Introduction
Lung cancer is a growing global health problem and
has become the most common type of cancer that results in mortality
in males and females in developed countries (1). Therapeutic strategies include surgery,
radiotherapy, chemotherapy, and targeted and combined therapies.
Despite advances in treatment, non-small cell lung cancer, which
accounts for 80–85% of all cases of lung cancer (2), remains an aggressive lung cancer with
poor patient survival rates. To date, chemotherapy has been the
most frequently used therapeutic strategy for lung cancer in
advanced stages. However, the outcome of chemotherapy in patients
with advanced lung cancer is poor. The median survival rate of
advanced lung cancer patients treated with standard platinum-based
chemotherapy is ~10 months (3).
Thus, a novel agent for lung cancer therapy is continually being
investigated.
With developments in phytochemistry, an increasing
number of individuals are acknowledging the importance of herbal
plants. Among the 155 small molecular anticancer drugs developed
between the 1940s and June 2006, 47% are natural products or their
derivatives (4). Examples of
plant-based therapeutic anticancer drugs are camptothecin from
Camptotheca acuminate, etoposide from Podophyllum
peltatum, vincristine from Catharanthus roseus and
paclitaxel from yews of the genus Taxus(5,6).
Polygonum cuspidatum, a traditional Chinese
medicinal herb commonly used for its root and rhizome, has been
officially listed in the Pharmacopoeia for a number of years.
3,4,5′-Trihydroxystilbene-3-β-D-mono-D-glucoside [polydatin (PD)],
the chemical structure of which is shown in Fig. 1, is one of the main effective
elements of P. cuspidatum. Previously, pharmacological
studies and clinical practice have demonstrated that PD has a
number of biological functions, such as protective effects against
shock (7–9), ischemia/reperfusion injury (10,11),
congestive heart failure (12) and
endometriosis (13). However, few
previous studies have analyzed the effects of PD on human cancer
cells. In the present study, the effects of PD on the
proliferation, cell cycle phase distribution and apoptosis of human
A549 and NCI-H1975 lung adenocarcinoma cancer cell lines and
potential mechanisms were investigated.
Materials and methods
Chemicals
LKT Laboratories, Inc. (St Paul, MN, USA) was the
supplier of the PD (cat. no. P5845) used. PD was dissolved in a
stock solution of 10 mmol/l dimethysulfoxide (DMSO) and directly
diluted in medium to appropriate concentrations prior to the
experiments. Thiazolyl blue tetrazolium bromide (MTT; cat. no.
M2128) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and
Annexin V-conjugated Alexa Fluor 488 apoptosis detection kits
(V-13245) were obtained from Molecular Probes, Inc. (Eugene, OR,
USA). Primary antibodies against Bcl-2, Bax and cyclin D1 and
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The Bio-Rad protein assay kit II was
supplied by Bio-Rad (Hercules, CA, USA) and the enhanced
chemiluminescent western blot detection reagents (cat. no. RPN2106)
were obtained from Amersham Pharmacia Biotech (Amersham, UK).
Cell lines and cell culture
Cancer cell lines were purchased from American Type
Culture Collection (Manassas, VA, USA). The cells were maintained
as a monolayer in DMEM or RPMI-1640 medium supplemented with 10%
fetal calf serum, 2 mmol/l glutamine, 100 μg/ml streptomycin and
100 U/ml penicillin, in a humidified atmosphere containing 5%
CO2. Cells in the logarithmic phase were used in the
experiments.
MTT viability assay
Determination of cell viability was performed using
an MTT assay as described previously (14). Briefly, cells were incubated in
flat-bottom, 96-well plates (6×103 cells/well)
overnight. Then, cells were treated with DMSO (0.1%) or an
increasing dosage of PD. Following 20, 44 and 68 h of treatment, 20
μl MTT (5 mg/ml) was added to each well and further incubated for 4
h. Cells were then solubilized in 150 μl DMSO. The absorbance
reading was obtained using a Dynatech 96-well spectrophotometer
(Dynatech Laboratories, Chantilly, VA, USA). The amount of MTT dye
reduction was calculated based on the difference between the
absorbances at 570 and 630 nm. The cell viability in treated cells
was expressed as the amount of dye reduction relative to that of
the untreated control cells.
