Introduction
Intradermal melanocytic nevi are common, benign,
pigmented skin tumors formed by proliferation of dermal
melanocytes. A number of notable, uncommon changes may be observed
in intradermal melanocytic nevi. In particular, their association
with lymphatic invasion is an extremely rare phenomenon. To date,
only two cases of an intradermal melanocytic nevus with lymphatic
invasion have been described in the literature (1,2).
The observation of nevus cell aggregates in the
lymph nodes has been of practical and academic significance
(3), and various hypotheses have
been presented to explain their origin. The current study presents
a case in which an aggregate of nevus cells was observed within a
lymphatic vessel of the upper dermis. The observation of a
lymphatic nevus cell embolus supports the hypothesis that the nevus
cells are likely to be transferred, via lymphatics, from a
cutaneous nevus to the draining lymph node.
Case report
A 26-year-old male presented to the Department of
Dermatology (Aerospace Medical Center, Republic of Korea Air Force,
Cheongju, Korea) with a slowly enlarging nodule on the back. The
patient had no personal or family history of malignant melanoma. A
physical examination revealed a brown-colored, pea-sized cutaneous
nodule on the back. A clinical diagnosis of melanocytic nevus was
determined and the lesion was excised with adjacent skin. For light
microscopic examination, the tissue was immediately preserved in
10% buffered formalin. Following 48 h of formalin fixation, the
specimen was embedded in paraffin, routinely processed and stained
with hematoxylin and eosin.
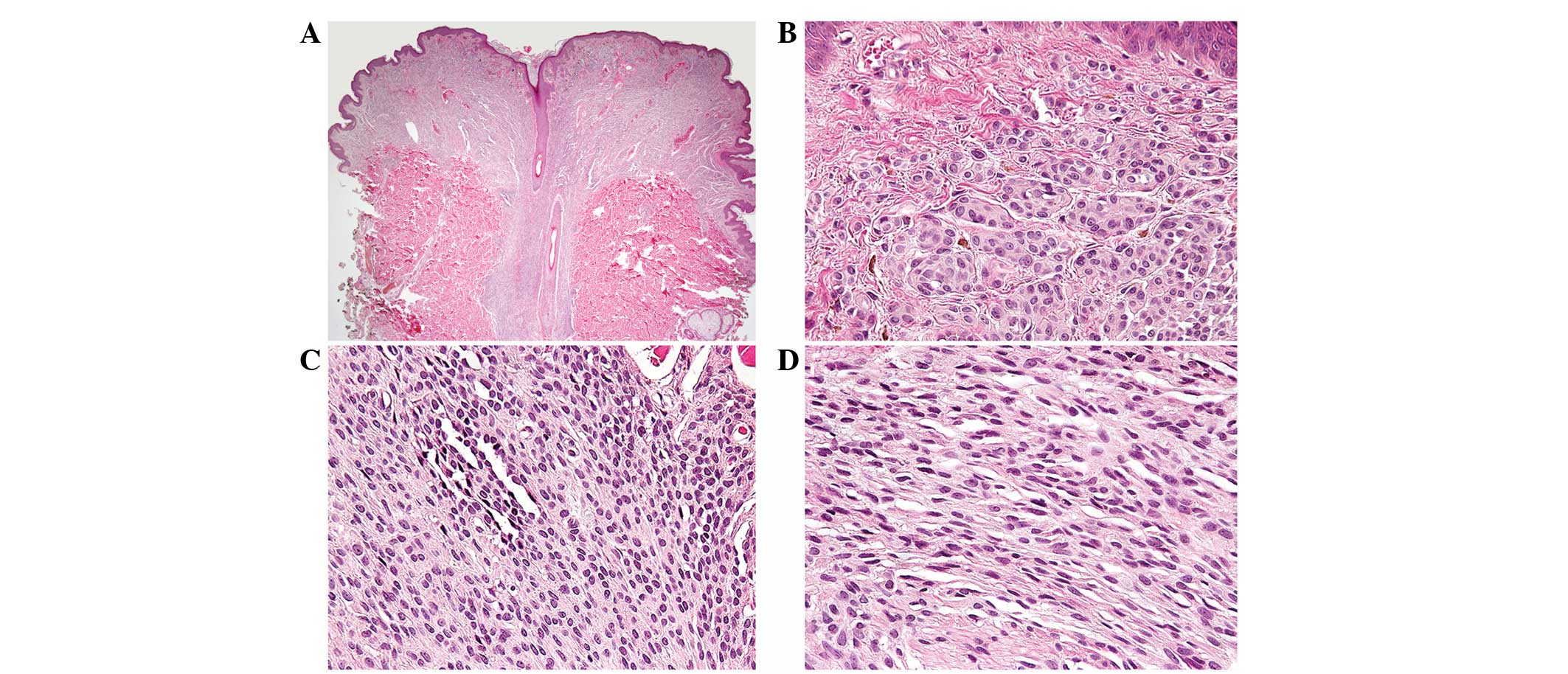
Grossly, the resected specimen consisted of
elliptical skin with a pigmented nodule measuring 1.0 cm in
diameter and 0.3 cm in height. Fig.
