Introduction
Novel understandings of the early molecular events
in prostatic carcinogenesis have emerged that may underlie the
genetic and clinical heterogeneity (1). Numerous molecular abnormalities have
been previously described in prostate cancer (CaP), including
chromosomal loss or gain, gene amplification, mutations leading to
the increase or decrease of gene expression levels and changes in
the function of proteins. A number of genes associated with
inflammation, cell cycle regulation, cell signaling pathways, cell
proliferation, steroid hormone metabolism and regulation of gene
expression have been implicated (2,3).
The binding of interleukin-6 (IL-6) cytokine family
ligands, first to the IL-6 receptor (IL-6R) α and then to the gp130
receptor complex, activates the Janus kinase (JAK)/signal
transducer and activator of transcription 3 (STAT3) signal
transduction pathway, where STAT3 is important in cell
proliferation, differentiation, survival, apoptosis, angiogenesis
and tumorigenesis. Circulating serum levels of IL-6 are raised in
hormone-refractory CaP patients and evidence from previous cell
line studies has suggested that the IL-6R/JAK/STAT3 pathway may be
involved in the development of CaP (4,5).
Tumorigenesis associated with IL-6 has been attributed to the
constitutive or aberrant activation of STAT3 (6). JAK/STAT signaling pathway is
well-known to be important in the carcinogenesis of several cell
types (7). Previous in vitro
functional experiments using CaP cell lines have shown that STAT3
is constitutively active in these cell lines and promotes the
metastatic progression of CaP (8,9).
Furthermore, tyrosine-phosphorylated (p)-STAT3 is observed in 82%
of human prostate tumors and expression levels correlate with the
Gleason score (8).
Blocking of the gp130 signaling pathway, at the JAK
level, may be a useful therapeutic approach against cancer owing to
the inhibition of STAT3 activity. JAK2 tyrosine kinase inhibitor
tyrphostin AG490 has been widely used as a method of blocking the
activation of STAT3 in vitro and in vivo (10–12).
In addition, a number of JAK2 inhibitors have been found to be
tolerable with no adverse impact on the quality of life of
patients. Therefore, JAK2 inhibitors are crucial in the management
of patients with CaP (13).
STAT proteins are cytoplasmic transcription factors
that transduce signals from cytokines/growth factors to the
nucleus. STAT3, a major member of the STAT family, is involved in
various biological cell processes. Therefore, it has become a focus
of interest as a new target for cancer therapy similar to that of
the JAK proteins (14,15). Previously, activated STAT3 has been
shown to promote cell proliferation, metastasis and angiogenesis
and to protect tumor cells from apoptosis by regulating associated
genes, including Bcl-xL, Bcl-2, Fas, cyclin D1, c-myc, vascular
endothelial growth factor (VEGF), matrix metalloproteinase
(MMP)-2/-9, myeloid cell leukemia sequence 1 (MCL-1) and survivin
(16–19). Abnormalities in the JAK/STAT3
pathway are critical in the oncogenesis of several types of cancer
(20) and are involved in the
survival, proliferation and metastases of CaP (21–23).
Inhibition of STAT3 has been shown to induce apoptosis in CaP cells
(13,24). S3I-201 is a chemical probe inhibitor
of STAT3 activity and inhibits STAT3:STAT3 protein dimer complex
formation and STAT3 DNA binding and transcriptional activities.
Furthermore, S3I-201 inhibits growth and induces apoptosis
preferentially in tumor cells that contain persistently activated
STAT3 (25,26) and in fibrotic kidney disease
(27).
VEGF expression has been found to correlate with
STAT3 activity in diverse human cancer cell lines. p-STAT3 is a
mediator and biomarker of endothelial activation that reports
VEGF-vascular endothelial growth factor receptor 2 (VEGFR2)
activity (28). Previous studies
(28) have provided evidence that
the VEGF gene is regulated directly by the STAT3 protein. In
addition, STAT3 is a common molecular target for blocking
angiogenesis induced by multiple signaling pathways in various
types of human cancer. Targeting STAT3 with a small molecule
inhibitor blocks hypoxia inducible factor-1 and VEGF expression
in vitro and inhibits tumor growth and angiogenesis in
vivo (16,29). Tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) inhibits messenger RNA (mRNA)
expression of VEGF, together with matrix metalloproteinase-2
(MMP-2) and tissue inhibitor of matrix metalloproteinases-2
(TIMP-2) in different human glioblastoma cell lines. Thus, the
TRAIL system may be regarded as a molecular target to be
investigated for innovative therapy of this type of tumor (30). Knockdown of osteopontin, a secreted
phosphoglycoprotein, may downregulate MMP-2 and -9 expression,
resulting in inhibition of the malignant physiological behaviors of
CaP PC-3 cells (31). STAT3 is a
mediator of angiogenic as well as antiapoptotic genes. Activation
of STAT3 in response to polyamine depletion increases the
transcription and subsequent expression of antiapoptotic Bcl-2 and
the inhibitors of apoptosis (IAP) family of proteins and thereby,
promotes the survival of cells against tumor necrosis factor
α-induced apoptosis (32). MCL-1 is
a member of the Bcl-2 family, which inhibits cell apoptosis by
sequestering proapoptotic proteins, Bim and Bid. MCL-1
overexpression has been associated with the progression of leukemia
and numerous solid tumors, including CaP (33). However, the regulatory mechanism for
MCL-1 expression in CaP cells remains elusive.
The purpose of the present study was to investigate
the inhibitory and apoptotic effects of AG490, S3I-201 and TRAIL
combinations on the JAK/STAT3 signaling pathway in a human
prostatic carcinoma cell line (LNCaP). Inhibition of the JAK/STAT3
signaling pathway may offer a novel strategy for CaP treatment and
as yet, has not been the subject of any study. In addition, the
study aimed to assess the biological fate of the LNCaP cells by
examining VEGFA, VEGFC, VEGFR2, STAT3, MMP-2, MCL-1 and caspase
(CASP) 3, CASP8 and CASP9 gene expression profiles.
Materials and methods
Cell line and culture conditions
The LNCaP, IL-6-negative, cell line was obtained
from the Marmara University, Faculty of Medicine, Department of
Urology (Istanbul, Turkey). The cell line was cultured in
Dulbecco’s modified Eagle media (HyClone, Logan, UT, USA)
supplemented with 2 mM L-glutamine, 10% heat-inactivated fetal
bovine serum, 100 U/ml penicillin G and 100 mg/ml streptomycin (all
obtained from HyClone) and kept at 37°C in a humidified incubator
with an atmosphere of 5% CO2 and 95% air.
