Introduction
Despite significant advances in screening techniques
that promote early detection of the disease, breast cancer is the
leading cause of cancer-related mortality among women worldwide
(1). The known risk factors for
breast cancer include family history, Li-Fraumeni syndrome,
atypical hyperplasia of the breast, a first full-term pregnancy at
an advanced age, early menarche and late menopause (2–4). As
these risk factors are not easily modifiable (such as genetic
predisposition), other strategies for reducing the risk of breast
cancer must be investigated. Although selective estrogen receptor
(ER) modulators (such as tamoxifen) are effective against
ER-positive breast cancers, these agents are ineffective against
ER-negative disease (5,6). Moreover, selective ER modulators have
severe side effects, including increased risk of uterine cancer,
thromboembolism, cataracts and perimenopausal symptoms (5,6).
Therefore, novel agents for the prevention and treatment of human
breast cancer, particularly hormone-independent breast cancer, are
required. Natural products have attracted increasing attention for
the discovery of novel anticancer and therapeutic agents (7).
Casticin is one of the active ingredients derived
from Fructus Viticis, the fruit of the traditional Chinese medicine
Vitex trifolia L. (Verbenaceae family) (8). A number of in vitro studies
have demonstrated that casticin exhibits anticarcinogenic activity
in breast (9), prostate (10), lung (11) and colon (12) cancer. Casticin has also been
reported to induce cell death of leukemia cells through the
induction of apoptosis or mitotic catastrophe (13). We recently reported casticin-induced
apoptosis of cervical cancer (14,15)
and hepatocellular carcinoma (16)
cells; however, the underlying mechanisms remain unclear.
The forkhead/winged helix box class O (FOXO)
transcription factors participate in a variety of cell processes,
including cell cycle progression, apoptosis, stress detoxification,
DNA repair, glucose metabolism and differentiation (17). In mammals, this family of proteins
consists of four members, FOXO1, 3, 4 and 6. These factors are
regulated by multiple mechanisms, including phosphorylation. The
phosphorylated FOXO proteins bind to 14-3-3 chaperone proteins and
are sequestered in the cytoplasm where they are unable to regulate
gene expression. When active, FOXOs induce cell cycle arrest and
apoptosis, negatively mediating oncogenic signaling and acting as
antiproliferative factors. Studies in mammalian cells have
identified important FOXO target genes involved in the regulation
of forkhead box protein M1 (FOXM1) and its downstream target genes,
including survivin, p27Kip1 and Bim (18). FOXO3a (also known as FOXO3) has been
described by several studies as a cell target for antitumor agents
in various types of cancers, including breast cancer (19,20)
and chronic myeloid leukemia (21).
However, the potential roles of FOXO3a/FOXM1 in casticin-induced
apoptosis in breast cancer cells had not yet been investigated.
Thus, this study aimed to investigate the role of FOXO3a in breast
cancer cells and examine the regulatory mechanisms of FOXO3a in
response to casticin treatment.
Materials and methods
Drugs and chemical reagents
Casticin was purchased from Chengdu Biopurify
Phytochemicals Ltd. (Chengdu, China). Casticin has a molecular
weight of 374.3, appears as yellow crystals and has a purity of
98.0%. Casticin was prepared in dimethylsulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) as a 10-mmol/l stock solution
and diluted in medium to the indicated concentration prior to use.
Mouse monoclonal antibodies against FOXM1, survivin and β-actin
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Mouse anti-human monoclonal antibodies FOXO3a and
phospho-FOXO3a-Thr32 were purchased from Millipore (Bedford, MA,
USA). The horseradish peroxidase-conjugated goat anti-mouse
secondary antibody was purchased from Santa Cruz Biotechnology,
Inc. Lipofectamine™ 2000 was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The protease inhibitor cocktail,
MTT, and all other chemicals were obtained from Sigma-Aldrich.