1A illustrates a scanning view of the lesion. Histologically,
the lesional cells in the upper, middle and lower dermis presented
characteristic morphological features of types A, B and C nevus
cells, respectively. The type A nevus cells (Fig. 1B) in the upper dermis were
round-to-cuboidal, showed voluminous cytoplasm containing variable
amounts of melanin granules and formed nests. Normal epidermis
overlying a nest of nevus cells was identified and junctional
activity was absent. The type B nevus cells (Fig. 1C) in the mid-dermis, which were
distinctly smaller than the type A nevus cells, were arranged in
well-defined aggregates or cords and contained less cytoplasm and
melanin. The type C nevus cells (Fig.
1D) in the lower dermis were elongated and possessed
spindle-shaped nuclei. The cells were arranged in strands and
rarely contained melanin. The decrease in cell size and
melanization and the progression from nests to cords to more
neuroidal spindle cells with dermal descent, often referred to as
maturation, were observed. No mitotic figures were identified.
These observations were typical of an intradermal melanocytic
nevus; the most notable feature of this nevus, however, was an
aggregate of nevus cells within a lymphatic vessel of the upper
dermis (Fig. 2A). The large lumen
of this lymphatic vessel contained proteinaceous fluid without red
blood cells. By contrast, adjacent small blood vessels contained
red blood cells and exhibited round, narrow lumina. The nevus cells
observed within the lymphatic lumen demonstrated characteristic
morphological features of type A nevus cells. The cells were
round-to-cuboidal, showed well-defined cell borders and abundant
cytoplasm and formed nests. In addition, the nevus cell aggregate
was lined by flattened endothelial cells, identical to the
endothelial cells lining the lymphatic lumen (Fig. 2B). Based on these observations, this
aggregate of nevus cells was considered to be a lymphatic nevus
cell embolus. No evidence of dysplastic change or malignancy was
observed. Serial sections for additional slides were performed to
confirm the lymphatic origin of the endothelial cells using
immunohistochemistry, but the nevus cell aggregate was no longer
detectable. The patient provided written informed consent.
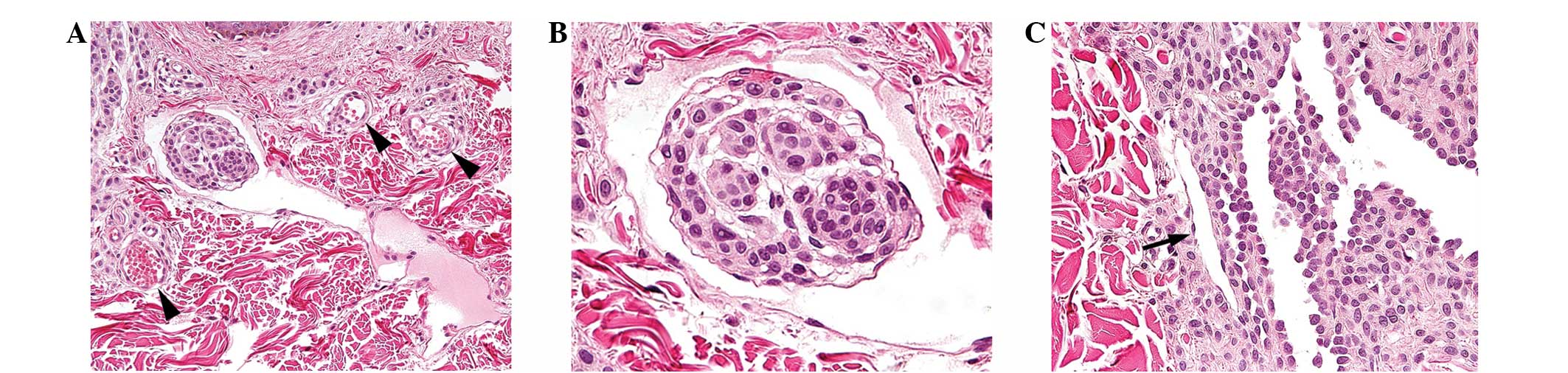 | Figure 2(A) An aggregate of nevus cells was
observed within a lymphatic vessel of the upper dermis. In contrast
to adjacent small blood vessels with red blood cells and small,
round lumina, indicated by the black arrowheads, the lymphatic
vessel contained proteinaceous fluid without red blood cells and
showed a large lumen (hematoxylin and eosin; magnification, ×100).