IC50 determination and cell
proliferation assay
The cells were seeded as 5×103 cells per
well in 200 μl complete culture medium containing various
concentrations of IL-6 (ranging between 1 and 100 ng/ml;
recombinant human IL-6; Raybiotech, Inc., Norcross, GA, USA),
AG-490 (ranging between 1 and 100 μM; Bioshop Canada, Inc.,
Burlington, ON, Canada), S3I-201 (ranging between 1 and 300 μM;
Calbiochem, Ukraine) and TRAIL (ranging between 25 and 1,000 ng/ml;
ABR; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells
were incubated for 24, 48 and 72 h, respectively, to determine
cytotoxic and apoptotic effects. Cells treated with 0.1% dimethyl
sulfoxide served as a solvent control. Each concentration of IL-6,
AG490, S3I-201 and TRAIL for each incubation period was repeatedly
incubated in four wells to identify the most efficient dose(s) and
incubation period(s). Viability IC50 values were
identified as the concentrations of the chemical agents causing 50%
decrease in cell viability. Cytotoxic activity was measured using
the water-soluble tetrazolium salt-1 (WST-1) assay (Roche
Diagnostics GmbH, Mannheim, Germany), following the manufacturer’s
instructions as previously described by Fortmüller et al
(34). The spectrophotometrical
absorbance of the samples was measured at 450 nm in a microplate
enzyme-linked immunosorbent assay (ELISA) reader (Spectramax M3;
Molecular Devices, LLC., Sunnyvale, CA, USA). Experiments were
conducted in triplicate and repeated at least three times. The
IC50 was set as the values obtained when AG490 and
S3I-201 concentrations were decreased to 50% of the control values.
The absorbance values were normalized by assigning the value of the
parent line in medium without drug to 1.0 and the value of the
no-cell control to 0. The most efficient incubation periods and
doses of IL-6 and TRAIL were calculated for cell viability. The
following IC50 values were used for the LNCaP cell line:
AG490, 50 μM; and S3I-201, 300 μM.
Isolated p-STAT3 protein ELISA
LNCaP cells were incubated with 25 ng/ml (optimal
effective dose) IL-6 for 3 h, followed by incubation with 300 μM
S3I-201 and 50 μM AG490 for various time periods (30 min to 6 h).
Cells were washed with ice-cold phosphate-buffered saline and cell
pellets were suspended in cell lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) containing 1 mM
phenylmethanesulfonyl fluoride and proteinase inhibitor cocktails
(Roche Diagnostics GmbH). Cell lysates were incubated on ice and
centrifuged for 10 min at 10,000 × g. Protein concentrations were
determined in all the cell extracts using the BCA Protein Assay
(Thermo Fisher Scientific).
ELISA assay [PathScan® pSTAT Sandwich
ELISA (Try 705); Cell Signaling Technology, Inc.] was then
performed according to the manufacturer’s instructions. Briefly,
standard ELISA procedures were performed using rabbit monoclonal
IgG primary antibodies against p-STAT3 with biotin-conjugated
anti-mouse IgG as the secondary antibody and horseradish
peroxidase-conjugated streptavidin. Quadruplicate assays were
performed on each sample and the absorbance at 450 nm was recorded
by ELISA reader (Spectramax M3; Molecular Devices, LLC.).
p-STAT3 protein western blotting
Lysates containing 100 μg protein were mixed with
loading buffer with 5% β-mercaptoethanol and heated for 5 min at
100°C. The protein samples were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto nitrocellulose membranes by semi-dry blotting.
SDS-PAGE was performed via standard procedures according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Membranes were incubated in blocking buffer
[Tris-buffered saline (TBS), 0.1% Tween 20 and 5% low-fat dry milk]
for 1 h at room temperature, followed by hybridization with
anti-p-STAT3 (tyr-705) antibody (1:1,000 dilution; Cell Signaling
Technology, Inc.), anti-STAT3 antibody (1:1,000 dilution; Cell
Signaling Technology, Inc.) and anti-β-actin antibody (internal
control; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
4°C overnight. Following three washes in TBS/0.1% Tween 20, the
membranes underwent hybridization with a horseradish
peroxidase-conjugated secondary antibody rabbit IgG (1:5,000
dilution; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Following extensive washing in TBS/0.1% Tween 20,
signals were detected by electrogenerated chemiluminescence
techniques using SuperSignal West Pico Chemilumiscent Substrate
(Thermo Fisher Scientific).
Quantitative polymerase chain reaction
(qPCR) analysis of VEGFA, VEGFC, VEGFR2, STAT3, MMP-2, MCL-1, CASP8
and CASP9 mRNA expression
Total RNA was isolated from each 96-well plate using
the High Pure RNA isolation kit (Roche Diagnostics GmbH) according
to the manufacturer’s instructions. The yield and quality of the
RNA of each sample was determined by measuring the absorbance at
260 and 280 nm using the Nanodrop spectrophotometer (NanoDrop
ND-1000; NanoDrop Technologies, Inc., Montchanin, DE, USA). Total
RNA (1 μg) was reverse-transcribed in a 20 μl reaction mixture
using random hexamers and Transcriptor First Strand cDNA synthesis
kit (Roche Diagnostics GmbH) according to the manufacturer’s
instructions.
VEGFA, VEGFC, VEGFR2, STAT3, MMP-2, MCL-1, CASP8 and
CASP9 mRNA expression levels were measured using qPCR method.
Probes and primer sets for each gene were designed at the
ProbeFinder Design Assay Center website (https://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp).