Cell culture
The MDA-MB-231 and MCF-7 cell lines were purchased
from the China Centre for Type Culture Collection (Wuhan, China)
and were maintained in Dulbecco’s modified Eagle’s medium (DMEM,
Invitrogen Life Technologies) supplemented with 10% fetal bovine
serum (FBS; Hyclone, Logan, UT, USA), 4 mM glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin. The cells were incubated at
37°C in a humidified atmosphere of 5% CO2.
Histone/DNA ELISA for detecting
apoptosis
The cell apoptosis ELISA detection kit (Roche, Palo
Alto, CA, USA) was used to detect apoptosis in cells treated with
casticin according to the manufacturer’s instructions. Briefly,
cells were seeded in 96-well plates at a density of
1×104 cells/well. When cells reached 70–80% confluence,
testing agents were added to the culture medium containing 10% FBS.
After 48 h of culture, the cytoplasm of the cells that was
extracted from the control or treatment groups was transferred to
96-well plates, which were pre-coated with streptavidin and
previously incubated with a biotinylated mouse anti-histone
monoclonal antibody and peroxidase-tagged mouse anti-human DNA
monoclonal antibody for 2 h at room temperature. The absorbance was
measured at 405 nm under the EXL-800-type enzyme-linked
immunosorbent apparatus (Bio-Tek, Winchester, VA, USA).
Flow cytometry using propidium iodide
(PI) staining
The cells were seeded at a density of
4×106 cells/well in 100 ml culture flasks for 24 h and
then treated with various concentrations (0.1, 0.5 and 1.0 μM) of
casticin for 48 h. PI staining for DNA content was performed as
described previously (22).
Briefly, the cells were collected and prepared as a single cell
suspension by mechanical blowing with PBS (Hyclone), washed twice
with cold PBS, fixed with 700 ml/l alcohol at 4°C for 24 h, stained
with PI (Sigma-Aldrich) and cell apoptosis was detected using flow
cytometry (FACS420, BD Biosciences, Franklin Lakes, NJ, USA).
DNA agarose gel electrophoresis
The cells were seeded at a density of
4×106 cells/well in 250 ml culture flasks for 48 h and
then treated with DMEM containing various concentrations (0.1, 0.5
and 1.0 μM) of casticin or DMSO and 10% FBS for 24 h. The assay was
performed as previously described (22). Briefly, cells were washed twice with
PBS and DNA was extracted with Apoptotic DNA Ladder Detection kit
(Bodataike Company, Beijing, China) according to the manufacturer’s
instructions. Extracted DNA was maintained at 4°C overnight.
Subsequently, 8.5 μl of the DNA sample was combined with 1.5 μl of
6× buffer solution (New England Biolabs Inc., Ipswich, MA, USA),
electrophoresed on 20 g/l agarose gel containing ethidium bromide
(BBI Solutions, Madison, WI, USA) at 40 V, and observed using the
DBT-08 gel image analysis system (VWR International Ltd., East
Grinstead, UK).
RNA interference
Control non-specific small interfering RNA (siRNA;
5′-UUCUCCGAACGUGUCACGUdTdT-3′) was purchased from Qiagen, Inc.
(Valencia, CA, USA). FOXO3A-targeted siRNA (5′-ACUCCGGGUCCAGCUC
CAC-3′) was purchased from Santa Cruz Biotechnology, Inc. The cells
were seeded in six-well plates and transfected at 50% confluence
with either 200 nmol/l of control non-specific siRNA or
FOXO3a-specific siRNA using Oligofectamine™ reagent (Invitrogen
Life Technologies) according to the manufacturer’s instructions.
After 24 h of transfection, the cells were treated with DMSO
(control) or 0.5 μM casticin for 48 h. The cells were then
collected and processed for western blotting and histone/DNA
ELISA.
