Introduction
Small cell lung cancer (SCLC) accounts for ~15% of
all lung cancer diagnoses in the United States. The histology is
unique and the disease is commonly referred to as a high-grade
neuroendocrine carcinoma. The cancer behaves differently than
non-small cell carcinomas (NSCLCs), as it is typically more
aggressive, with metastatic disease usually present at diagnosis
(1). In addition, SCLC is
characteristically initially very responsive to chemotherapy and/or
radiation, with response rates in the 60–80% range (2). However, once it has relapsed, SCLC is
rather resistant to chemotherapy. This resistance to therapy
equates to a poor median survival time of ~9–11 months in patients
treated for metastatic disease (3).
Understanding both innate and acquired resistance in SCLC is key to
developing improved therapies for this disease.
Class III β-tubulin (TUBB3) is an isotype of tubulin
in normal and malignant cells that contributes to mitosis via
construction of the mitotic spindle. TUBB3 is normally very highly
expressed in neuronal tissues (4).
There has been great interest in TUBB3 in cancer, as it correlates
with resistance to therapy. TUBB3 has been shown to be highly
expressed in a variety of types of cancer, including NSCLC
(5), breast cancer (6), ovarian cancer (7), head and neck cancers (8) and cancers of unknown primary site
(9). There is a growing body of
translational and clinical literature that shows a marked link
between high TUBB3 expression and resistance to the standard
microtubule inhibitor (MTI) class of drugs, the taxanes (docetaxel
and paclitaxel) and vinca alkaloids (vinorelbine) (10).
Resistance to MTIs via TUBB3 expression has been
best described in NSCLC. In early-stage, operable disease, in
patients treated with adjuvant platinum therapy plus paclitaxel,
high TUBB3 expression has been shown to correlate with decreased
survival (11). In addition, in
advanced, inoperable disease, treatment with taxane-based therapy
has been associated with poorer response rates, decreased
progression-free survival (PFS) and decreased overall survival (OS)
in NSCLCs that highly express TUBB3 (12–14).
Furthermore, an analysis of 202 patients with advanced NSCLC
revealed that high TUBB3 expression was found more often in
patients with adenocarcinoma histology, large cell carcinoma
histology, diagnosis at a younger age (<59 years) and higher
stage disease at diagnosis (stage IV vs. III) (14,15).
Based on these data, it has been suggested that high TUBB3 may
correlate with more aggressive disease in advanced NSCLC. As a
result, there is expanding interest in evaluating TUBB3 as both a
prognostic and predictive biomarker in NSCLC and a number of other
cancers.
There is little data on TUBB3 expression and its
clinical significance in SCLC. One small study that evaluated TUBB3
expression in neuroendocrine tumors included an analysis of 19
primary and 20 metastatic SCLC specimens (16). This study revealed a mean labeling
index (MLI) of 75% (range, 45–92%) in malignant cells of the
primary lesions and 96% (range, 1–99%) in the malignant cells of
the metastatic lesions. This was comparable to that which was
observed in large cell neuroendocrine tumors (80% MLI), but
markedly higher than that which was observed in atypical carcinoid
tumors (25% MLI) and typical carcinoid tumors (3% MLI). There was
no analysis to evaluate correlative clinical characteristics of the
patients in this study. Based on these findings, we hypothesize
that TUBB3 is highly expressed in SCLC. To test our hypothesis,
viable SCLC specimens from the Department of Pathology, Minneapolis
VA Healthcare System (Minneapolis, MN, USA) were analyzed for TUBB3
via immunohistochemistry (IHC). In addition, the degree of
expression was compared with baseline patient characteristics and
outcomes to identify factors that may correlate with the expression
of TUBB3 in SCLC.
Materials and methods
Patients and tumor specimens
Patients with a diagnosis of small cell carcinoma
were identified through the Minneapolis VA Hospital Tumor Registry.
The five-year period from January 1, 2006, to December 31, 2010,
was selected to ensure that adequate patient data and evaluable
tumor specimens were available. Patients with a clinical and
confirmed pathologic diagnosis of small cell carcinoma were
included. We included those with lung primaries as well as those
with extra-pulmonary small cell carcinomas (EPSCC), provided that
the histology was consistent with small cell carcinoma or poorly
differentiated (high grade or anaplastic) neuroendocrine carcinoma
consistent with small cell carcinoma. Any patients who had mixed
histology (i.e. small cell carcinoma with adenocarcinoma), a
questionable or speculative diagnosis of small cell carcinoma, or
inadequate tissue for staining were excluded.
Patient clinical data were accessed via the tumor
registry and verified with the electronic medical record.
Demographic data, including age at diagnosis, gender and race, were
recorded. Smoking status was also included and was defined
according to the following categories: No history of smoking, past
history of smoking, and actively smoking at the time of diagnosis.