PCR primers (exon-exon junction to allow discrimination between
cDNA and gDNA) and Universal Probe Library (UPL) numbers are
provided in Table I. The 10 ml
reaction mixture, prepared in borosilicate glass capillaries,
contained 1X LightCycler TaqMan Master reaction mixture (Roche
Diagnostics GmbH), 2.5 pmol of each primer, 1 pmol UPL probe, 4 mM
MgCl2 and 1 μl cDNA. The qPCR assay included a
no-template control. PCR reactions were performed in the
LightCycler 1.5 instrument (Roche Diagnostics GmbH) using the
following denaturing conditions: 95°C for 10 min, followed by 50
cycles at 95°C for 10 sec, 60°C for 20 sec and a cooling step to
40°C. To normalize the results obtained by qPCR, the expression of
the GAPDH housekeeping gene was analyzed. Each sample was tested in
triplicate. PCR efficiency for each gene was tested by serial
dilutions of all genes. Amplification efficiencies of target genes
and those of GAPDH were approximately equal.
 | Table IGene-specific primer sequences and
probe numbers. |
Table I
Gene-specific primer sequences and
probe numbers.
| Gene probe | Forward primer | Reverse primer | UPL |
|---|
| GAPDH |
5′-AGCCACATCGCTCAGACAC-3′ |
5′-GCCCAATACGACCAAATCC-3′ | 60 |
| VEGFA |
5′-AGTGTGTGCCCACTGAGGA-3′ |
5′-GGTGAGGTTTGATCCGCATA-3′ | 9 |
| VEGFC |
5′-TGCCAGCAACACTACCACAG-3′ |
5′-GTGATTATTCCACATGTAATTGG-3′ | 27 |
| VEGFR2 |
5′-GAACATTTGGGAAATCTCTTGC-3′ |
5′-CGGAAGAACAATGTAGTCTTTGC-3′ | 18 |
| STAT3 |
5′-CCCCGCACTTTAGATTCATT-3′ |
5-CATGTCAAAGGTGAGGGACTC-3′ | 18 |
| MMP-2 |
5′-CCCCAAAACGGACAAAGAG-3′ |
5′-CTTCAGCACAAACAGGTTGC-3′ | 43 |
| MCL-1 |
5′-AAGCCAATGGGCAGGTCT-3′ |
5′-TGTCCAGTTTCCGAAGCAT-3′ | 4 |
| CASP8 |
5′-TCCAAATGCAAACTGGATGA-3′ |
5′-TCCCAGGATGACCCTCTTCT-3′ | 62 |
| CASP9 |
5′-CCATATGATCGAGGACATCCA-3′ |
5′-GACTCCCTCGAGTCTCCAGAT-3′ | 27 |
CASP3 protein ELISA
CASP3 family of proteases are key effectors in the
apoptosis of mammalian cells. The measurement of CASP3 activity was
determined using a luminescent assay according to the
manufacturer’s instructions [PathScan® Cleaved CASP3
(Asp175) Sandwich ELISA; Cell Signaling Technology, Inc.]. Cells
were incubated with 25 ng/ml IL-6 for 3 h to detect the
proapoptotic effects of the optimum doses (300 μM S3I-201; 50 μM
AG490) of reagents alone and in combination with/without 100 ng/ml
TRAIL. Adding the reagent to the wells resulted in cell lysis,
followed by CASP cleavage of the substrate and generation of a
luminescent signal produced by luciferase which is proportional to
the amount of present CASP activity. The CASP3 activity was
analyzed by reading the absorbance at 450 nm using a microplate
ELISA reader (Tecan Austria GmbH, Grödig, Austria).
DAPI/terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) double staining
assay
DNA fragmentation was detected by TUNEL, using the
DeadEnd™ Fluorometric TUNEL System assay (Promega Corporation,
Madison, WI, USA). Cells (5×103) were plated into
96-well flat bottom plates (Corning Inc., Acton, MA, USA) and
allowed to attach overnight prior to treatment with IL-6 (25
ng/ml), IL-6 + AG490 (50 μM), IL-6 + S3I-201 (300 μM), IL-6 + AG490
+ TRAIL (25 ng/ml optimal effective dose) and IL-6 + S3I-201 +
TRAIL for 24 h in fresh complete medium. The assay was performed as
previously described by Kristjansdottir et al (35). Experiments were performed in
triplicate.
Statistical analysis
Statistical significance levels of differences in
mRNA expression were analyzed by the pair-wise fixed reallocation
randomization test. The REST software tool 2009 version 2.013
(36) was used for group-wise
comparison and statistical analysis of relative expression results.
Cell viability and alterations in apoptosis were analyzed using the
one-way analysis of variance (ANOVA). Multiple comparison analysis
was performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL,
USA). P<0.01 was considered to indicate a statistically
significant difference. Data are expressed as the mean ± standard
deviation.
Results
Cytotoxic activity
IL-6 (ranging between 1 and 100 ng/ml) treatment
resulted in a dose- and time-dependent stimulation of LNCaP cell
proliferation compared with the untreated controls. In the 25 ng/ml
concentration of IL-6, increase in the cell viability was 20, 45
and 46% at 24, 48 and 72 h, respectively. Cell viability
percentages for >25 ng/ml IL-6 were 21, 40 and 39% at 24, 48 and
72 h, respectively. In other words, the most profound effect of
IL-6 was observed when cells treated with a 25 ng/ml concentration
of IL-6 yielded a relatively higher cell viability than cells
treated with concentrations of >25 ng/ml (data not shown).
The IC50 values of AG490 and S3I-201 were
measured for the LNCaP cell line. Percentages of viability were
51.27, 51.54 and 52.62% at 24, 48 and 72 h, respectively, for a 50
μM concentration of AG490. On the other hand, percentages of
viability were 52.27, 53.54 and 54.62% at 24, 48 and 72 h,
respectively, for a 300 μM concentration of S3I-201. The
IC50 values were verified by WST-1 assay when the cells
in culture were treated with 25 ng/ml IL-6 alone and combinations
of IL-6 + AG490 and IL-6 + S3I-201 for all incubation periods.
The addition of TRAIL per se, did not affect
the percentage of the cell viability even when the cells were
treated with the highest concentration (1,000 ng/ml). The addition
of TRAIL to the combination [IL-6 (25 ng/ml) + AG490 (50 μM) +
TRAIL (100 ng/ml; mean value of TRAIL concentrations)] did not
cause a significant change as cell viability percentages were 49,
50 and 51.2% at 24, 48 and 72 h, respectively. Furthermore, when
the combination of IL-6 + S3I-201 (300 μM) + TRAIL was used, cell
viability percentages were 53.42, 53.32 and 52.26% at 24, 48 and 72
h, respectively (data not shown).
AG490 and S3I-201 inhibit IL-6-induced
STAT3 phosphorylation
To determine whether AG490 and S3I-201 inhibit
p-STAT3 (STAT3 activation) human LNCaP cells which do not
constitutively express activated STAT3, IL-6 was exogenously added
to induce the phosphorylation of STAT3 at Tyr705. Firstly, to
confirm the most effective dose of IL-6, dose and time changes were
measured in the level of p-STAT3 by ELISA assay. The highest level
of p-STAT3 was found following 3 h of incubation with IL-6, which
resulted in an 18-fold increase in comparison with the control
(P<0.01; Fig. 1A). Among the
doses studied, the highest level of p-STAT3 which indicated a
30-fold increase (Fig. 1B;
P<0.01) was observed at 25 ng/ml IL-6 treatment. Therefore, the
dose previously found to be most effective by WST-1 assay was
confirmed.