Apoptosis assays and cell cycle
distribution analysis
The percentage of cells that actively underwent
apoptosis was analyzed using Annexin V-phycoerythrin-based
immunofluorescence, as described previously (15). Briefly, the cells were incubated in
six-well plates (2.5×105 cells/well) overnight. The
cells were then treated with DMSO or PD for 48 h. Adherent and
floating cells were collected, washed in cold phosphate-buffered
saline (PBS) twice and stained with Annexin V-PE, according to the
manufacturer’s instructions. Cells were identified using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Cells for cell cycle analysis were washed once with PBS and fixed
in 70% cold ethanol for ≥4 h. The fixed cells were then washed
twice with PBS and resuspended in 500 μl propidium iodide (10
mg/ml) containing 300 μg/ml RNase. Cell cycle distribution was
calculated from 10,000 cells with ModFit LT software (Verity
Software House, Topsham, ME, USA), using FACSCalibur.
Western blot analysis
Western blot analyses were performed as described
previously (16). Cells were
treated with DMSO (0.1%) or PD and, following 48 h of treatment,
were harvested and lysed. The protein concentration in the lysates
was quantified using Bio-Rad Protein Assay reagent (Bio-Rad)
following the manufacturer’s instructions. An equal amount of
protein was separated by electrophoresis on SDS-polyacrylamide gels
(Bio-Rad) and transferred to PVDF membranes (Santa Cruz
Biotechnology, Inc.). Following blocking with 5% non-fat milk, the
membranes were incubated with the desired primary antibodies
overnight at the following dilutions: Anti-Bcl-2, 1:500; anti-Bax,
1:1,000; anti-cyclin D1, 1:1,000; and anti-β-actin, 1:20,000.
Subsequently, the membranes were incubated with appropriate
secondary antibodies. The immunoreactive bands were visualized
using enhanced chemiluminescence, according to the manufacturer’s
instructions.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed by multifactorial analysis of
variance using SPSS software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
PD has a wide anticancer spectrum and is
more potent in eliminating cancer cells than non-cancer cells
The cytotoxicity of PD in 10 cancer cell lines was
first determined by MTT assay. The decrease in absorbance in this
assay was due to cell death or reduction in cell proliferation. As
shown in Table I, PD exhibits
broad-spectrum growth inhibition against 10 cancer cell lines. A
dose-dependent and time-dependent inhibition of human lung cancer
cells was shown (Figs. 2A and B).
By comparing the effects of PD in inhibiting cell growth between
cancer and non-cancer cells (Fig.
2C), it was found that 6 μmol/l PD caused 65% (48 h) loss of
cell viability in A549 lung cancer cells and 66% (48 h) loss in
NCI-H1975 lung cancer cells. However, at the same concentration,
loss of cell viability in human bronchial epithelial (HBE) cells
derived from normal HBE cells was 28% (48 h). This result indicated
that PD is more potent in eliminating cancer cells than non-cancer
cells.
 | Table IIC50 values of PD in
various cancer cell lines. |
Table I
IC50 values of PD in
various cancer cell lines.