As many patients had quit smoking near the diagnosis of their
cancer, a patient had to have quit smoking for over one month prior
to their diagnosis to be considered a past smoker.
Cancer-specific data regarding stage and disease
burden at diagnosis were evaluated. The Veterans Administration
Lung Study Group staging categories of limited- or extensive-stage
disease were used to classify stage at diagnosis (17). Disease burden was measured by
calculating the number of metastatic sites involved beyond the lung
and included metastases to the lymph node, bone, brain, liver, soft
tissue and other visceral sites. Information pertaining to the
pathology specimen was also obtained. Biopsy site, as defined by
biopsy of a lung lesion, lymph node or metastatic site, was
included. The type of biopsy was also evaluated in terms of whether
it was a core biopsy, cytology, bone marrow biopsy or autopsy
specimen.
As this study was not performed in the context of a
clinical trial, standard RECIST responses to therapy were not
available (18). For this analysis,
imaging responses to therapy were classified as a response (any
measurable decrease in the size of tumor target lesions), stable
disease (no measurable change in the size of tumor target lesions)
and progressive disease (any measurable increase in the size of
tumor target lesions or the development of new lesions). OS for
each patient was calculated from diagnosis of their cancer to
mortality from any cause. PFS was defined as the time period from
diagnosis to first documentation of disease progression or
mortality from any cause. Subjects were censored at the time of
last follow-up if mortality or progression were not observed during
the follow-up period. The study was reviewed and approved by the
Institutional Review Board of Minneapolis VA Healthcare System
(Minneapolis, MN, USA). All patient and tumor data were
de-identified prior to analysis according to VA standards.
Immunohistochemical staining and
assessment
Formalin-fixed, paraffin-embedded (FFPE) tumor
specimens were obtained from the Department of Pathology,
Minneapolis VA Healthcare System. The archival FFPE slides stained
with hematoxylin-eosin were reviewed by a pathologist (C.I.) to
assess tumor volume and adequacy for immunostaining on both tissue
biopsies and cytology cell-blocks. Sections (5-μm) were obtained
from the corresponding FFPE blocks and stained using antibodies
against neuronal Class B-tubulin (TUJ1) (1:400 dilution;
monoclonal; cat. no. MMS-435P; Covance Inc., Princeton, NJ, USA)
following the manufacturer’s instructions in a Leica Bond Max
automated immunostainer (Leica Microsystems, Inc., Buffalo Grove,
IL, USA). In brief, heat-induced epitope retrieval was performed
with ethylenediaminetetraacetic acid-Tris base buffer (pH 8.9–9.1;
Leica Microsystems, Inc.); primary antibody incubation time was 15
min, and a Bond polymer refined detection kit (Leica Bond; Leica
Microsystems, Inc.) was used. Brain tissue was used as the positive
control and muscle tissue was used as the negative control, as
recommended in the antibody manual. Tumor staining was assessed by
a trained pathologist (C.I.) with no knowledge of patient’s
clinical data, under the light microscope at low- and high-power
magnifications (×20–×200).
No validated expression scoring system exists for
TUBB3. The scoring system in this analysis was derived from
previously reported scoring systems of TUBB3 ‘positivity’ in NSCLC
(12,14,15).
These studies classify positivity for TUBB3 as >50% of malignant
cells staining positive for TUBB3 with 2+ intensity or greater on a
0–3+ intensity scale. For the purpose of our study, we aimed to
evaluate classes of expression in addition to positivity.
Therefore, expression of TUBB3 was classified as the composite of
distribution of malignant cells staining positive for the TUBB3
immunostain and the intensity of staining. Distribution of staining
was classified as the percentage of malignant cells staining
positive (range, 0–100%; 10% increments). Intensity of staining was
defined as: 0, no staining; 1+, weak staining; 2+, moderate
staining; and 3+, strong staining. Tumor samples were determined to
have high expression of TUBB3 if >50% of malignant cells were
positive with 3+ intensity. Moderate expression of TUBB3 was
defined as >50% of malignant cells staining positive with 2+
intensity. Low expression of TUBB3 was defined as ≤50% of malignant
cells staining positive and/or ≤2+ intensity of staining. Brain
tissue served as the reference specimen for high expression (3+
intensity in 100% of cells). Muscle tissue served as the reference
specimen for low expression (0 intensity in 0% of cells).
Statistical analysis
The association between TUBB3 expression and
clinical/biopsy characteristics was summarized by frequencies and
percentages. Fisher’s exact test was used for formal hypothesis
testing due to the small sample size. OS and PFS were summarized
for high and low or moderate TUBB3 expression by Kaplan-Meier
survival curves. Differences in survival were tested using Cox
proportional hazards regression adjusting for initial therapy.