Following 3 h of incubation with IL-6, the cells
were treated with various doses of AG490 and S3I-201 inhibitors.
Among the doses studied, the lowest level of p-STAT3 was observed
at 50 μM AG490 and 300 μM S3I-201 (data not shown). The doses
previously found by WST-1 assay were verified. Treatment with
combinations of IL-6 + AG490 and IL-6 + S3I-201 for the indicated
time periods led to the lowest level of p-STAT3 at 6 h with 50 μM
AG490 and 3 h with 300 μM S3I-201 (Fig.
2; P<0.01).
Compared with the control cells, western blot
analysis revealed that p-STAT3 and STAT3 protein levels in LNCaP
cells were significantly downregulated following treatment with
combinations of IL-6 (3 h; 25 ng/ml) + AG490 (6 h; 50 μM) and IL-6
(3 h; 25 ng/ml) + S3I-201 (3 h; 300 μM). Levels of β-actin remained
unchanged in response to treatment with inhibitors (Fig. 3).
AG490 and S3I-201 inhibit
JAK/STAT3-mediated gene expression
Alterations of gene expression in LNCaP cells
treated with IL-6 alone and combinations of IL-6 (3 h; 25 ng/ml) +
AG490 (6 h; 50 μM) and IL-6 (3 h; 25 ng/ml) + S3I-201 (3 h; 300 μM)
at the mRNA level were verified for all selected genes. It was
shown that the results for these genes were consistent with the
apoptosis and cell proliferation inhibition analysis. Following
treatment with combinations of IL-6 + AG490 and IL-6 + S3I-201 for
24 h, statistically significant differences were identified in the
mRNA expression levels of the VEGFA, STAT3, MCL-1, CASP8 and CASP9
genes when compared with sole IL-6 treatment (Fig. 4; P<0.01). As shown in Fig. 4, use of 25 ng/ml IL-6 alone resulted
in an increase in STAT3, VEGFA and MCL-1, but a decrease in CASP8
and CASP9. Notably, the VEGFC and MMP-2 mRNA levels in LNCaP cells
were found to be extremely low. Furthermore, no differences were
observed in the mRNA levels of VEGFR2 between the sole IL-6
treatment group and treatment with combinations of IL-6 + AG490 and
IL-6 + S3I-201 for 24 h (P>0.05). In the presence of the two
inhibitors, gene expression of MCL-1 decreased significantly
compared with the IL-6 alone group (P<0.01). Treatment with
combination of IL-6 + 50 μM AG490 resulted in a decrease in STAT3
(4.22-fold), VEGFA (1.39-fold) and MCL-1 (4.47-fold) at 24 h.
Treatment with combination of IL-6 + 300 μM S3I-201 resulted in a
decrease in STAT3 (5.01-fold), VEGFA (2.41-fold) and MCL-1
(14.66-fold) at 24 h. These decreases were greater than the
decreases observed following treatment with combination of IL-6 +
50 μM AG490 at 24 h. By contrast, treatment with combination of
IL-6 + 50 μM AG490 resulted in an increase in CASP8 (18.93-fold)
and CASP9 (23.25-fold) at 24 h. Treatment with combination of IL-6
+ 300 μM S3I-201 also resulted in an increase in CASP8 (16.12-fold)
and CASP9 (14.74-fold) at 24 h. These results suggested that AG490
and S3I-201 regulate the expression of specific genes involved in
cell growth, angiogenesis and apoptosis at the mRNA level in a
dose- and time-dependent manner.
 | Figure 4Effects of AG490 and S3I-201 on mRNA
levels of the genes in the LNCaP cell line. Real-time polymerase
chain reaction was performed using specific primers for VEGFA,
VEGFC, VEGFR2, STAT3, MMP-2, MCL-1, CASP8 and CASP9 with total RNA
isolated from the prostate cancer LNCaP cell line.
*P<0.01, vs. IL-6 only. (*)Cells previously treated
with 25 ng/ml IL-6 for 3 h. VEGFA, vascular endothelial growth
factor A; VEGFC, vascular endothelial growth factor C; VEGFR2,
vascular endothelial growth factor receptor 2; STAT3, signal
transducer and activator of transcription 3; MMP-2, matrix
metalloproteinase-2; MCL-1, myeloid cell leukemia sequence 1;
CASP8, caspase 8; CASP9, caspase 9; IL-6, interleukin-6. |
AG490 and S3I-201 promotes IL-6-induced
activation of CASP3
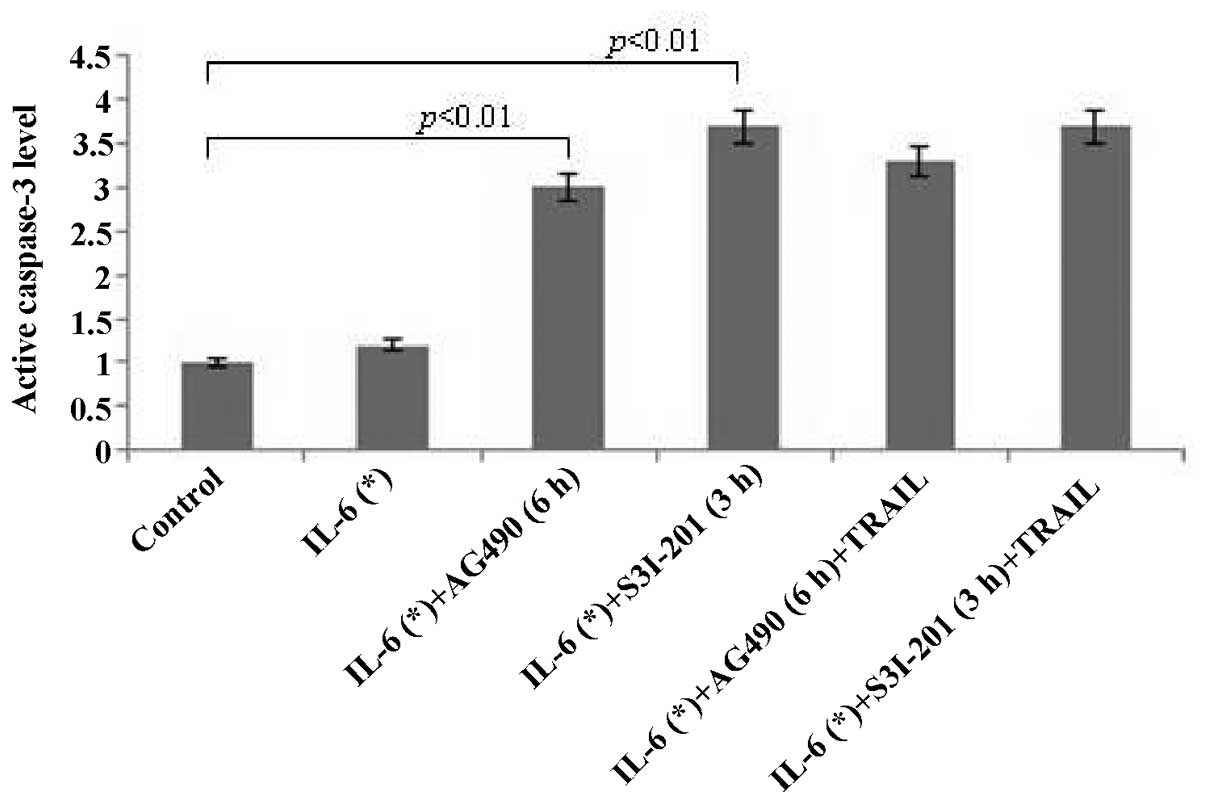
The activity level of CASP3 (Fig. 5) greatly increased in LNCaP cells
exposed to combinations of IL-6 (3 h; 25 ng/ml) + AG490 (6 h; 50
μM) and IL-6 (3 h; 25 ng/ml) + S3I-201 (3 h; 300 μM) for 24 h
(P<0.01). These results indicated that AG490 and S3I-201 promote
IL-6-induced apoptosis via CASP-dependent apoptotic pathways in
prostate tumor cells. No statistically significant differences were
identified in the CASP3 expression levels between treatment with
inhibitors alone and combinations of inhibitor + TRAIL (Fig. 5).
TUNEL analysis of apoptotic cells
To validate whether AG490 and S3I-201 enhance
IL-6-induced activation of CASP activity in human CaP cells, TUNEL
assay was used to quantify the effects of the inhibitors on the
number of IL-6-induced apoptotic cells. The number of apoptotic
cells following treatment with combinations of IL-6 (3 h; 25 ng/ml)
