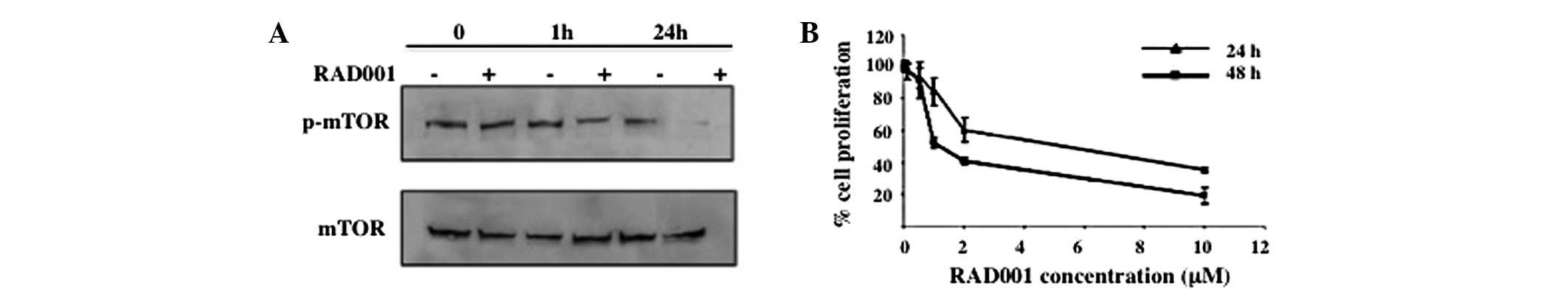
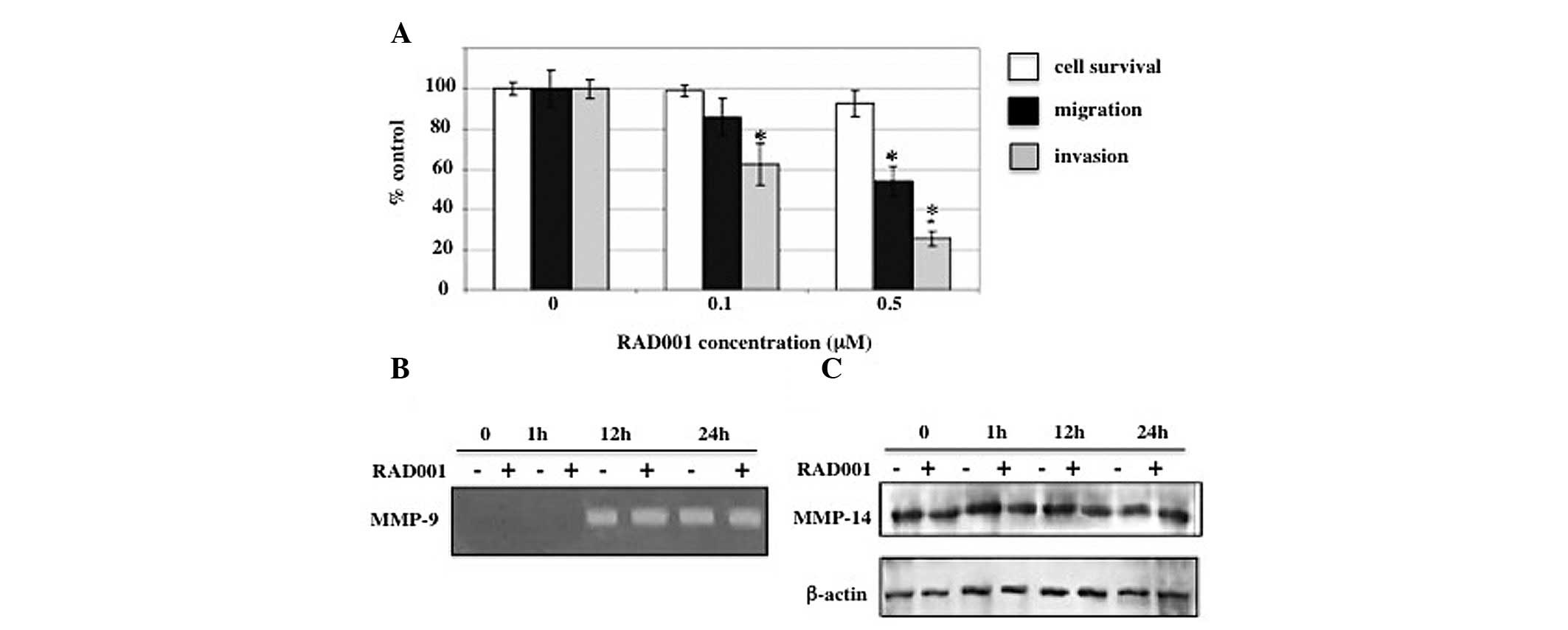
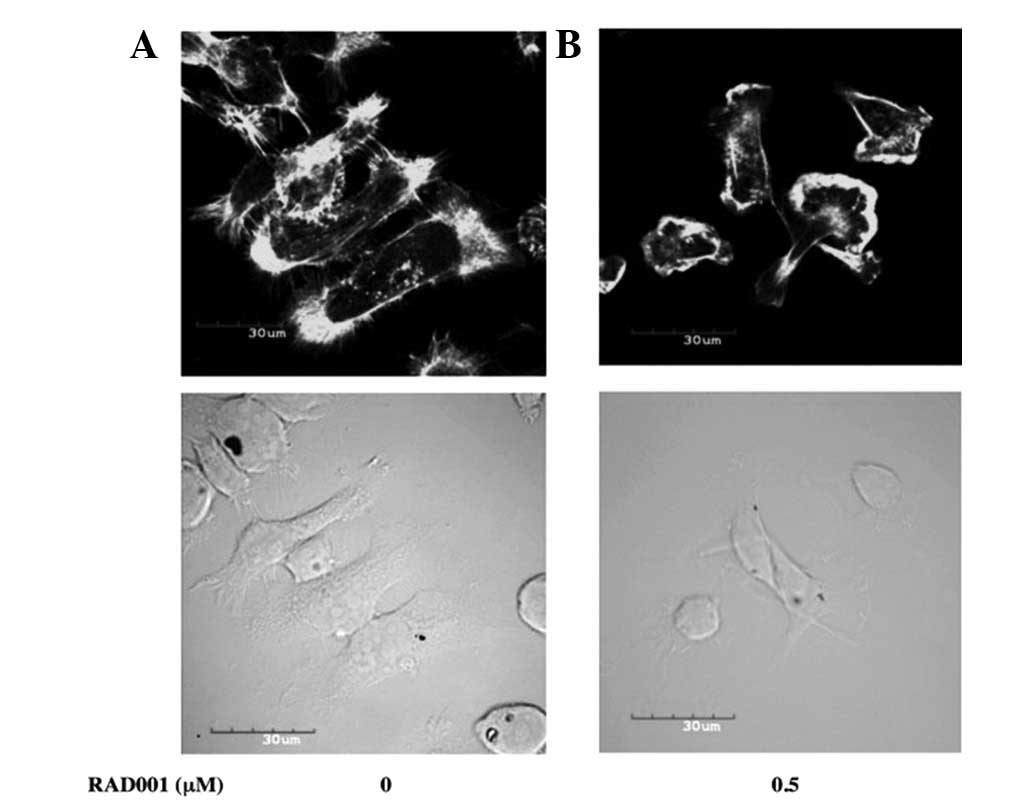
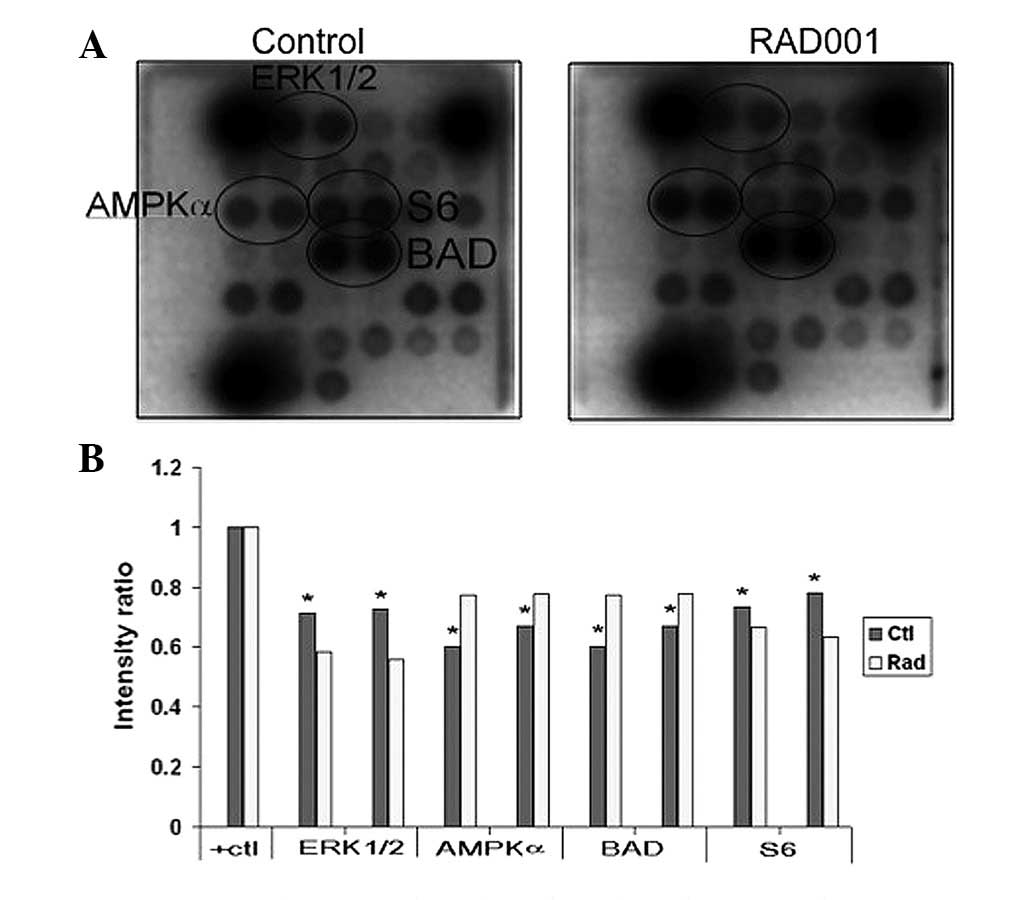
|
1
|
Sano T, Shimada K, Sakamoto Y, Yamamoto J,
Yamasaki S and Kosuge T: One hundred two consecutive hepatobiliary
resections for perihilar cholangiocarcinoma with zero mortality.
Ann Surg. 244:240–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leelawat K, Leelawat S, Narong S and
Hongeng S: Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4
induced cholangiocarcinoma cell invasion. World J Gastroenterol.
13:1561–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alvarez M, Roman E, Santos ES and Raez LE:
New targets for non-small-cell lung cancer therapy. Expert Rev
Anticancer Ther. 7:1423–1437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan ZQ, Sun M, Feldman RI, et al:
Frequent activation of AKT2 and induction of apoptosis by
inhibition of phosphoinositide-3-OH kinase/Akt pathway in human
ovarian cancer. Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beugnet A, Wang X and Proud CG: Target of
rapamycin (TOR)-signaling and RAIP motifs play distinct roles in
the mammalian TOR-dependent phosphorylation of initiation factor
4E-binding protein 1. J Biol Chem. 278:40717–40722. 2003.
View Article : Google Scholar
|
|
7
|
Sehgal SN, Baker H and Vézina C: Rapamycin
(AY-22, 989), a new antifungal antibiotic. II Fermentation,
isolation and characterization. J Antibiot (Tokyo). 28:727–732.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreno A, Akcakanat A, Munsell MF, Soni A,
Yao JC and Meric-Bernstam F: Antitumor activity of rapamycin and
octreotide as single agents or in combination in neuroendocrine
tumors. Endocr Relat Cancer. 15:257–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Escudier B, Oudard S, et al:
Efficacy of everolimus in advanced renal cell carcinoma: a double
blind, randomised, placebo-controlled phase III trial. Lancet.
372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Paolo S, Teutonico A, Ranieri E,
Gesualdo L and Schena PF: Monitoring antitumor efficacy of
rapamycin in Kaposi sarcoma. IS J Kidney Dis. 49:462–470.
2007.PubMed/NCBI
|
|
11
|
Oshiro N, Yoshino K, Hidayat S, et al:
Dissociation of raptor from mTOR is a mechanism of
rapamycin-induced inhibition of mTOR function. Genes Cells.
9:359–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schöniger-Hekele M and Müller C: Pilot
study: rapamycin in advanced hepatocellular carcinoma. Aliment
Pharmacol Ther. 32:763–768. 2010.PubMed/NCBI
|
|
13
|
Okada T, Sawada T and Kubota K: Rapamycin
inhibits growth of cholangiocarcinoma cells.