Patients receiving only best supportive care were excluded from the
analysis of OS and PFS. P-values less than 0.05 were considered to
indicate a statistically significant difference. All calculations
were completed using R version 2.15.1 (http://www.R-project.org/).
Results
Patient and sample characteristics
A total of 106 patients with small cell carcinoma
were diagnosed during the study period. Of these 106 patients, a
total of 66 had specimens that met our inclusion criteria for IHC
staining. Patient and sample characteristics are outlined in
Table I. There were no EPSCCs in
our cohort, as all cases represented primary SCLCs. No recurrent
small cell carcinomas were included. Regarding treatment, a total
of 41 patients (62%) received standard chemotherapy (cisplatin or
carboplatin in combination with etoposide or irinotecan), 12
patients (18%) received chemotherapy with concurrent radiation and
12 patients (18%) opted for best supportive care. At the time of
analysis, 61 (92%) of the patients had died and 5 (8%) were still
living.
 | Table IPatient and sample
characteristics. |
Table I
Patient and sample
characteristics.
| Characteristics | High TUBB3 (n=56), n
(%) | Low or moderate TUBB3
(n=10), n (%) | P-value |
|---|
| Age (years) | | | 0.342 |
| ≤60 | 8 (14) | 0 (0) | |
| >60 | 48 (86) | 10 (100) | |
| Smoking status | | | 0.492 |
| Past smoker | 24 (43) | 6 (60) | |
| Smoker at
diagnosis | 32 (57) | 4 (40) | |
| Gender | | | 1.000 |
| Male | 56 (100) | 10 (100) | |
| Female | 0 (0) | 0 (0) | |
| Ethnicity | | | 1.000 |
| Caucasian | 56 (100) | 7 (70) | |
| Other | 0 (0) | 3 (30) | |
| Stage at
diagnosis | | | 0.672 |
| Limited | 12 (21) | 1 (10) | |
| Extensive | 44 (79) | 9 (90) | |
| Number of metastatic
organ sites | | | 1.000 |
| ≤2 | 33 (59) | 6 (60) | |
| >2 | 23 (41) | 4 (40) | |
| Biopsy site | | | 0.254 |
| Lung | 32 (57) | 7 (70) | |
| Lymph node | 11 (20) | 3 (30) | |
| Distant
metastasis | 13 (23) | 0 (0) | |
| Type of sample | | | 0.004 |
| Core biopsy | 40 (71) | 2 (20) | |
| Cytology | 14 (25) | 8 (80) | |
| Bone marrow
biopsy | 1 (2) | 0 (0) | |
| Autopsy | 1 (2) | 0 (0) | |
TUBB3 expression in SCLC
Of the 66 patients evaluated, 85% (95% CI,
73.9–92.5%) had high IHC expression of TUBB3. Only 4% (95% CI,
0.9–12.7%) had low TUBB3 expression and 11% (95% CI, 4.4–20.6) had