+ AG490 (6 h; 50 μM) and IL-6 (3 h; 25 ng/ml) + S3I-201 (3 h; 300
μM) for 24 h were ~4-fold higher than the number of apoptotic cells
following treatment with IL-6 alone (Fig. 6). However, addition of TRAIL to the
combinations did not affect the number of apoptotic cells. An
independent Giemsa analysis of apoptotic cells revealed similar
quantitative results (data not shown).
Discussion
The JAK/STAT3 signaling pathway is crucial in
regulating a number of pathways in tumorigenesis. During malignant
transformation, STAT3 is frequently overexpressed and
constitutively activated by tyrosine phosphorylation. Certain
studies have previously shown that activated STAT3 is overexpressed
in cancer tissues and cell lines (37–40).
Despite the apparent importance of STAT3 in cell proliferation,
metastasis and survival in CaP, its potential molecular mechanisms
involving CaP tumorigenesis have not been entirely characterized.
This may yield promising results for the future of cancer in terms
of adjuvant cancer therapies involving IL-6-induced LNCaP in human
CaP cells. Due to the pleiotropy of IL-6, the outcome of this
cytokine-targeting therapy may be unpredictable in CaP patients,
ranging from a lack of response to beneficial or detrimental
effects (41). Therefore, the
present study focused on AG490 and S3I-201 inhibitors of the
JAK/STAT3 signaling pathway.
In LNCaP cells where STAT3 is not constitutively
active, the exogenous addition of IL-6 induced the phosphorylation
of STAT3 at Tyr705 which had been reduced by JAK/STAT3 inhibitors.
AG490 is a well-known JAK2 inhibitor which is able to effectively
block STAT3 activation in different cancer cell lines (42–44).
On the other hand, NSC 74859, one of the novel STAT3 inhibitors,
significantly suppresses tumor growth in vitro and in
vivo (45,46). The aim of the current study was to
determine whether AG490 and S3I-201 are effective in inhibiting
STAT3 phosphorylation in LNCaP cells. This effect may modulate the
expression of a number of the well-known angiogenic and
apoptotic/antiapoptotic genes. It was revealed that the addition of
AG490 (50 μM) and S3I-201 (300 μM) to the IL-6-stimulated cells
resulted in suppression of the tyrosine phosphorylation of STAT3 in
a time-dependent manner. Such downregulation was paralleled by an
evident decrease of p-STAT3 protein expression. p-STAT3 protein
levels were found to be significantly lower in cells treated with
AG490 and S3I-201, compared with the IL-6-stimulated cells. Western
blot analysis showed weak expression levels (the intensity of
immunoreactivity bands) of STAT3 protein when treated with
inhibitors. Nevertheless, no change in the level of STAT3 was
observed following stimulation with IL-6 only compared with the
control, which suggested that the upregulation of p-STAT3 may be
attributed to the activation of upstream factors. Previous studies
have demonstrated that the AG490 does not affect the total protein
levels of STAT3 (12,37). Notably, in contrast to this, the
present study found that STAT3 protein levels in LNCaP cells were
significantly downregulated following treatment with AG490 (50 μM)
and S3I-201 (300 μM). The differences between the observations of
the current study and those of Seo et al (12) and Huang et al (37) stem from various factors, including
incubation periods and instructions, the different molecular
mechanisms used to induce apoptosis on the various types of
cultured cells and laboratory conditions. In healthy human and
animal cells, ligand-dependent activation of STATs is a transient
process, lasting for several minutes to several hours. By contrast,
the results of the current study are consistent with those of a
previous study by Zhang et al (19). Treatment of hepatoma cell lines with
NSC 74859 resulted in the downregulation of p-STAT3 levels, whereas
no change was identified in the levels of total STAT3 (46). A novel small molecule, LLL12, which
targets STAT3, was found to inhibit STAT3 phosphorylation and
induce apoptosis in various breast, pancreatic and glioblastoma
cancer cell lines (47). Downstream
targets of STAT3, cyclin D1, Bcl-2 and survivin, were also
downregulated by LLL12 at the protein and mRNA levels. The results
of the present study with regard to S3I-201 are consistent with
those of a previous study by Lin et al (47). It was not possible to compare the
results of the current study with those of other studies as no
previous studies have analyzed the protein expression levels of
STAT3 and p-STAT3 following IL-6 + AG490 and IL-6 + S3I-201
administration in LNCaP cells. The present study has demonstrated
that AG490 and S3I-201 markedly suppress STAT3 activity and that
IL-6 promotes STAT3 activity in LNCaP cell lines.