| Cell lines | IC50 (48
h) |
|---|
| Lung cancer |
| A549 | 2.95±0.37 |
| NCI-H1975 | 3.23±0.46 |
| Breast cancer |
| MDA-MB-231 | 2.66±0.73 |
| MCF-7 | 1.49±0.26 |
| Cervical cancer |
| Hela | 2.13±0.52 |
| Ovarian cancer |
| SKOV-3 | 4.44±0.89 |
| Liver cancer |
| SMMC-7721 | 2.43±0.27 |
| Nasopharyngeal
cancer |
| CNE-1 | 5.62±1.28 |
| Leukemia |
| HL-60 | 1.63±0.91 |
| K562 | 1.91±0.37 |
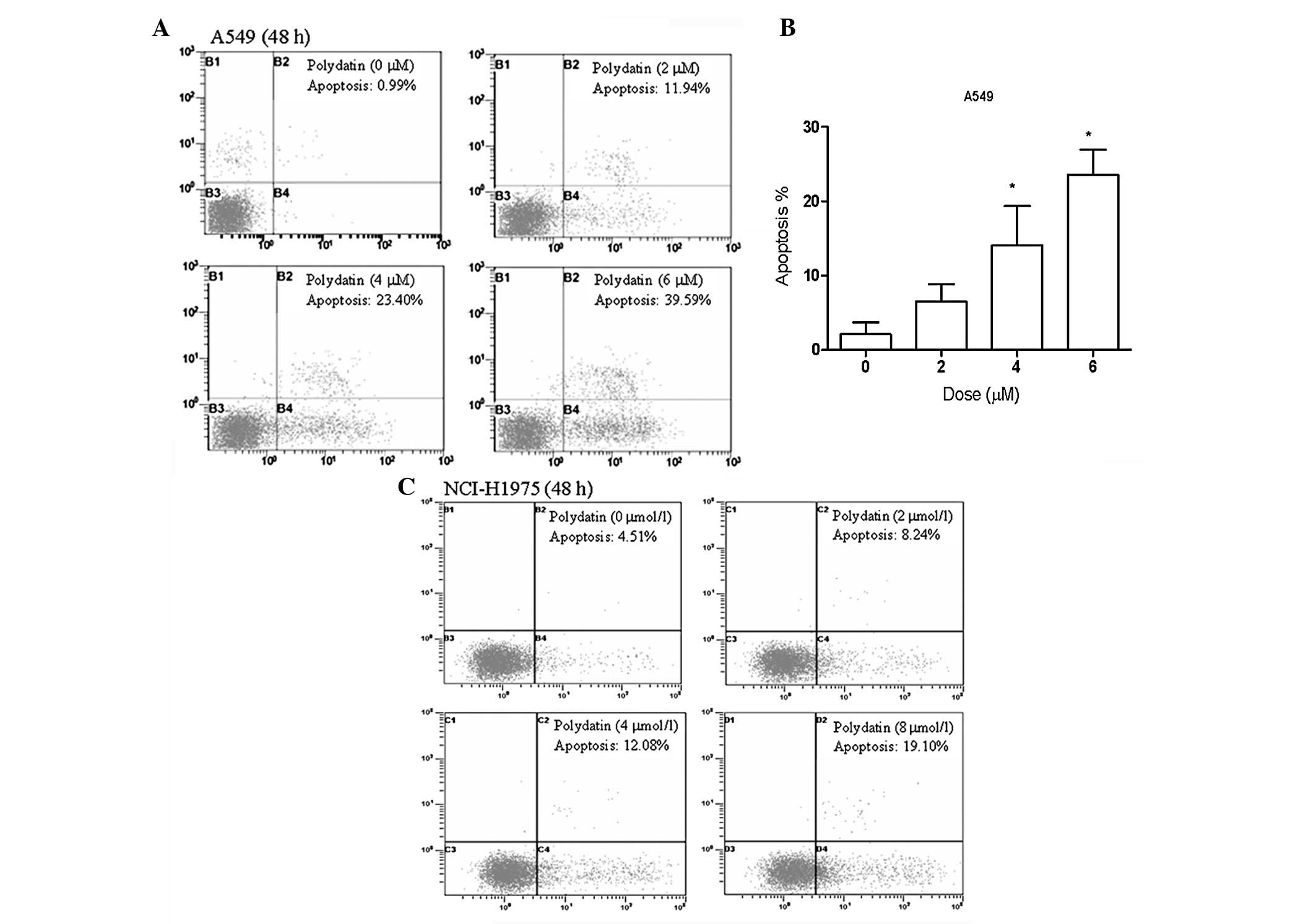
PD induces apoptosis in lung cancer
cells
To investigate the features of PD-induced lung
cancer cell growth inhibition, A549 and NCI-H1975 lung cancer cells
were treated with various concentrations of PD for 48 h.
Subsequently, apoptosis was detected by flow cytometry. As shown in
Figs. 3A and B, PD activates
apoptosis in A549 lung cancer cells in a dose-dependent manner. The
percentage of cells undergoing apoptotic cell death increased from
0.99% in the control culture to 39.5% following exposure to 6
μmol/l PD for 48 h in A549 lung cancer cells. Similar results were
observed in NCI-H1975 cell lines (Fig.