moderate TUBB3 expression. The mean distribution of malignant cells
staining positive for TUBB3 was also high at 87.3 ± 1.8% (mean ±
SE). Based on previously reported scoring systems defined in NSCLC
(12,14,15),
the majority (n=63, 95%) of our specimens were positive for TUBB3.
All samples had some degree of expression of TUBB3, with the lowest
expression being observed in one specimen with 40% of the malignant
cells staining positive at 1+ intensity. Conversely, there were 25
specimens (38%) that had 100% of the malignant cells staining
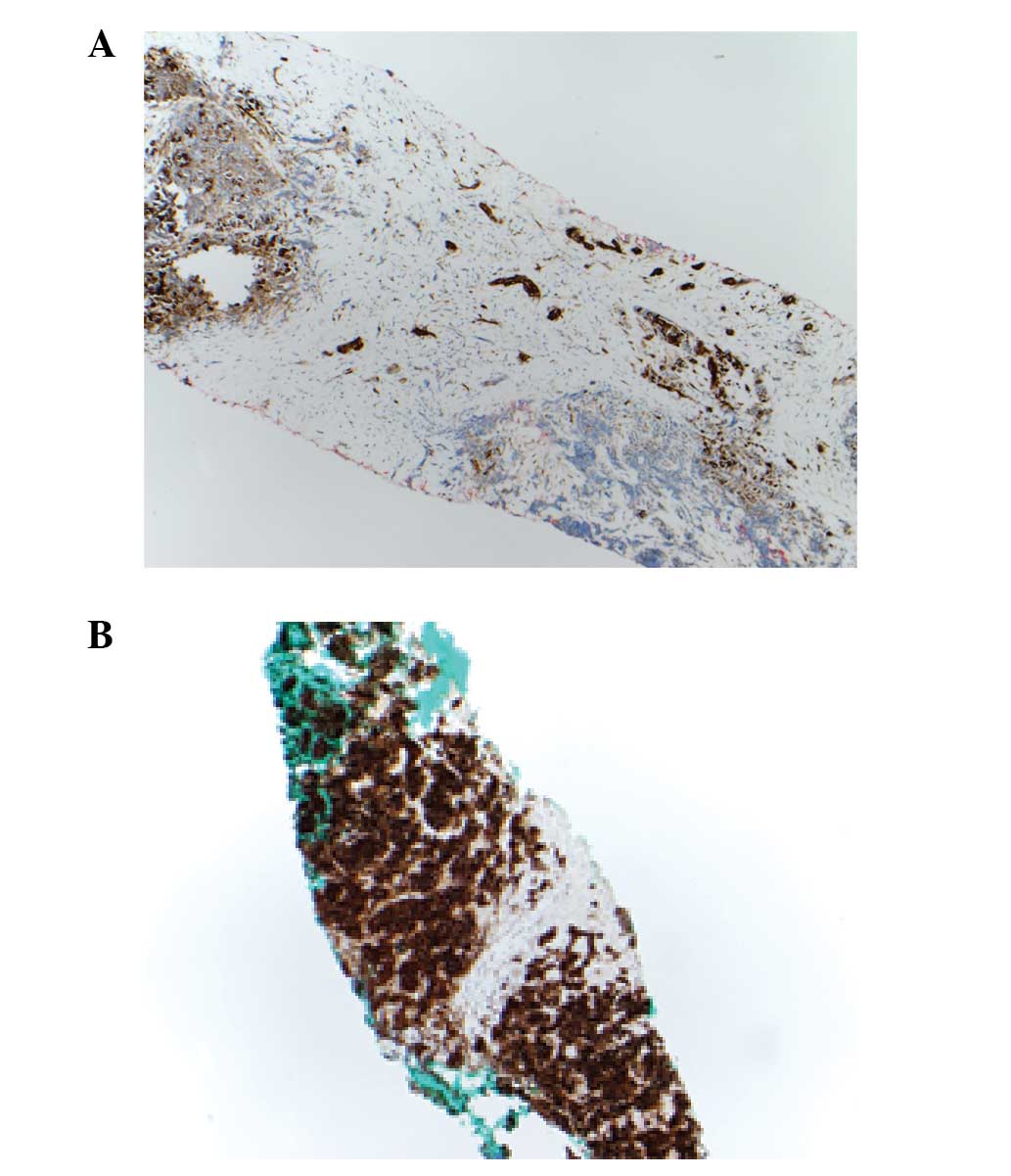
positive at 3+ intensity, similar to brain tissue. Fig. 1 displays the staining pattern and
intensity of a specimen with low TUBB3 expression and high TUBB3
expression.
Correlation of TUBB3 expression with
clinical and biopsy characteristics
Comparisons of TUBB3 expression (high vs. low or
moderate) based on biopsy site and type of sample are outlined in
Table I. Overall, there were no
significant differences found among clinical characteristics
including age, gender, ethnicity, smoking status, stage at
diagnosis and disease burden. Additional analysis revealed that
there were no significant differences in TUBB3 expression when
comparing patients who presented with CNS, bone, liver or soft
tissue metastases to those who did not. There was no significant
difference in TUBB3 expression based on the site of the biopsy;
however, there was a trend towards higher expression in the
metastatic lesions biopsied. The only significant difference in
TUBB3 expression was with regard to to biopsy type. Core biopsies
showed significantly higher expression overall when compared to
cytology specimens (P=0.004).
TUBB3 expression and survival
Initial therapies in our cohort included standard
chemotherapy (62%), chemotherapy with concurrent radiation (18%)
and best supportive care (18%). Treatment and survival data were
not available for one patient due to transfer of care to another
institution. Data on treatment response was available for 49 of the
53 patients that received therapy. Of these, 46 (94%) responded to
therapy and 3 (6%) progressed despite therapy. We were unable to
complete a formal hypothesis test comparing response rate by TUBB3
expression due to the uniformly high response rate for both
groups.