Inappropriate activation of STAT3 may be responsible
for CaP progression by regulating the expression of angiogenic and
apoptotic/antiapoptotic genes. The functional inactivation of STAT3
by inhibitors may inhibit cell proliferation and promote the
apoptosis of CaP cells. In the present study, the use of AG490 and
S3I-201 markedly reduced VEGFA, STAT3 and MCL-1 mRNA expression in
LNCaP cells. The regulatory mechanism for MCL-1 expression in CaP
cells remains elusive due to the limited information available on
its expression profile. AG490-induced apoptosis in leukemic large
granular lymphocytes was independent of Bcl-xL or Bcl-2 expression.
However, Epling-Burnette et al (48) previously found that the Bcl-2 family
protein, MCL-1, was significantly reduced by AG490 treatment.
Results of the current study demonstrated that induction of
apoptosis involved the inhibition of STAT3 and p-STAT3 activity by
downregulating the expression of a known STAT3 target gene, MCL-1.
The decrease in the transcriptional expression level of the MCL-1
gene confirms that LNCaP cells are directed toward apoptosis. In
the present study, LNCaP cells expressed the mRNA of VEGFA but not
of VEGFC. A previous study by Zhang et al (33) detected no VEGFR1 expression in
ARCaPM, ARCaPM-C2, PC3,
LNCaP, C4–2 and C4–2 B cell lines, but found
a low expression level for VEGFR2 in only one cell line
(ARCaPM-C2). However, it remains
controversial whether VEGF exhibits significant autocrine effects
in CaP cells, since the ‘classical’ VEGFRs, i.e., VEGFR1 and
VEGFR2, are undetectable in the majority of established CaP cell
lines (49). The results of the
current study are in line with those of a previous study by Zhang
et al (33) which exhibited
the low expression of VEGFR2 in LNCaP cells. These results suggest
that neuropilin-1 may be the major receptor mediating VEGF effects
in CaP cells. Previously, it has been shown that AG490 markedly
inhibits angiotensin II-induced STAT3 activation and the expression
of MMP-2 and VEGF in gastric cancer cells (50). Furthermore, the use of AG490
markedly reduced MMP-2 mRNA expression in human pancreatic cells
(SW1990) (37). However, the
current study unexpectedly found no MMP-2 and VEGFC mRNA expression
in LNCaP cells. CASPs are the central executors of the apoptotic
process and CASP3, CASP8 and CASP9 are considered to be markers of
different apoptotic pathways. CASP8 and CASP9 expression at mRNA
level was investigated to determine the efficacy of AG490 and
S3I-201 via the CASP cascade-mediated pathway in LNCaP cells. For
CASP8 and CASP9, the increase in the mRNA expression levels in
comparison with the controls (treated with IL-6 alone) was found to
be statistically significant (P<0.01). Again, it was not
possible to compare the results of the present study with those of
other studies as no previous studies have analyzed the mRNA
expression levels of CASP8 and CASP9 following the administration
of inhibitors to LNCaP cells. The mRNA results of the current study
showed that IL-6 + AG490 treatment is more effective than the IL-6
+ S3I-201 treatment in terms of leading the LNCaP cells to
apoptosis. On the other hand, IL-6 + S3I-201 treatment plays a
regulatory role on the angiogenic and antiapoptotic genes. The two
treatments may trigger apoptosis in cells through the extrinsic or
intrinsic pathways. A decrease in the mRNA level of STAT3 following
the two treatments led to the hypothesis that these inhibitors may
affect the phosphorylation of STAT3 and more directly the STAT3
gene itself. Similar results were achieved for the active CASP3
protein level which revealed that AG490 and S3I-201 caused an
increase in the CASP3 protein level. This may be attributed to
activation of the apoptotic signaling pathway. To validate these
results, TUNEL assays were used to quantify the effect of AG490 and
S3I-201 with/without TRAIL on the number of cells previously
treated with IL-6. The observations suggested that the cells are
directed towards apoptosis upon administration of inhibitors alone
and with combinations of TRAIL. In addition, the observations were
consistent with those of other studies which have explored
different cell lines (8,47).
Lanuti et al (43) previously reported that the
combinatorial effect of TRAIL and AG490 on T-cell leukemia was
characterized by a significant inhibition of STAT3 phosphorylation
compared with the controls or TRAIL alone-treated samples (43). However, cell viability, CASP3
activity and TUNEL assay results of the present study showed that
LNCaP cells are resistant to TRAIL-induced apoptosis. There are a
number of possible causes of resistance to TRAIL as follows: higher
expression of cellular IAP proteins (51); interference of the
phosphatidylinositide 3-kinase/Akt pathway with an apoptotic signal
by inhibiting the processing of Bid (52); and TRAIL receptor DR4 protein
degradation by the ubiquitin-proteasome (53).
The current study focused on IL-6 + AG490 and IL-6 +
S3I-201 treatments but not on the combined IL-6 + AG490 + S3I-201
treatment due to the evidence that each inhibitor affects the
JAK/STAT pathway during different phases. In addition, the trials
involving the latter combination did not provide comparatively
significant results in terms of leading the cells to apoptosis
(data not shown).
The observations of the present study shed light on
a novel biological effect produced by the chemotherapeutic drugs,
AG490 and S3I-201. To the best of our knowledge, the study shows
for the first time that the selective JAK2 inhibitor, AG490 and
STAT3 inhibitor, S3I-201, downregulate MCL-1, STAT3 and VEGFA and
that this process is accompanied by the decreased proliferation and
increased apoptosis of LNCaP cancer cells. In addition, it was
shown that VEGFA exhibits a primary role in the JAK/STAT3 pathway
and that its activation is not dependent on VEGFR2. These
inhibitors may be effective target molecules for antiangiogenic
therapy. Future treatments that combine antiangiogenic treatments
with conventional therapy may lead to increased clinical efficacy
for the benefit of CaP patients. These observations may aid the
development of new anticancer strategies. Since preclinical results
have been promising, additional animal model studies must be
undertaken to determine whether overexpression of the JAK/STAT
pathway is ‘driving’ prostate carcinogenesis.