(B) The lymphatic nevus cell embolus demonstrated characteristic
morphological features of type A nevus cells; a round to cuboidal
shape, well-defined cell borders, abundant cytoplasm and nest
formation. In addition, it was lined by flattened endothelial
cells, identical to the endothelial cells lining the lymphatic
lumen (hematoxylin and eosin; magnification, ×400). (C) A clear
distinction in morphology was identified between a true lymphatic
vessel, indicated by the black arrow and the pseudovascular spaces
formed by nevus cells, indicated in the right half of the image.
The lumina of the pseudovascular spaces anastomosed irregularly
with each other and were lined by nevus cells with cuboidal
contours and round-to-oval nuclei, and not by the vascular
endothelial cells with flattened nuclei and greatly attenuated
cytoplasm. Moreover, the nevus cells formed intraluminal papillary
projections and specific floating individual nevus cells were also
present (hematoxylin and eosin; magnification, ×200). |
Discussion
Prior to the diagnosis of a lymphatic nevus cell
embolus in the present study, the possibility of pseudovascular
space being misidentified as a lymphatic vessel was considered.
Melanocytic nevi are commonly associated with clefts or slits of
nests, resembling lymphatic or vascular spaces. These
pseudovascular spaces have been mainly identified as artifacts of
tissue processing and may simulate the lymphatic invasion of
malignant melanoma. In the present case, the vascular channel
exhibiting a nevus cell embolus contained no red blood cells,
showed a large lumen and was lined by a single layer of flattened
endothelial cells, indicating a true lymphatic vessel. In addition,
Fig. 2C (obtained from the slide
that exhibited a lymphatic nevus cell embolus) clearly demonstrates
the morphological differences between a true lymphatic vessel and
pseudovascular space formed by nevus cells. The lumina of the
latter showed intraluminal papillary projections and a few floating
nevus cells. The lumina anastomosed irregularly with each other and
were lined by nevus cells with cuboidal contours and round-to-oval
nuclei, and not by vascular endothelial cells with flattened nuclei
and greatly attenuated cytoplasm. These observations clearly
identified the space as a true lymphatic vessel and excluded the
possibility of a pseudovascular space.
Since the first description in 1931 (3), there have been a number of cases of
benign nevus cells within lymph nodes reported in the literature
(3–8). However, the histogenesis of this
lesion remains unclear. The prevailing theories concerning the
mechanism by which nevus cells become incorporated into lymph nodes
include arrested migration of neural crest progenitor cells during
embryonic development (5,8) and the transfer of nevus cell emboli,
via lymphatics, from a cutaneous nevus to the draining regional
lymph node, also termed mechanical transport or benign metastasis
(8,9).
The following are among the observations that
favored arrested migration (10):
a) The concurrence of embryonic migration of melanocytic precursors
and development of the lymphatic system (11); b) the presence of a blue nevus in
non-cutaneous sites, such as the uterine cervix, vagina, prostate,
spermatic cord and seminal vesicles (2,8); c)
the usual capsular location with sparing of the sinuses; d) the
rarity of observing metastatic melanoma and nevus cells in the same
lymph node (9); and e) the common
observation of congenital nevi in association with nodal nevus cell
aggregates, indicating that anomalous migration is responsible for
the nodal and cutaneous observations (11). Moreover, the morphological pattern
of the distribution of nevus cells in nests and strands in the
collagenous capsule and trabeculae of lymph nodes is distinct from
the occupation of the subcapsular sinus observed in metastatic
disease.