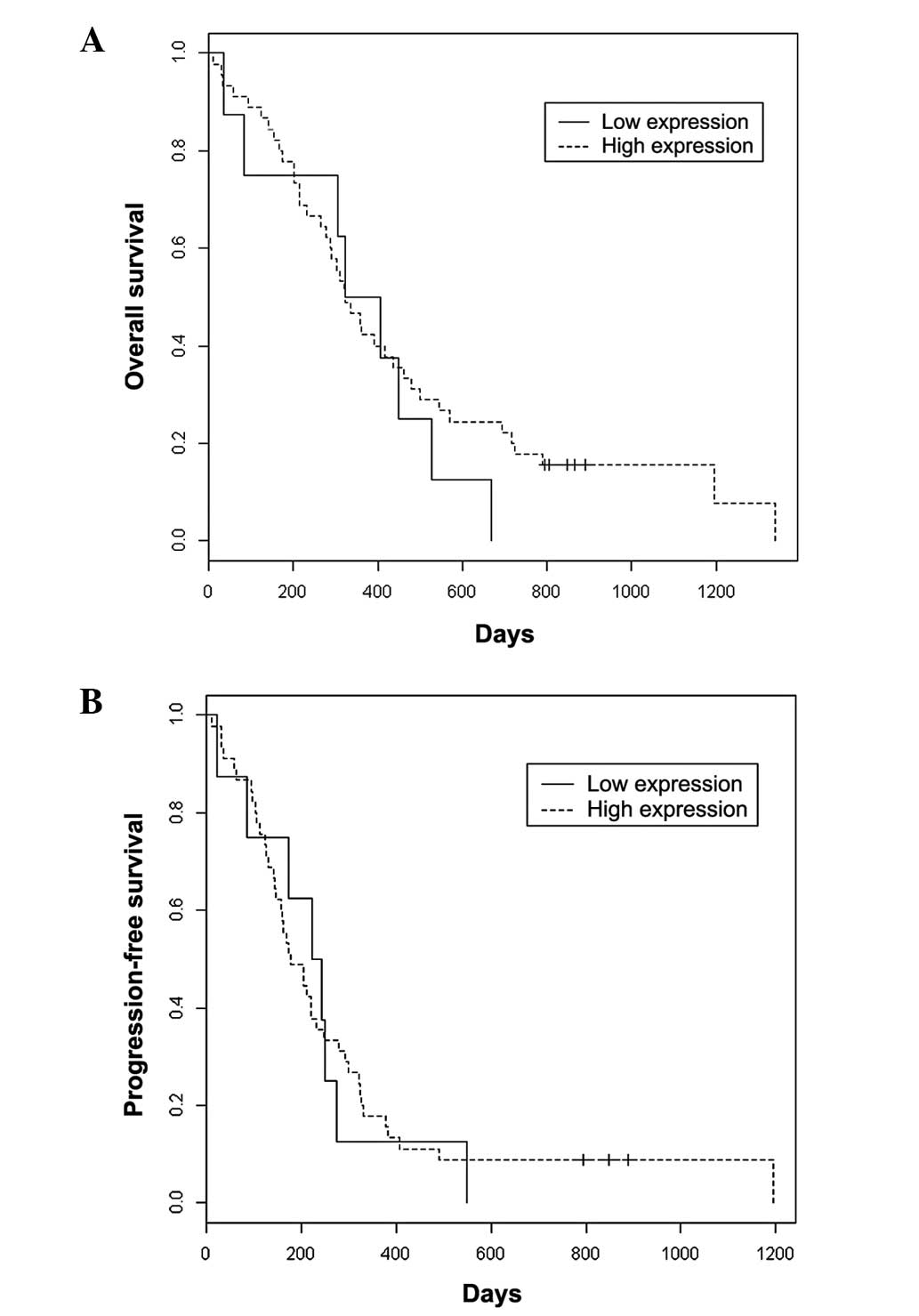
Kaplan-Meier survival curves for OS and PFS
comparing high TUBB3 expression versus low or moderate expression
can be observed in Fig. 2. There
was no significant difference in OS (median OS, 322 vs. 364 days;
P=0.452) or PFS (median PFS, 178 vs. 233 days; P=0.845) in patients
with high TUBB3 expression versus patients with low or moderate
expression, respectively.
Discussion
TUBB3 is emerging as a potential biomarker in NSCLC.
Its role as a predictive and prognostic biomarker remains to be
defined in a number of malignancies. In the present study, we aimed
to define TUBB3 expression and correlative clinical and pathologic
findings in patients with SCLC. Ultimately, we found that TUBB3 is
highly expressed in SCLC, with 56 of 66 patients (85%) displaying
high expression of TUBB3 in their tumors. Furthermore, using the
definition of TUBB3 positivity as utilized in the NSCLC literature
(12,14,15),
we found that almost all of our specimens (63 of 66; 96%) were
positive for TUBB3.
We were unable to identify any significant
correlations between baseline clinical factors, OS or PFS in
patients with high TUBB3 expression when comparing them with SCLC
patients with moderate or lower expression. Other studies in NSCLC
have revealed that differences in histology (adenocarcinoma and
large cell carcinoma), younger age and more advanced stage at
diagnosis are correlated with higher TUBB3 expression (15). Our findings do not disprove TUBB3 as
a predictive biomarker in other cancers, but its role as a
biomarker in SCLC may be questionable, as it has such uniformly
high expression. However, the fact that TUBB3 is so highly
expressed in SCLC may be one reason why the disease tends to behave
more aggressively than other types of lung cancer.