In conclusion, the current study holds therapeutic
promise for JAK/STAT inhibitors for the treatment of CaP by
targeting constitutively activated STAT3. The JAK/STAT3 signaling
pathway is likely to be an important mediator in the pathogenesis
of CaP. Therefore, we hypothesized that the future use of the JAK2-
and STAT3-specific inhibitors may be a new approach in blocking the
development of CaP.
Acknowledgements
The present study was supported by a grant from the
Gazi University Research Fund (no. 01/2010-74).
References
|
1
|
Cazares LH, Drake RR, Esquela-Kirscher A,
Lance RS, Semmes OJ and Troyer DA: Molecular pathology of prostate
cancer. Cancer Biomark. 9:441–459. 2010.
|
|
2
|
Foley R, Hollywood D and Lawler M:
Molecular pathology of prostate cancer: the key to identifying new
biomarkers of disease. Endocr Relat Cancer. 11:477–488. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen MM and Abate-Shen C: Molecular
genetics of prostate cancer: new prospects for old challenges.
Genes Dev. 24:1967–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tam L, McGlynn LM, Traynor P, Mukherjee R,
Bartlett JM and Edwards J: Expression levels of the JAK/STAT
pathway in the transition from hormone-sensitive to
hormone-refractory prostate cancer. Br J Cancer. 97:378–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chun JY, Nadiminty N, Dutt S, Lou W, Yang
JC, Kung HJ, et al: Interleukin-6 regulates androgen synthesis in
prostate cancer cells. Clin Cancer Res. 15:4815–4822. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Constantinescu SN, Girardot M and Pecquet
C: Mining for JAK-STAT mutations in cancer. Trends Biochem Sci.
33:122–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barton BE, Karras JG, Murphy TF, Barton A
and Huang HF: Signal transducer and activator of transcription 3
(STAT3) activation in prostate cancer: Direct STAT3 inhibition
induces apoptosis in prostate cancer lines. Mol Cancer Ther.
3:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal C, Tyagi A, Kaur M and Agarwal R:
Silibinin inhibits constitutive activation of Stat3, and causes
caspase activation and apoptotic death of human prostate carcinoma
DU145 cells. Carcinogenesis. 28:1463–1470. 2007. View Article : Google Scholar
|
|
10
|
Samanta AK, Lin H, Sun T, Kantarjian H and
Arlinghaus RB: Janus kinase 2: a critical target in chronic
myelogenous leukemia. Cancer Res. 66:6468–6472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuke H, Shiraki K, Sugimoto K, Tanaka J,
Beppu T, Yoneda K, et al: Jak inhibitor induces S phase cell-cycle
arrest and augments TRAIL-induced apoptosis in human hepatocellular
carcinoma cells. Biochem Biophys Res Commun. 363:738–744. 2007.
View Article : Google Scholar
|
|
12
|
Seo IA, Lee HK, Shin YK, Lee SH, Seo SY,
Park JW and Park HT: Janus Kinase 2 Inhibitor AG490 Inhibits the
STAT3 Signaling Pathway by Suppressing Protein Translation of
gp130. Korean J Physiol Pharmacol. 13:131–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shodeinde AL and Barton BE: Potential use
of STAT3 inhibitors in targeted prostate cancer therapy: future
prospects. Oncol Targets Ther. 5:119–125. 2012.PubMed/NCBI
|
|
14
|
Flowers LO, Subramaniam PS and Johnson HM:
A SOCS-1 peptide mimetic inhibits both constitutive and IL-6
induced activation of STAT3 in prostate cancer cells. Oncogene.
24:2114–2120. 2005. View Article : Google Scholar
|
|
15
|
Klampfer L: Signal transducers and
activators of transcription (STATs): Novel targets of
chemopreventive and chemotherapeutic drugs. Curr Cancer Drug
Targets. 6:107–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, et al: Constitutive Stat3 activity up-regulates VEGF
expression and tumor angiogenesis. Oncogene. 21:2000–2008. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Zhang D, Luan X, Xie G and Pan X:
Inhibition of the signal transducers and activators of
transcription (STAT) 3 signalling pathway by AG490 in laryngeal
carcinoma cells. J Int Med Res. 38:1673–1681. 2010. View Article : Google Scholar
|
|
20
|
Huang C, Yang G, Jiang T, Huang K, Cao J
and Qiu Z: Effects of IL-6 and AG490 on regulation of Stat3
signaling pathway and invasion of human pancreatic cancer cells in
vitro. J Exp Clin Cancer Res. 29:512010. View Article : Google Scholar
|
|
21
|
Azare J, Leslie K, Al-Ahmadie H, Gerald W,
Weinreb PH, Violette SM and Bromberg J: Constitutively activated
Stat3 induces tumorigenesis and enhances cell motility of prostate
epithelial cells through integrin beta 6. Mol Cell Biol.
27:4444–4453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdulghani J, Gu L, Dagvadorj A, Lutz J,
Leiby B, Bonuccelli G, et al: Stat3 promotes metastatic progression
of prostate cancer. Am J Pathol. 172:1717–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun M, Liu C, Nadiminty N, Lou W, Zhu Y,
Yang J, et al: Inhibition of Stat3 activation by sanguinarine
suppresses prostate cancer cell growth and invasion. Prostate.
72:82–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rojas A, Liu G, Coleman I, Nelson PS,
Zhang M, Dash R, et al: IL-6 promotes prostate tumorigenesis and
progression through autocrine cross-activation of IGF-IR. Oncogene.
30:2345–2355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddiquee K, Zhang S, Guida WC, Blaskovich
MA, Greedy B, Lawrence HR, et al: Selective chemical probe
inhibitor of Stat3, identified through structure-based virtual
screening, induces antitumor activity. Proc Natl Acad Sci USA.
104:7391–7296. 2007. View Article : Google Scholar
|
|
26
|
Zhang X, Yue P, Fletcher S, Zhao W,
Gunning PT and Turkson J: A novel small-molecule disrupts Stat3 SH2
domain-phosphotyrosine interactions and Stat3-dependent tumor
processes. Biochem Pharmacol. 79:1398–1409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang M, Ma L, Gong R, Tolbert E, Mao H,
Ponnusamy M, et al: A novel STAT3 inhibitor, S3I-201, attenuates
renal interstitial fibroblast activation and interstitial fibrosis
in obstructive nephropathy. Kidney Int. 78:257–268. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen SH, Murphy DA, Lassoued W, Thurston
G, Feldman MD and Lee WM: Activated STAT3 is a mediator and
biomarker of VEGF endothelial activation. Cancer Biol Ther.