3C).
PD induces S-phase cell cycle arrest in
lung cancer cells
To determine whether interference with cell cycle
progression is mediated by the PD-based growth inhibition of lung
cancer cells, the effects of PD on cell cycle progression were
examined in an exponentially dividing culture of A549 and NCI-H1975
cells. The treatment of cells with varying concentrations of PD for
48 h resulted in the increased accumulation of cells in the S phase
and a corresponding decrease in the G0/G1 and G2/M phases. PD at a
concentration of 6 μmol/l increased the S phase population from
19.91±2.34 to 31.71±1.83% in A549 cells and from 21.41±8.72 to
36.37±3.56% in NCI-H1975 cells (Fig.
4). The typical flow histogram of sub-G1 apoptotic peaks was
also detected.
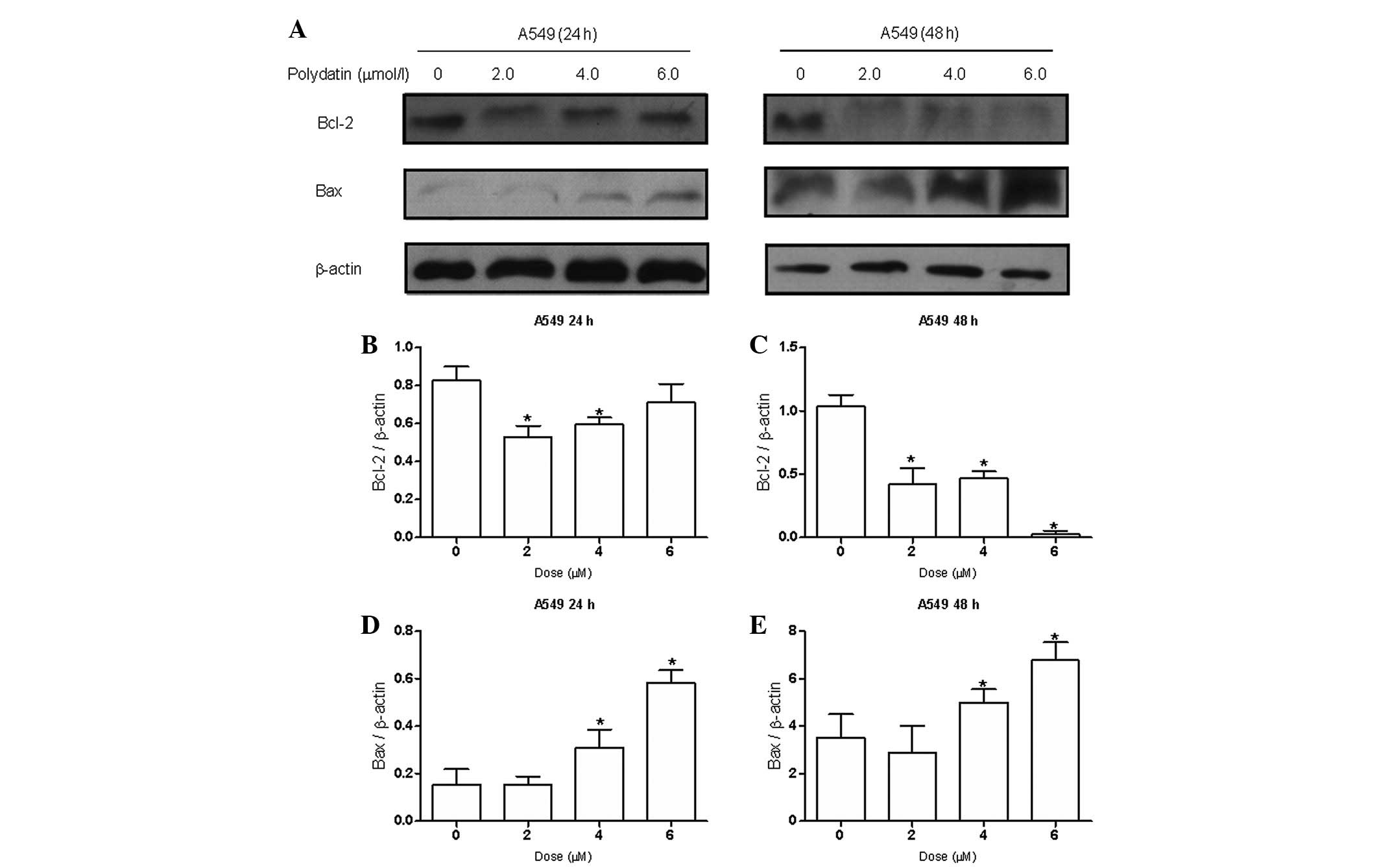
PD downregulates Bcl-2 and cyclin D1 and