However, certain aspects of the association provide
equally compelling support for the mechanical transport theory,
including the following arguments (10): a) there are examples of intranodal
deposits of other tissues, such as endometrium, endosalpinx and
breast epithelium; b) nevus cells are not found in lymph nodes
draining non-cutaneous sites (9);
c) non-cutaneous nevus cell aggregates are found only in
association with lymph nodes (12);
d) melanocytes arrested in their migration through the dermis are
bipolar, whereas, in the majority of cases, nevus cells in lymph
nodes have the oval or cuboidal contours encountered in
conventional cutaneous melanocytic nevi (13); and e) nevus cell clusters are found
within cutaneous lymphatics and in afferent lymphatics of lymph
nodes (1,2,11,14),
which are not known to have a role in the embryonic migration of
neural crest-derived cells. Consistent with the observations of two
previous cases (1,2), an endosalpinx aggregate of benign
nevus cells in association with intradermal melanocytic nevus was
observed in the present case report. The histological findings may
be regarded as support for the mechanical transport theory.
While the observation made in the current study
contributes a piece to the puzzle of nodal nevi, it does not yield
definitive information concerning the mechanism underlying this
phenomenon. Considerable research is required prior to being able
to fully understand the process of nodal involvement by nevus
cells. However, it is clear that such cases require investigation
with great care, and considerable caution must be exercised in
interpreting the results. Moreover, it is essential that
pathologists educate surgeons and oncologists with regard to the
occurrence of nevus cell aggregates in lymphatics and lymph
nodes.
In conclusion, the current report presents a unique
case of an intralymphatic nevus cell aggregate in association with
an intradermal melanocytic nevus. To the best of our knowledge, the
current report presents the third case of a lymphatic nevus cell
embolus observed in an intradermal melanocytic nevus. The
histological observation presented may be regarded as support for
the mechanical transport theory, which posits lymphatic transfer of
nevus cell emboli from a cutaneous nevus to the draining regional
lymph node. This observation must not be misinterpreted as evidence
of malignancy, but must be assessed in context with the associated
histological features of the lesion.
Acknowledgements
The authors would like to thank the medical
librarian Ja Ok Kim of the Aerospace Medical Library (Aerospace
Medical Center, Republic of Korea Air Force) for her assistance in
searching for literature.
The views and opinions expressed in this article are
those of the authors and do not reflect the official policy or
position of the Republic of Korea Air Force or Republic of Korea
Ministry of National Defense.
References
|
1
|
Katsumata M, Matsunaga T, Maruyama R and
Ezoe K: Lymphatic invasion of nevus cells observed in intradermal
nevus. J Dermatol. 17:264–265. 1990.PubMed/NCBI
|
|
2
|
Subramony C and Lewin JR: Nevus cells
within lymph nodes: possible metastases from a benign intradermal
nevus. Am J Clin Pathol. 84:220–223. 1985.
|
|
3
|
Stewart FW and Copeland MM: Neurogenic
sarcoma. Am J Cancer. 15:12351931.
|
|
4
|
Erlandson RA and Rosen PP: Electron
microscopy of a nevus cell aggregate associated with axillary lymph
node. Cancer. 49:269–272. 1982. View Article : Google Scholar
|
|
5
|
Hart WR: Primary nevus of lymph node. Am J
Clin Pathol. 55:88–92. 1971.
|
|
6
|
McCarthy SW, Palmer AA, Bale PM and Herst
E: Nevus cells in lymph nodes. Pathology. 6:351–358. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ridolfi RL, Rosen PP and Thaler H: Nevus
cell aggregates associated with lymph nodes: estimated frequency
and clinical significance. Cancer. 39:164–171. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson WT and Helwig EB: Benign nevus
cells in the capsule of lymph nodes. Cancer. 23:747–753. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carson KF, Wen DR, Li PX, et al: Nodal
nevi and cutaneous melanomas. Am J Surg Pathol. 20:834–840. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patterson JW: Nevus cell aggregates in
lymph nodes. Am J Clin Pathol. 121:13–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fontaine D, Parkhill W, Greer W and Walsh
N: Nevus cells in lymph nodes. Am J Dermatopathol. 24:1–5. 2002.
View Article : Google Scholar
|
|
12
|
Bautista NC, Cohen S and Anders KH: Benign
melanocytic nevus cells in axillary lymph nodes: a prospective
incidence and immunohistochemical study with literature review. Am
J Clin Pathol. 102:102–108. 1994.
|
|
13
|
Azzopardi JG, Ross CM and Frizzera G: Blue
naevi of lymph node capsule. Histopathology. 1:451–461. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bell ME, Hill DP and Bhargava MK:
Lymphatic invasion in pigmented nevi. Am J Clin Pathol. 72:97–100.
1979.PubMed/NCBI
|