7:1994–2003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Q, Briggs J, Park S, Niu G, Kortylewski
M, Zhang S, et al: Targeting Stat3 blocks both HIF-1 and VEGF
expression induced by multiple oncogenic growth signaling pathways.
Oncogene. 24:5552–5560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantarella G, Risuglia N, Dell’eva R,
Lempereur L, Albini A, Pennisi G, et al: TRAIL inhibits
angiogenesis stimulated by VEGF expression in human glioblastoma
cells. Br J Cancer. 94:1428–1435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu H, Chen A, Guo F and Yuan L: A
short-hairpin RNA targeting osteopontin downregulates MMP-2 and
MMP-9 expressions in prostate cancer PC-3 cells. Cancer Lett.
295:27–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharya S, Ray RM and Johnson LR:
STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents
apoptosis in polyamine-depleted cells. Biochem J. 392:335–344.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Zhau HE, Osunkoya AO, Iqbal S,
Yang X, Fan S, et al: Vascular endothelial growth factor regulates
myeloid cell leukemia-1 expression through neuropilin-1-dependent
activation of c-MET signaling in human prostate cancer cells. Mol
Cancer. 9:92010. View Article : Google Scholar
|
|
34
|
Fortmüller K, Alt K, Gierschner D, Wolf P,
Baum V, Freudenberg N, et al: Effective targeting of prostate
cancer by lymphocytes redirected by a PSMA×CD3 bispecific
single-chain diabody. Prostate. 71:588–596. 2011.PubMed/NCBI
|
|
35
|
Kristjansdottir K, Kim K, Choi JS, Horan
TC, Brard L, Moore RG and Singh RK: 7 Methyl indole ethyl
isothiocyanate causes ROS mediated apoptosis and cell cycle arrest
in endometrial cancer cells. Gynecol Oncol. 126:252–258. 2012.
View Article : Google Scholar
|
|
36
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar
|
|
37
|
Huang C, Huang R, Chang W, Jiang T, Huang
K, Cao J, et al: The expression and clinical significance of
pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma. Neoplasma.
59:52–61. 2012. View Article : Google Scholar
|
|
38
|
Liu X, He Z, Li CH, Huang G, Ding C and
Liu H: Correlation analysis of JAK-STAT pathway components on
prognosis of patients with prostate cancer. Pathol Oncol Res.
18:17–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang T, Yan XB, Zhao JJ, Ye J, Jiang ZF,
Wu DR, et al: Gene associated with retinoid-interferon-induced
mortality-19 suppresses growth of lung adenocarcinoma tumor in
vitro and in vivo. Lung Cancer. 72:287–293. 2011. View Article : Google Scholar
|
|
40
|
Lavecchia A, Di Giovanni C and Novellino
E: STAT-3 inhibitors: state of the art and new horizons for cancer
treatment. Curr Med Chem. 18:2359–2375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santer FR, Malinowska K, Culig Z and
Cavarretta IT: Interleukin-6 trans-signalling differentially
regulates proliferation, migration, adhesion and maspin expression
in human prostate cancer cells. Endocr Relat Cancer. 17:241–253.
2010. View Article : Google Scholar
|
|
42
|
Yu JH, Kim KH and Kim H: Suppression of
IL-1beta expression by the Jak 2 inhibitor AG490 in
cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol.
72:1555–1562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lanuti P, Bertagnolo V, Pierdomenico L,
Bascelli A, Santavenere E, Alinari L, et al: Enhancement of TRAIL
cytotoxicity by AG-490 in human ALL cells is characterized by
downregulation of cIAP-1 and cIAP-2 through inhibition of
Jak2/Stat3. Cell Res. 19:1079–1089. 2009. View Article : Google Scholar
|
|
44
|
Shu M, Zhou Y, Zhu W, Wu S, Zheng X and
Yan G: Activation of a pro-survival pathway IL-6/JAK2/STAT3
contributes to glial fibrillary acidic protein induction during the
cholera toxin-induced differentiation of C6 malignant glioma cells.
Mol Oncol. 5:265–272. 2011. View Article : Google Scholar
|
|
45
|
Lin L, Amin R, Gallicano GI, Glasgow E,
Jogunoori W, Jessup JM, et al: The STAT3 inhibitor NSC 74859 is
effective in hepatocellular cancers with disrupted TGF-beta
signaling. Oncogene. 28:961–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen W, Shen X, Xia X, Xu G, Ma T, Bai X,
et al: NSC 74859-mediated inhibition of STAT3 enhances the
anti-proliferative activity of cetuximab in hepatocellular
carcinoma. Liver Int. 32:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin L, Hutzen B, Li PK, Ball S, Zuo M,
DeAngelis S, et al: A novel small molecule, LLL12, inhibits STAT3
phosphorylation and activities and exhibits potent
growth-suppressive activity in human cancer cells. Neoplasia.
12:39–50. 2010.
|
|
48
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al:
Inhibition of STAT3 signaling leads to apoptosis of leukemic large
granular lymphocytes and decreased Mcl-1 expression. J Clin Invest.
107:351–362. 2001. View Article : Google Scholar
|
|
49
|
Kitagawa Y, Dai J, Zhang J, Keller JM, Nor
J, Yao Z, et al: Vascular endothelial growth factor contributes to
prostate cancer- mediated osteoblastic activity. Cancer Res.
65:10921–10929. 2005. View Article : Google Scholar
|
|
50
|
Huang W, Yu LF, Zhong J, Wu W, Zhu JY,
Jiang FX and Wu YL: Stat3 is involved in angiotensin II-induced
expression of MMP2 in gastric cancer cells. Dig Dis Sci.
54:2056–2062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kang J, Bu J, Hao Y and Chen F: Subtoxic
concentration of doxorubicin enhances TRAIL-induced apoptosis in
human prostate cancer cell line LNCaP. Prostate Cancer Prostatic
Dis. 8:274–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nesterov A, Lu X, Johnson M, Miller GJ,
Ivashchenko Y and Kraft AS: Elevated AKT activity protects the
prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J
Biol Chem. 276:10767–10774. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Horndasch M and Culig Z: SOCS-3
antagonizes pro-apoptotic effects of TRAIL and resveratrol in
prostate cancer cells. Prostate. 71:1357–1366. 2011.
|