Western blot analysis
The cells (1×106) were seeded in 100-mm
culture dishes, allowed to attach by overnight incubation and
treated with DMSO (control) or 0.5 μM casticin for the specified
time periods. Cell lysates were prepared as previously described
(22). Lysates were cleared by
centrifugation at 16,873 × g for 30 min. Lysate proteins were
resolved by 10 or 12.5% SDS-PAGE (Millipore) and transferred to
polyvinylidene fluoride membranes. The membranes were incubated
with Tris-buffered saline containing 0.05% Tween 20 and 5% (w/v)
non-fat dry milk. The membranes were then treated with the desired
primary antibody for 1 h at room temperature or overnight at 4°C.
Following treatment with the horseradish peroxidase-conjugated goat
anti-mouse secondary antibody the immunoreactive bands were
visualized using an enhanced chemiluminescence kit (Amersham
Pharmacia Biotech, Piscataway, USA). The blots were stripped and
re-probed with anti-actin antibody to normalize for differences in
protein loading. Changes in the level of the desired protein were
determined by densitometric scanning of the immunoreactive band and
corrected for the β-actin loading control. Immunoblotting for each
protein was performed at least twice using independently prepared
lysates to ensure reproducibility of the results.
Statistical analysis
The data were analyzed using SPSS software, version
15.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the means
± standard deviation. The means of multiple groups were compared
with one-way analysis of variance, after the equal check of
variance, and the comparisons among the means were performed using
the least significant difference method. Statistical comparison was
also performed with Dunnett’s two-tailed t-test when appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of casticin on breast cancer cell
apoptosis
It has previously been reported that casticin
inhibits the growth of MCF-7 human breast cancer cells and induces
G2/M cell cycle arrest (9). Thus,
whether casticin exerts any effect on apoptosis of the
estrogen-responsive MCF-7 or the estrogen-independent MDA-MB-231
breast cancer cell lines was investigated. ER-positive MCF-7 cells
were originally isolated from pleural effusion of a stage IV
invasive ductal carcinoma. These cells are aneuploid with high
chromosomal instability and are defective for the G1 and mitotic
spindle checkpoints. However, the cells express wild-type p53
(23). The MDA-MB-231 cell line,
which was derived from a stage IV invasive ductal carcinoma, is
ER-negative, partially proficient for all cell cycle checkpoints
and expresses mutant p53 (23).
After 48 h of exposure, casticin significantly
induced histone/DNA fragmentation in a concentration-dependent
manner in MCF-7 (Fig. 1A) and
MDA-MB-231 cells (Fig. 1B). Agarose
gel electrophoresis revealed a typical ladder pattern of
internucleosomal DNA fragmentation in MDA-MB-231 cells treated with
0.5 and 1.0 μM casticin (Fig. 1C).
Flow cytometry analysis showed that casticin treatment resulted in
increased sub-G1 population in MCF-7 (Fig. 1D and F) and MDA-MB-231 (Fig. 1E and G) cells (P<0.05) in a
concentration-dependent manner. Overall, these findings suggest
that casticin induces breast cancer cell apoptosis.