upregulates Bax expression in lung cancer cell lines
Due to the effects of PD on apoptosis, the impact of
PD on the expression of Bcl-2 and Bax, two key apoptosis regulatory
proteins, were examined by western blot analysis. The results
indicated (Fig. 5) that PD
dose-dependently downregulated the expression of antiapoptotic
protein Bcl-2 and upregulated the expression of proapoptotic
protein Bax. Following treatment with 6 μmol/l PD, the Bax/Bcl-2
ratio, which favors apoptosis (17), increased significantly in the A549
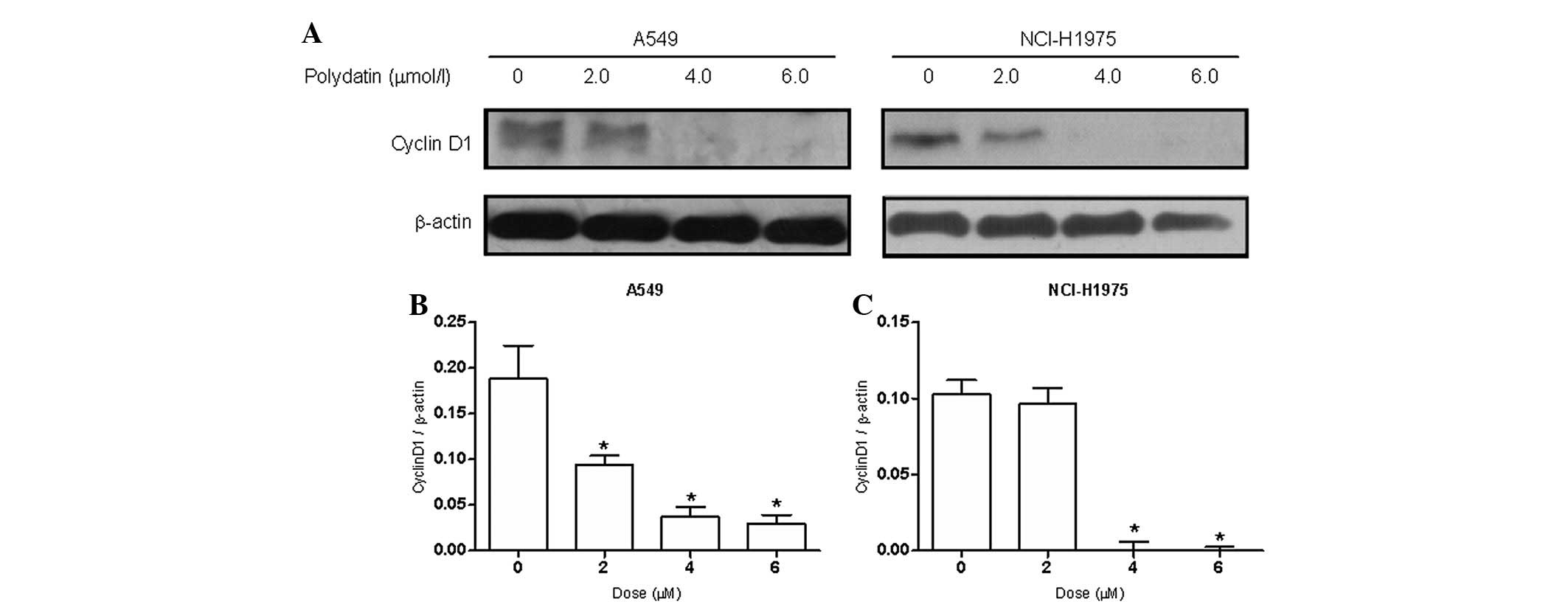
cells. To explore the mechanism of the effects of PD on S phase
cell cycle arrest, the expression levels of cell cycle-related
protein cyclin D1 were examined. The results showed (Fig. 6) that the expression of protein
cyclin D1 decreased significantly following the treatment of A549
and NCI-H1975 cells with PD for 48 h.