Hepatogastroenterology. 56:6–10. 2009.
|
|
14
|
Agarwala SS and Case S: Everolimus
(RAD001) in the treatment of advanced renal cell carcinoma: a
review. Oncologist. 15:236–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rattanasinganchan P, Leelawat K,
Treepongkaruna SA, et al: Establishment and characterization of a
cholangiocarcinoma cell line (RMCCA-1) from a Thai patient. World J
Gastroenterol. 12:6500–6506. 2006.PubMed/NCBI
|
|
16
|
Cybulski N and Hall MN: TOR complex 2: a
signaling pathway of its own. Trends Biochem Sci. 34:620–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vousden KH and Lu X: Live or let die: the
cell’s response to p53. Nat Rev Cancer. 2:594–604. 2002.
|
|
18
|
Delhalle S, Duvoix A, Schnekenburger M,
Morceau F, Dicato M and Diederich M: An introduction to the
molecular mechanisms of apoptosis. Ann N Y Acad Sci. 1010:1–8.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao CF, Xie Q, Su YL, et al: Proliferation
and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA.
102:10528–10533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pei D and Weiss SJ: Transmembrane-deletion
mutants of the membrane-type matrix metalloproteinase-1 process
progelatinase A and express intrinsic matrix-degrading activity. J
Biol Chem. 271:9135–9140. 1996. View Article : Google Scholar
|
|
21
|
Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M
and Okada Y: Membrane type 1 matrix metalloproteinase digests
interstitial collagens and other extracellular matrix
macromolecules. J Biol Chem. 272:2446–2451. 1997. View Article : Google Scholar
|
|
22
|
Koshikawa N, Giannelli G, Cirulli V,
Miyazaki K and Quaranta V: Role of cell surface metalloprotease
MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol.
148:615–624. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mabuchi S, Altomare DA, Cheung M, et al:
RAD001 inhibits human ovarian cancer cell proliferation, enhances
cisplatin-induced apoptosis, and prolongs survival in an ovarian
cancer model. Clin Cancer Res. 13:4261–4270. 2007. View Article : Google Scholar
|
|
24
|
Brown VI, Fang JJ, Barr R, Alcorn K, Kim J
and Grupp SA: The mTOR inhibitors rapamycin and RAD001 are active
in experimental models of ALL and are antagonized by interleukin-7.
Blood. 100:761a2002.
|
|
25
|
Hirashima K, Baba Y, Watanabe M, et al:
Aberrant activation of the mTOR pathway and anti-tumour effect of
everolimus on oesophageal squamous cell carcinoma. Br J Cancer.
106:876–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ida S, Miki Y, Ono K, et al: Synergistic
anti-tumor effects of RAD001 with MEK inhibitors in neuroendocrine
tumors: a potential mechanism of therapeutic limitation of mTOR
inhibitor. Mol Cell Endocrinol. 350:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leelawat K, Narong S, Udomchaiprasertkul
W, Leelawat S and Tungpradubkul S: Inhibition of PI3K increases
oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell
Int. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gorshtein A, Rubinfeld H, Kendler E, et
al: Mammalian target of rapamycin inhibitors rapamycin and RAD001
(everolimus) induce anti-proliferative effects in GH-secreting
pituitary tumor cells in vitro. Endocr Relat Cancer. 16:1017–1027.
2009. View Article : Google Scholar
|
|
29
|
Thomas G, Siegmann M and Gordon J:
Multiple phosphorylation of ribosomal protein S6 during transition
of quiescent 3T3 cells into early G1, and cellular
compartmentalization of the phosphate donor. Proc Natl Acad Sci
USA. 76:3952–3956. 1979. View Article : Google Scholar
|
|
30
|
Fitzgerald JP, Nayak B, Shanmugasundaram
K, et al: Nox4 mediates renal cell carcinoma cell invasion through
hypoxia-induced interleukin 6- and 8- production. PLoS One.
7:e307122012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bergmann A: Survival signaling goes BAD.
Dev Cell. 3:607–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schürmann A, Mooney AF, Sanders LC, et al:
p21-activated kinase 1 phosphorylates the death agonist bad and
protects cells from apoptosis. Mol Cell Biol. 20:453–461.
2000.PubMed/NCBI
|
|
33
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y, et al: Akt phosphorylation of BAD couples survival signals
to the cellintrinsic death machinery. Cell. 91:231–241. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Datta SR, Katsov A, Hu L, Petros A, Fesik
SW, Yaffe MB, et al: 14-3-3 proteins and survival kinases cooperate
to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 6:41–51.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng EH, Wei MC, Weiler S, et al: BCL-2,
BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and
BAK-mediated mitochondrial apoptosis. Mol Cell. 8:705–711. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Willis SN, Wei A, et al:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar
|