In our cohort, samples obtained by core needle
biopsy were more likely to have high TUBB3 expression when compared
with cytology specimens. Preparation of cytology specimens into
cell blocks generally has more fixation variability than that of
standard core biopsies. As a result, less positive TUBB3 staining
in these specimens may be observed. As TUBB3 evolves as a
biomarker, the sample source should be taken into account, as
variability in different sample types could lead to erroneous
results. In addition, although it was not significant, there was a
trend towards higher expression of TUBB3 in metastatic lesions that
were biopsied. In the previous analysis of SCLC specimens, it was
also noted that there was a higher percentage of positive TUBB3
staining cells in metastatic lesions (16). This raises the question of which
lesions are best to sample in studies evaluating TUBB3 as a
biomarker. Beyond these points, there was no other significant
difference in biopsy or pathological characteristics between the
high and low/moderate TUBB3 expression groups.
The high expression of TUBB3 in our cohort is
markedly higher than that which has been reported in the literature
for NSCLC. Numerous studies evaluating TUBB3 expression in NSCLC
have defined high (or positive) expression as >50% of malignant
cells staining positive (12,14,15). A
review of TUBB3 expression included an analysis of three studies
that utilized this 50% cut-off. This revealed that 105 of 203 (52%)
cases of NSCLC specimens had high expression of TUBB3. The cut-off
we selected in our study was more stringent and included staining
intensity. By including only those with the highest intensity
staining (3+) and using the cut-off of >50% of malignant cells
staining positive, we still had 85% of our specimens with high
expression. If we included those with moderate-intensity staining
(2+), this number rose to 96% of our specimens. Comparing our
findings to other tumors in the same review, the degree of high
TUBB3 expression in our group was higher than that which has been
observed in breast cancer (43% of 196 cases) and cancer of unknown
primary site (55% of 40 cases) (10). Other studies using more permissive
guidelines for high expression than those in our study have also
revealed lower rates of high TUBB3 expression in head and neck
cancer (40% of 80 cases) (8) and
gastric cancer (30% of 20 cases) (19). The majority of these studies have
correlated high TUBB3 expression with resistance to taxane-based
therapies, and, in a number of cases, poorer outcomes.
It remains unclear why TUBB3 expression is higher in
certain tumors such as NSCLC and SCLC. Normal lung tissue does not
highly express TUBB3. One study evaluating tubulin isoform mRNA
expression in normal and malignant tissues revealed that TUBB3 mRNA
represented <5% of tubulin isoform mRNA in normal lung tissue.
In the same study, the TUBB3 mRNA jumped to nearly 16% of tubulin
isoform mRNA expressed in lung malignancies (20). This is much higher than that
observed in normal brain tissue, where the highest expression of
TUBB3 mRNA (8% of tubulin isoform mRNA) can be observed. One study
in an ovarian cancer cell line revealed that hypoxia, via
hypoxia-inducible factor-1a (HIF-1a), is able to induce TUBB3
expression (21). A more recent
study in NSCLC showed that activating KRAS mutations correlated
with a 40% increase in TUBB3 protein expression and subsequent
inactivation of this pathway correlated with downregulation of
TUBB3 protein expression (22).
Despite these studies, there is limited knowledge of how TUBB3 is
expressed in normal and malignant tissues and how it may be
involved in carcinogenesis.
There were several limitations to our study. First,
the number of samples viable for staining was limited. This is due
to the natural history of SCLC and the fact that it is rarely
treated with surgery. Therefore, even in early-stage disease, core
needle biopsies or fine needle aspirates are often used for
diagnosis (1). This limits the
amount of stainable tissue for IHC studies. Due to the limited
amount of tissue, the sample size for our secondary outcomes was
limited. In addition, the scoring system for TUBB3 expression has
not been clearly defined in the literature. Scoring for expression
has differed among analyses, with some using only the percentage of
malignant cells staining positive, and others utilizing intensity
of staining as well. We selected our scoring system to fit with the
literature available on NSCLC, as the data is most developed for
this disease. A better method would be to use a more quantifiable
method of scoring. Alternative studies have attempted to do this by
quantifying TUBB3 mRNA in lieu of IHC. However, mRNA does not
always correlate with the degree of protein expression (23). Therefore, the majority of studies
have used IHC as the preferred measurement of TUBB3. As TUBB3
emerges as a biomarker, it is imperative that an easily
reproducible and effective measurement technique is developed.
Several hypotheses can be generated from our study.
The first is that high TUBB3 expression in SCLC may correlate with
innate resistance to taxanes. As a single agent, paclitaxel has a
response rate in the 30–50% range in chemotherapy naïve patients
with SCLC (24–26). When the drug was combined with
platinum agents (carboplatin and cisplatin), the overall response
rates (ORRs) were 65–68% (27,28).