Discussion
PD is a glycoside of resveratrol, in which the
glycoside group is bonded in the C-3 position, substituting a
hydroxyl group. This substitution leads to conformational changes
in the molecule, resulting in changes in its biological properties.
PD is more efficiently absorbed (18,19)
and more resistant to enzymatic oxidation than resveratrol
(20) and is soluble in hot water.
In contrast to resveratrol, which penetrates cells passively, PD
enters cells via an active mechanism using glucose carriers
(21). These properties provide PD
with greater bioavailability than resveratrol.
Previous studies have demonstrated the
chemopreventive and anticancer activities of resveratrol (22–31).
However, little is known concerning the antitumor activity of PD.
For the first time, the current study examined the cytotoxic effect
of PD in various cancer cell lines and PD was found to have potent
growth inhibitory effects on leukemia, breast, lung, cervical,
ovarian, liver and nasopharyngeal cancer cells. In particular, PD
had less toxicity to non-neoplastic HBE cells. This suggests that
PD may be a potent chemotherapeutic agent.
Apoptosis, also known as programmed cell death, is
morphologically characterized by cell shrinkage, membrane
remodeling, cell blebbing, chromatin condensation and DNA
fragmentation with apoptotic bodies (32). Apoptosis activation is considered to
be a good target in cancer therapies (33,34). A
number of anticancer drugs act through the induction of apoptosis
to prevent tumor promotion and progression. In general, apoptosis
is regulated by proapoptotic and antiapoptotic proteins of the
Bcl-2 family, and is executed through caspases (or
cysteine-aspartic proteases). The induction of apoptosis in tumor
cells has been proposed to result from the inability of Bcl-2 to
form heterodimers with Bax. Bax overexpression increases the
sensitivity of cells to anticancer drugs due to the lack of Bcl-2
in the cell. An increase in the ratio of Bax/Bcl-2 stimulates the
release of cytochrome c from the mitochondria into the
cytosol, which leads to the activation of caspase-3 (35,36).
The results of the present study showed that PD induces apoptosis
in lung cancer cells effectively. The induction of apoptosis was
accompanied by an increase in Bax expression and a decrease in
Bcl-2 expression. The results support the development of PD for
lung cancer prevention and treatment.
The control of cell cycle progression in cancer
cells is a potentially effective strategy to arrest tumor growth
(37,38). Cyclin D1, an important regulator of
cell cycle progression, functions as a transcriptional coregulator
(39). Overexpression of cyclin D1
has been described in a wide spectrum of human cancer types, such
as breast, lung, liver and brain cancer (40–42).
Cyclin D1 levels must be high during the G1 phase to initiate DNA
synthesis, but must be suppressed to low levels during the S phase
for efficient DNA synthesis. To continue cell proliferation, cyclin
D1 must be induced once again during the G2 phase (43). The in vitro results of the
current study indicated that the treatment of A549 and NCI-H1975
cells with PD results in the S-phase arrest of cell cycle
progression. Western blot analysis showed that the expression level
of cyclin D1 was inhibited, whereas, cyclin A, B1 and E expression
levels were not affected (data not shown). These results suggest
that PD inhibits the proliferation of cancer cells by inhibition of
cyclin D1 expression, thereby, reducing cell cycle progression by
arresting the cells at S phase.
The present study performed a preliminary
investigation of the inhibitory effect of PD on lung cancer cells.