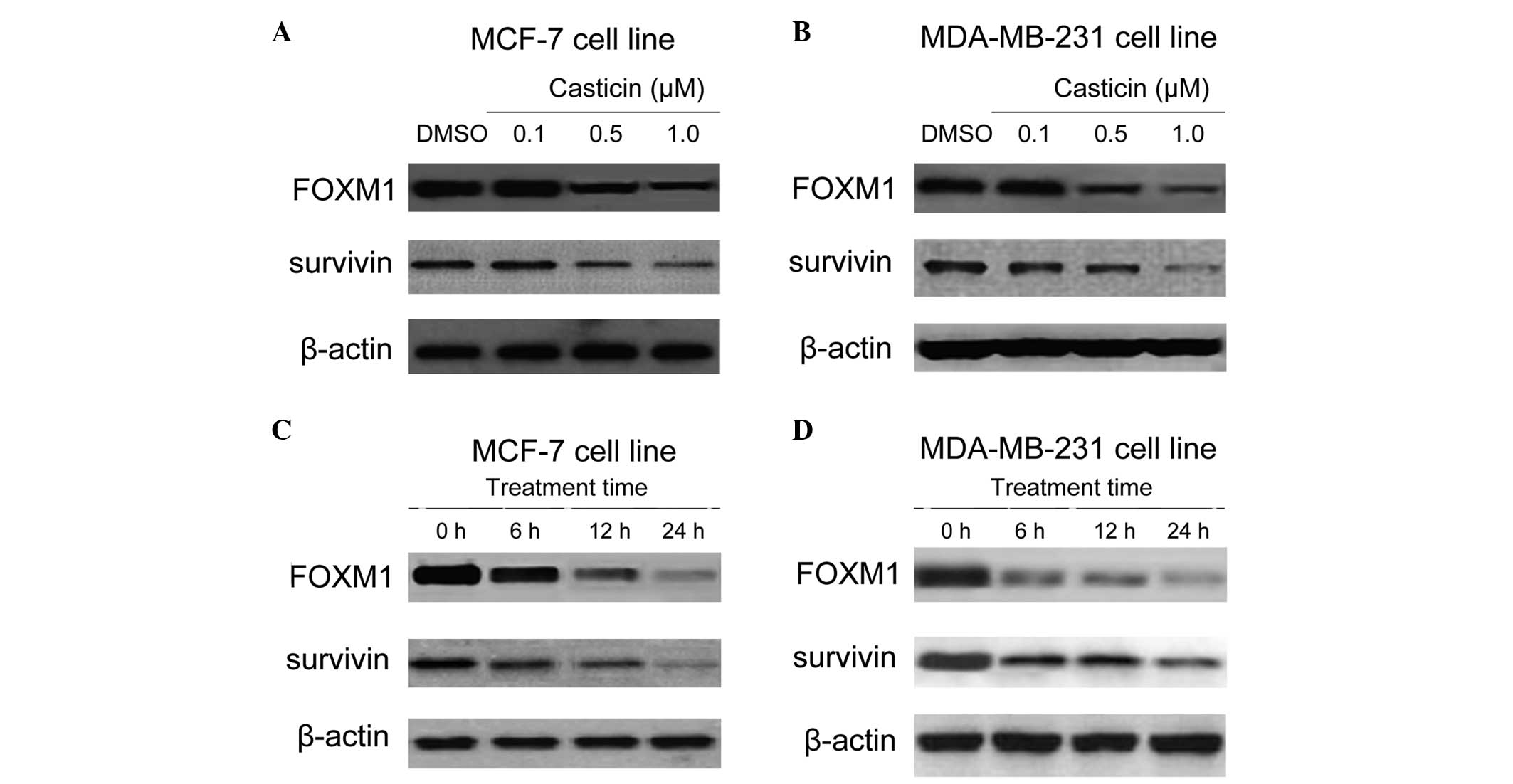
Effects of casticin on the expression of
FOXM1 in breast cancer cells
Previous research, including a study by Wang et
al (24), has demonstrated that
FOXM1 is a novel target of natural active compounds (24,25).
Thus, whether FOXM1 is a downstream signaling target of casticin in
breast cancer cells was investigated. Dose titration of casticin in
the MCF-7 and MDA-MB-231 cell lines was performed, and the effects
on FOXM1 and its downstream target survivin were assayed (Fig. 2A and B). A dose of 0.5 μM was
selected for subsequent experiments. The MCF-7 and MDA-MB-231 cells
were then treated with 0.5 μM casticin for 0, 6, 12 and 24 h.
Western blot analysis revealed that casticin treatment decreased
FOXM1 expression and this coincided with a decrease in the FOXM1
target, survivin (Fig. 2C and D).
Collectively, these findings suggest that FOXM1 is a cellular
target of casticin in breast cancer cells.
Effects of casticin on the
phosphorylation of FOXO3a in breast cancer cells
FOXO3a is an upstream regulator of FOXM1.
