US solid organ transplant
recipients. JAMA. 306:1891–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kasiske BL, Snyder JJ, Gilbertson DT and
Wang C: Cancer after kidney transplantation in the United States.
Am J Transplant. 4:905–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar
|
|
4
|
Zhao Q, Shentu J, Xu N, et al: Phase I
study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine
kinase inhibitor, in patients with advanced NSCLC and other solid
tumors. Lung Cancer. 73:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor L, Hughes RA and McPherson K: The
risk of cancer from azathioprine as a treatment for multiple
sclerosis. Eur J Neurol. 11:1412004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kauffman HM, Cherikh WS, McBride MA, Cheng
Y and Hanto DW: Post-transplant de novo malignancies in renal
transplant recipients: the past and present. Transpl Int.
19:607–620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webster AC, Lee VW, Chapman JR and Craig
JC: Target of rapamycin inhibitors (sirolimus and everolimus) for
primary immunosuppression of kidney transplant recipients: a
systematic review and meta-analysis of randomized trials.
Transplantation. 81:1234–1248. 2006. View Article : Google Scholar
|
|
8
|
Campistol JM, Eris J, Oberbauer R, et al:
Sirolimus therapy after early cyclosporine withdrawal reduces the
risk for cancer in adult renal transplantation. J Am Soc Nephrol.
17:581–589. 2006. View Article : Google Scholar
|
|
9
|
Kahan BD, Yakupoglu YK, Schoenberg L, et
al: Low incidence of malignancy among
sirolimus/cyclosporine-treated renal transplant recipients.
Transplantation. 80:749–758. 2005. View Article : Google Scholar
|
|
10
|
Kauffman HM, Cherikh WS, Cheng Y, Hanto DW
and Kahan BD: Maintenance immunosuppression with
target-of-rapamycin inhibitors is associated with a reduced
incidence of de novo malignancies. Transplantation. 80:883–889.
2005. View Article : Google Scholar
|
|
11
|
Barber NA and Ganti AK: Pulmonary
toxicities from targeted therapies: a review. Target Oncol.
6:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singer SJ, Tiernan R and Sullivan EJ:
Interstitial pneumonitis associated with sirolimus therapy in
renal-transplant recipients. N Engl J Med. 343:1815–1816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pham PT, Pham PC, Danovitch GM, et al:
Sirolimus-associated pulmonary toxicity. Transplantation.
77:1215–1220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morelon E, Stern M, Israel-Biet D, et al:
Characteristics of sirolimus-associated interstitial pneumonitis in
renal transplant patients. Transplantation. 72:787–790. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5899. 2005.
View Article : Google Scholar
|
|
16
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
17
|
Gemma A: Drug-induced interstitial lung
diseases associated with molecular-targeted anticancer agents. J
Nippon Med Sch. 76:4–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hotta K, Kiura K, Takigawa N, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
Patients with non-small cell lung cancer: the Okayama Lung Cancer
Study Group experience. J Thorac Oncol. 5:179–184. 2010. View Article : Google Scholar
|
|
19
|
Cohen MH, Williams GA, Sridhara R, et al:
United States Food and Drug Administration Drug Approval summary:
Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 10:1212–1218.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ando M, Okamoto I, Yamamoto N, et al:
Predictive factors for interstitial lung disease, antitumor
response, and survival in non-small-cell lung cancer patients
treated with gefitinib. J Clin Oncol. 24:2549–2556. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hotta K, Kiura K, Tabata M, et al:
Interstitial lung disease in Japanese patients with non-small cell
lung cancer receiving gefitinib: an analysis of risk factors and
treatment outcomes in Okayama Lung Cancer Study Group. Cancer J.
11:417–424. 2005. View Article : Google Scholar
|
|
22
|
Takano T, Ohe Y, Kusumoto M, et al: Risk
factors for interstitial lung disease and predictive factors for
tumor response in patients with advanced non-small cell lung cancer
treated with gefitinib. Lung Cancer. 45:93–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danson S, Blackhall F, Hulse P and Ranson
M: Interstitial lung disease in lung cancer: separating disease
progression from treatment effects. Drug Saf. 28:103–113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki M, Asahina H, Konishi J, Yamazaki K
and Nishimura M: Recurrent gefitinib-induced interstitial lung
disease. Intern Med. 47:533–536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buck E, Eyzaguirre A, Brown E, et al:
Rapamycin synergizes with the epidermal growth factor receptor
inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and
breast tumors. Mol Cancer Ther. 5:2676–2684. 2006. View Article : Google Scholar
|
|
26
|
Costa LJ, Gemmill RM and Drabkin HA:
Upstream signaling inhibition enhances rapamycin effect on growth
of kidney cancer cells. Urology. 69:596–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reardon DA, Desjardins A, Vredenburgh JJ,
et al: Phase 2 trial of erlotinib plus sirolimus in adults with
recurrent glioblastoma. J Neurooncol. 96:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flaig TW, Costa LJ, Gustafson DL, et al:
Safety and efficacy of the combination of erlotinib and sirolimus
for the treatment of metastatic renal cell carcinoma after failure
of sunitinib or sorafenib. Br J Cancer. 103:796–801. 2010.
View Article : Google Scholar
|
|
29
|
Kris MG, Riely GJ, Azzoli CG, et al:
Combined inhibition of mTOR and EGFR with everolimus (RAD001) and
gefitinib in patients with non-small cell lung cancer who have
smoked cigarettes: A phase II trial. J Clin Oncol. 25(Suppl 18):
403S2007.
|
|
30
|
Weir MR, Diekmann F, Flechner SM, et al:
mTOR inhibition: the learning curve in kidney transplantation.
Transpl Int. 23:447–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ajithkumar TV, Parkinson CA, Butler A and
Hatcher HM: Management of solid tumours in organ-transplant
recipients. Lancet Oncol. 8:921–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan F, Shen X, Wang D, et al: Icotinib
(BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays
potent efficacy in preclinical studies. Lung Cancer. 76:177–182.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang GC, Lin JY, Wang Z, et al: Epidermal
growth factor receptor double activating mutations involving both
exons 19 and 21 exist in Chinese non-small cell lung cancer
patients. Clin Oncol (R Coll Radiol). 19:499–506. 2007. View Article : Google Scholar
|
|
34
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|