While this ORR is comparable to the ‘gold standard’ regimen of
cisplatin or carboplatin plus etoposide, the complete response (CR)
rates were lower, with only ~5–10% achieving a CR with platinum
plus paclitaxel versus ~20–40% achieving a CR with platinum plus
etoposide (29,30). This suggests that there may be some
innate resistance to taxanes in SCLC. Based on our finding of high
TUBB3 expression in SCLC and the fact that this has correlated with
taxane resistance in other tumors, it is possible that TUBB3 is
involved in taxane resistance.
High expression of TUBB3 may make it a potential
target for novel microtubule inhibitors. The epothilones are a
novel class of MTIs that have been shown to be active in
taxane-resistant malignancies (31). There are several taxane resistance
mechanisms that this class of drug can overcome, one of which is
high expression of TUBB3. In fact, the epothilones show
preferential targeting of TUBB3 over other isoforms of tubulin
(32). Based on the fact that our
SCLC cohort showed high expression of TUBB3 throughout the majority
of samples, further evaluation of this drug class in SCLC may be
worthwhile.
In conclusion, our study reveals that TUBB3 is
highly expressed in SCLC. This finding may elucidate why the
disease is less responsive to taxane chemotherapy. In addition,
TUBB3 may be a potential target for novel microtubule inhibitor
therapy, such as epothilones. Further evaluation of TUBB3 as a
biomarker in SCLC is warranted.
Acknowledgements
The authors thank Patricia Albrecht, the Minneapolis
VA Tumor Registrar, for assisting in data identification and
extraction from the local tumor registry. Also, the authors thank
Dennis Knapp from the Minneapolis VA Immunohistochemistry Lab for
specimen acquisition, preparation and staining. Funding was
provided by the Minneapolis VA Healthcare System.
References
|
1
|
Argiris A and Murren JR: Staging and
clinical prognostic factors for small-cell lung cancer. Cancer J.
7:437–447. 2001.PubMed/NCBI
|
|
2
|
Chute JP, Chen T, Feigal E, Simon R and
Johnson BE: Twenty years of phase III trials for patients with
extensive-stage small-cell lung cancer: perceptible progress. J
Clin Oncol. 17:1794–1801. 1999.PubMed/NCBI
|
|
3
|
Smyth JF: Chemotherapy of small cell lung
cancer. Chest. 96(Suppl 1): 61S–62S. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsetos CD, Legido A, Perentes E and Mörk
SJ: Class III beta-tubulin isotype: a key cytoskeletal protein at
the crossroads of developmental neurobiology and tumor
neuropathology. J Child Neurol. 18:851–866. 2003. View Article : Google Scholar
|
|
5
|
Burkhart CA, Kavallaris M and Horwitz SB:
The role of beta-tubulin isotypes in resistance to antimitotic
drugs. Biochim Biophys Acta. 1471:1–9. 2001.PubMed/NCBI
|
|
6
|
Bernard-Marty C, Treilleux I, Dumontet C,
et al: Microtubule-associated parameters as predictive markers of
docetaxel activity in advanced breast cancer patients: results of a
pilot study. Clin Breast Cancer. 3:341–345. 2002. View Article : Google Scholar
|
|
7
|
Ohishi Y, Oda Y, Basaki Y, et al:
Expression of beta-tubulin isotypes in human primary ovarian
carcinoma. Gynecol Oncol. 105:586–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koh Y, Kim TM, Jeon YK, et al: Class III
beta-tubulin, but not ERCC1, is a strong predictive and prognostic
marker in locally advanced head and neck squamous cell carcinoma.
Ann Oncol. 20:1414–1419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sève P, Reiman T, Lai R, et al: Class III
beta-tubulin is a marker of paclitaxel resistance in carcinomas of
unknown primary site. Cancer Chemother Pharmacol. 60:27–34.
2007.PubMed/NCBI
|
|
10
|
Sève P and Dumontet C: Is class III
beta-tubulin a predictive factor in patients receiving
tubulin-binding agents? Lancet Oncol. 9:168–175. 2008.PubMed/NCBI
|
|
11
|
Sève P, Lai R, Ding K, et al: Class III
beta-tubulin expression and benefit from adjuvant
cisplatin/vinorelbine chemotherapy in operable non-small cell lung
cancer: analysis of NCIC JBR.10. Clin Cancer Res. 13:994–999.
2007.PubMed/NCBI
|
|
12
|
Dumontet C, Isaac S, Souquet PJ, et al:
Expression of class III beta tubulin in non-small cell lung cancer
is correlated with resistance to taxane chemotherapy. Bull Cancer.
92:E25–E30. 2005.PubMed/NCBI
|
|
13
|
Rosell R, Scagliotti G, Danenberg KD, et
al: Transcripts in pretreatment biopsies from a three-arm
randomized trial in metastatic non-small-cell lung cancer.