Additionally, the antiproliferative and apoptotic effects of
genistein, an isoflavone derived from soybeans, were partly
mediated through the regulation of Akt/FOXO3a signaling (26). Thus, phosphorylated FOXO3a protein
was examined in order to determine whether differences in the
expression or activity of signaling regulators may enhance the
effect of casticin on FOXM1. Western blot analysis revealed that
treatment with casticin led to a decrease in FOXO3a phosphorylation
and a corresponding reduction in FOXM1 and its target, survivin
(Fig. 3A and B). These findings
suggest that the casticin-induced repression of FOXM1 may be
associated with FOXO3a activation.
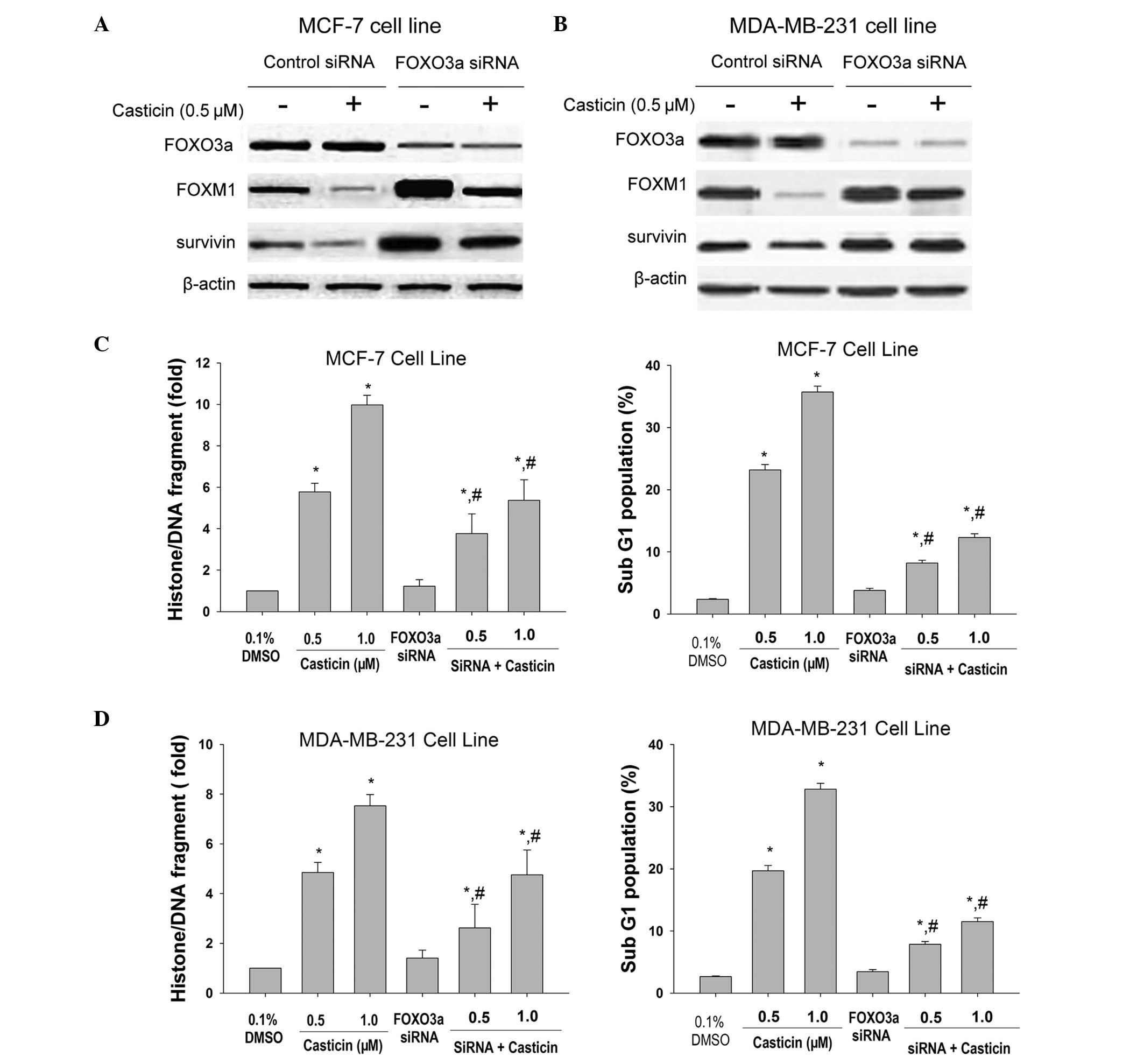
Effects of FOXO3a silencing on
casticin-mediated apoptosis of breast cancer cells
In order to determine the importance of FOXO3a in
the cellular response to casticin, the MCF-7 and MDA-MB-231 cells,
which express high protein levels of FOXO3a, were transfected with
specific siRNAs. As shown in Fig. 4A
and B, FOXM1 and survivin proteins were increased in
FOXO3a-knockdown cells. The decrease of FOXO3a significantly
attenuated the apoptotic effects of casticin in breast cancer cells
(Fig. 4C and D). These findings
support the hypothesis that casticin induces breast cancer cell
apoptosis by inducing FOXO3a activity, which represses FOXM1.