The antiproliferation effect of PD involves the suppression of cell
cycle progression and induction of apoptosis in human lung cancer
cells. Apoptosis was initiated by upregulating Bax levels together
with downregulating Bcl-2 levels. However, the anti-tumor effect
and toxicity of PD in vivo is unknown. Future studies on the
in vivo effect of PD are necessary. Current investigations
on the mechanism and the in vivo anticancer efficacy of PD
are in progress.
Acknowledgements
The current study was supported by grants from the
National Natural Science Foundation of China (nos. 81071906 and
81172127), Suzhou Key Laboratory of Radiation Oncology (no.
SZS0802), Suzhou Science and Technology Program (SYS201345) and the
Priority Academic Program Development (PAPD) of Jiangsu Higher
education institutions.
References
|
1
|
Spiro SG, Tanner NT, Silvestri GA, et al:
Lung cancer: progress in diagnosis, staging and therapy.
Respirology. 15:44–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stinchcombe TE, Fried D, Morris DE and
Socinski MA: Combined modality therapy for stage III non-small cell
lung cancer. Oncologist. 11:809–823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luan J, Duan H, Liu Q, Yagasaki K and
Zhang G: Inhibitory effects of norcantharidin against human lung
cancer cell growth and migration. Cytotechnology. 62:349–355. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007.PubMed/NCBI
|
|
5
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
|
|
6
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao KS, Jin C, Huang X, et al: The
mechanism of Polydatin in shock treatment. Clin Hemorheol
Microcirc. 29:211–217. 2003.PubMed/NCBI
|
|
8
|
Wang X, Song R, Chen Y, Zhao M and Zhao
KS: Polydatin - a new mitochondria protector for acute severe
hemorrhagic shock treatment. Expert Opin Investig Drugs.
22:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Song R, Bian HN, Brunk UT, Zhao M
and Zhao KS: Polydatin, a natural polyphenol, protects arterial
smooth muscle cells against mitochondrial dysfunction and lysosomal
destabilization following hemorrhagic shock. Am J Physiol Regul
Integr Comp Physiol. 302:R805–R814. 2012. View Article : Google Scholar
|
|
10
|
Cheng Y, Zhang HT, Sun L, et al:
Involvement of cell adhesion molecules in polydatin protection of
brain tissues from ischemia-reperfusion injury. Brain Res.
1110:193–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miao Q, Wang S, Miao S, Wang J, Xie Y and
Yang Q: Cardioprotective effect of polydatin against
ischemia/reperfusion injury: roles of protein kinase C and mito
K(ATP) activation. Phytomedicine. 19:8–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao JP, Chen CX, Gu WL, Wu Q, Wang Y and
Lü J: Effects of polydatin on attenuating ventricular remodeling in
isoproterenol-induced mouse and pressure-overload rat models.
Fitoterapia. 81:953–960. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Indraccolo U and Barbieri F: Effect of
palmitoylethanolamide-polydatin combination on chronic pelvic pain
associated with endometriosis: preliminary observations. Eur J
Obstet Gynecol Reprod Biol. 150:76–79. 2010. View Article : Google Scholar
|
|
14
|
Fan S, Wang JA, Yuan RQ, et al: BRCA1 as a
potential human prostate tumor suppressor: modulation of
proliferation, damage responses and expression of cell regulatory
proteins. Oncogene. 16:3069–3082. 1998. View Article : Google Scholar
|
|
15
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
16
|
Fan S, Gao M, Meng Q, et al: Role of
NF-kappaB signaling in hepatocyte growth factor/scatter
factor-mediated cell protection. Oncogene. 24:1749–1766. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu JY, Meng QH, Chong Y, et al:
Sanguinarine inhibits growth of human cervical cancer cells through
the induction of apoptosis. Oncol Rep. 28:2264–2270.