Oncogene. 22:3548–3553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sève P, Isaac S, Trédan O, et al:
Expression of class III {beta}-tubulin is predictive of patient
outcome in patients with non-small cell lung cancer receiving
vinorelbine-based chemotherapy. Clin Cancer Res. 11:5481–5486.
2005.
|
|
15
|
Sève P, Mackey J, Isaac S, et al: Class
III beta-tubulin expression in tumor cells predicts response and
outcome in patients with non-small cell lung cancer receiving
paclitaxel. Mol Cancer Ther. 4:2001–2007. 2005.
|
|
16
|
Katsetos CD, Kontogeorgos G, Geddes JF, et
al: Differential distribution of the neuron-associated class III
beta-tubulin in neuroendocrine lung tumors. Arch Pathol Lab Med.
124:535–544. 2000.PubMed/NCBI
|
|
17
|
Zelen M: Keynote address on biostatistics
and data retrieval. Cancer Chemother Rep 3. 4:31–42.
1973.PubMed/NCBI
|
|
18
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Urano N, Fujiwara Y, Doki Y, et al:
Clinical significance of class III beta-tubulin expression and its
predictive value for resistance to docetaxel-based chemotherapy in
gastric cancer. Int J Oncol. 28:375–381. 2006.
|
|
20
|
Leandro-García LJ, Leskelä S, Landa I, et
al: Tumoral and tissue-specific expression of the major human
beta-tubulin isotypes. Cytoskeleton (Hoboken). 67:214–23.
2010.PubMed/NCBI
|
|
21
|
Raspaglio G, Filippetti F, Prislei S, et
al: Hypoxia induces class III beta-tubulin gene expression by
HIF-1alpha binding to its 3′ flanking region. Gene. 409:100–108.
2008.PubMed/NCBI
|
|
22
|
Levallet G, Bergot E, Antoine M, et al:
High TUBB3 expression, an independent prognostic marker in patients
with early non-small cell lung cancer treated by preoperative
chemotherapy, is regulated by K-Ras signaling pathway. Mol Cancer
Ther. 11:1203–1213. 2012. View Article : Google Scholar
|
|
23
|
Pentheroudakis G, Batistatou A, Kalogeras
KT, et al: Prognostic utility of β-tubulin isotype III and
correlations with other molecular and clinicopathological variables
in patients with early breast cancer: a translational Hellenic
Cooperative Oncology Group (HeCOG) study. Breast Cancer Res Treat.
127:179–193. 2011.
|
|
24
|
Ettinger DS, Finkelstein DM, Sarma RP and
Johnson DH: Phase II study of paclitaxel in patients with
extensive-disease small-cell lung cancer: an Eastern Cooperative
Oncology Group study. J Clin Oncol. 13:1430–1435. 1995.
|
|
25
|
Kirschling RJ, Grill JP, Marks RS, et al:
Paclitaxel and G-CSF in previously untreated patients with
extensive stage small-cell lung cancer: a phase II study of the
North Central Cancer Treatment Group. Am J Clin Oncol. 22:517–522.
1999. View Article : Google Scholar
|
|
26
|
Graziano SL, Herndon JE II, Socinski MA,
et al: Phase II trial of weekly dose-dense paclitaxel in
extensive-stage small cell lung cancer: cancer and leukemia group B
study 39901. J Thorac Oncol. 3:158–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas P, Castelnau O, Paillotin D, et al:
Phase II trial of paclitaxel and carboplatin in metastatic
small-cell lung cancer: a Groupe Français de Pneumo-Cancérologie
study. J Clin Oncol. 19:1320–1325. 2001.PubMed/NCBI
|
|
28
|
Stinchcombe TE, Mauer AM, Hodgson LD, et
al: Phase II trial of paclitaxel and cisplatin in patients with
extensive stage small cell lung cancer: Cancer and Leukemia Group B
Trial 9430. J Thorac Oncol. 3:1301–1307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Evans WK, Shepherd FA, Feld R, Osoba D,
Dang P and Deboer G: VP-16 and cisplatin as first-line therapy for
small-cell lung cancer. J Clin Oncol. 3:1471–1477. 1985.PubMed/NCBI
|
|
30
|
Smith IE, Evans BD, Gore ME, et al:
Carboplatin (Paraplatin; JM8) and etoposide (VP-16) as first-line
combination therapy for small-cell lung cancer. J Clin Oncol.
5:185–189. 1987.PubMed/NCBI
|
|
31
|
Rivera E, Lee J and Davies A: Clinical
development of ixabepilone and other epothilones in patients with
advanced solid tumors. Oncologist. 13:1207–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dumontet C, Jordan MA and Lee FF:
Ixabepilone: targeting beta III-tubulin expression in
taxane-resistant malignancies. Mol Cancer Ther. 8:17–25. 2009.
View Article : Google Scholar
|