Discussion
This study demonstrated that the polymethoxyflavone
compound, casticin, induces apoptosis through the activation of
FOXO3a. This correlates with casticin-mediated inhibition of FOXM1
and survivin, which are downstream targets of FOXO3a. Inhibition of
FOXO3a by siRNA predominantly blocks casticin-induced apoptosis.
Previous studies have demonstrated the antiproliferative and
pro-apoptotic effects of casticin in prostate (10), cervical (14,15),
lung (11) and colon (12) cancer. This study investigated the
role of FOXO transcription factors in mediating the effects of
casticin. As casticin is a non-toxic polyphenolic compound, it is
safe to use for the treatment and/or prevention of breast
cancer.
In the present study, the role and regulation of
FOXM1 in response to casticin treatment in breast cancer cells was
investigated. Our findings demonstrated that casticin repressed the
expression of FOXM1 in breast cancer cells, which was associated
with the downregulation of FOXM1 activity, revealed by the
concomitant decrease in expression of its downstream target,
survivin. As casticin targets FOXM1 through FOXO3a in breast
cancer, it is possible to increase the efficacy of casticin by
targeting FOXM1. FOXM1 has been reported as a valid target for the
development of anticancer therapeutics (17). For example, a novel thiazole
antibiotic, thiostrepton, selectively induced cell cycle arrest and
cell death in breast cancer cells through the downregulation of
FOXM1 expression (26). Similarly,
other native compounds, such as resveratrol and genistein, have
also been found to repress the expression of FOXM1 and cell
proliferation (26–28). Furthermore, a cell-permeable ARF
peptide inhibitor of FOXM1 has been shown to selectively induce
apoptosis in human hepatocellular carcinoma cell lines and mouse
models (29).
FOXO transcription factors play important roles in
the regulation of apoptosis (30).
In the present study, FOXO3a was key in the regulation of the
anti-apoptotic gene, survivin. In accordance with our findings,
FOXO silencing has been shown to decrease the expression levels of
Bim, TNF-related apoptosis-inducing ligand, Fas ligand (FasL) and
p27Kip1, which are all FOXO target genes controlling the
cell cycle and apoptosis (31–33).
Inhibition of the PI3K/Akt and MEK/ERK pathways act synergistically
to regulate the anti-angiogenic effects of epigallocatechin
gallate, resveratrol and sulforaphane through activation of FOXO
transcription factors (25,34,35).
The FOXO transcription factors regulate tissue homeostasis in the
pancreas and in individuals with diabetes and cancer. FOXO
regulates apoptotic genes, such as Bim, FasL and survivin (36). Collectively, those findings suggest
that activation of FOXO transcription factors by chemopreventive
agents may regulate apoptosis. Akt and ERK have been shown to
directly phosphorylate and inactivate FOXO transcription factors
resulting in cytoplasmic retention, inactivation and inhibition of
the expression of FOXO-regulated genes. This enables the control of
various cell processes, such as metabolism, cell cycle, cell death
and oxidative stress (37). Our
findings suggested that casticin inhibits the cytoplasmic
phosphorylation of FOXO3a. Depletion of FOXO3a levels by siRNA
abrogates casticin-induced apoptosis. Overall, these results
demonstrate that the activation of FOXOs has significant
implications for the treatment and prevention of breast cancer.