2012.PubMed/NCBI
|
|
18
|
Hollman PC, de Vries JH, van LSD,
Mengelers MJ and Katan MB: Absorption of dietary quercetin
glycosides and quercetin in healthy ileostomy volunteers. Am J Clin
Nutr. 62:1276–1282. 1995.PubMed/NCBI
|
|
19
|
Paganga G and Rice-Evans CA: The
identification of flavonoids as glycosides in human plasma. FEBS
Lett. 401:78–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Regev-Shoshani G, Shoseyov O, Bilkis I and
Kerem Z: Glycosylation of resveratrol protects it from enzymic
oxidation. Biochem J. 374:157–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krasnow MN and Murphy TM: Polyphenol
glucosylating activity in cell suspensions of grape (Vitis
vinifera). J Agric Food Chem. 52:3467–3472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903. 2002.
|
|
24
|
Rezk YA, Balulad SS, Keller RS and Bennett
JA: Use of resveratrol to improve the effectiveness of cisplatin
and doxorubicin: study in human gynecologic cancer cell lines and
in rodent heart. Am J Obstet Gynecol. 194:e23–e26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu PL, Tsai JR, Charles AL, et al:
Resveratrol inhibits human lung adenocarcinoma cell metastasis by
suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway
and subsequently downregulating expression of matrix
metalloproteinases. Mol Nutr Food Res. 54(Suppl 2): S196–S204.
2010. View Article : Google Scholar
|
|
26
|
Shibata MA, Akao Y, Shibata E, et al:
Vaticanol C, a novel resveratrol tetramer, reduces lymph node and
lung metastases of mouse mammary carcinoma carrying p53 mutation.
Cancer Chemother Pharmacol. 60:681–691. 2007. View Article : Google Scholar
|
|
27
|
Liu HS, Pan CE, Yang W and Liu XM:
Antitumor and immunomodulatory activity of resveratrol on
experimentally implanted tumor of H22 in Balb/c mice. World J
Gastroenterol. 9:1474–1476. 2003.PubMed/NCBI
|
|
28
|
Zhou HB, Chen JJ, Wang WX, Cai JT and Du
Q: Anticancer activity of resveratrol on implanted human primary
gastric carcinoma cells in nude mice. World J Gastroenterol.
11:280–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan MH, Gao JH, Lai CS, et al: Antitumor
activity of 3,5,4′-trimethoxystilbene in COLO 205 cells and
xenografts in SCID mice. Mol Carcinog. 47:184–196. 2008.
|
|
30
|
Li T, Fan GX, Wang W, Li T and Yuan YK:
Resveratrol induces apoptosis, influences IL-6 and exerts
immunomodulatory effect on mouse lymphocytic leukemia both in vitro
and in vivo. Int Immunopharmacol. 7:1221–1231. 2007. View Article : Google Scholar
|
|
31
|
Chen JC, Chen Y, Lin JH, Wu JM and Tseng
SH: Resveratrol suppresses angiogenesis in gliomas: evaluation by
color Doppler ultrasound. Anticancer Res. 26:1237–1245.
2006.PubMed/NCBI
|
|
32
|
Wyllie AH: Apoptosis: an overview. Br Med
Bull. 53:451–465. 1997. View Article : Google Scholar
|
|
33
|
Neto CC, Amoroso JW and Liberty AM:
Anticancer activities of cranberry phytochemicals: an update. Mol
Nutr Food Res. 52(Suppl 1): S18–S27. 2008.PubMed/NCBI
|
|
34
|
Kaur M and Agarwal R: Transcription
factors: molecular targets for prostate cancer intervention by
phytochemicals. Curr Cancer Drug Targets. 7:355–367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
38
|
Pavletich NP: Mechanisms of
cyclin-dependent kinase regulation: structures of Cdks, their
cyclin activators, and Cip and INK4 inhibitors. J Mol Biol.
287:821–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alao JP: The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gillett C, Smith P, Gregory W, et al:
Cyclin D1 and prognosis in human breast cancer. Int J Cancer.
69:92–99. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hall M and Peters G: Genetic alterations
of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human
cancer. Adv Cancer Res. 68:67–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Molenaar JJ, Ebus ME, Koster J, et al:
Cyclin D1 and CDK4 activity contribute to the undifferentiated
phenotype in neuroblastoma. Cancer Res. 68:2599–2609. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang K, Hitomi M and Stacey DW: Variations
in cyclin D1 levels through the cell cycle determine the
proliferative fate of a cell. Cell Div. 1:322006. View Article : Google Scholar : PubMed/NCBI
|