Notably, the MDA-MB-231 triple-negative breast
cancer (TNBC) cell line was sensitive to casticin treatment. TNBC
is clinically characterized as more aggressive and less responsive
to standard treatments. Searching for effective strategies for the
treatment of TNBC has become a high priority in breast cancer
therapy. Our results warrant further investigation to determine
whether casticin may serve as a novel candidate agent for the
management of TNBC. Identification of casticin as a potent
anti-TNBC agent may have a significant effect on developing novel
therapeutic strategies for the treatment of TNBC.
In summary, our study suggests that
FOXO3a/FOXM1/survivin are cellular targets and markers of casticin
action in breast cancer. Furthermore, FOXM1 functions downstream of
FOXO3a in response to casticin. These findings may have important
implications for the development of therapeutic agents for breast
cancer.
Acknowledgements
The authors would like to thank Dr Jian-Guo Cao for
the critical reading of this study. This study was supported by the
Project of Scientific Research of Hunan Province the Administration
Bureau of Traditional Chinese Medicine (no. 2010081), the Hunan
Province Science and Technology Project (no. 2011FJ4144), the
program for Excellent Talents in Hunan Normal University (no.
ET13107) and the Construct Program of the Key Discipline of Basic
Medicine in Hunan Province and Research Fund for the Doctoral
Program of Hunan Normal University (no. 110656).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Kelsey JL, Gammon MD and John EM:
Reproductive factors and breast cancer. Epidemiol Rev. 15:36–47.
1993.PubMed/NCBI
|
|
3
|
Hulka BS and Stark AT: Breast cancer:
cause and prevention. Lancet. 346:883–887. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelsey JL and Bernstein L: Epidemiology
and prevention of breast cancer. Annu Rev Public Health. 17:47–67.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher B, Costantino JP, Wickerham DL, et
al: Tamoxifen for prevention of breast cancer: report of the
National Surgical Adjuvant Breast and Bowel Project P-1 Study. J
Natl Cancer Inst. 90:1371–1388. 1998. View Article : Google Scholar
|
|
6
|
Cuzick J, Forbes J, Edwards R, et al:
First results from the International Breast Cancer Intervention
Study (IBIS-I): a randomised prevention trial. Lancet. 360:817–824.
2002. View Article : Google Scholar
|
|
7
|
Newman DJ, Cragg GM and Snader KM: Natural
products as sources of new drugs over the period 1981–2002. J Nat
Prod. 66:1022–1037. 2003.PubMed/NCBI
|
|
8
|
Zeng X, Fang Z, Wu Y and Zhang H: Chemical
constituents of the fruits of Vitex trifolia L. Zhongguo
Zhong Yao Za Zhi. 21:167–168. 1911996.(In Chinese).
|
|
9
|
Haïdara K, Zamir L, Shi QW and Batist G:
The flavonoid Casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006.PubMed/NCBI
|
|
10
|
Weisskopf M, Schaffner W, Jundt G, Sulser
T, Wyler S and Tullberg-Reinert H: A Vitex agnus-castus
extract inhibits cell growth and induces apoptosis in prostate
epithelial cell lines. Planta Med. 71:910–916. 2005.
|
|
11
|
Koh DJ, Ahn HS, Chung HS, et al:
Inhibitory effects of casticin on migration of eosinophil and
expression of chemokines and adhesion molecules in A549 lung
epithelial cells via NF-kappaB inactivation. J Ethnopharmacol.
136:399–405. 2011. View Article : Google Scholar
|
|
12
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar
|
|
13
|
Shen JK, Du HP, Yang M, Wang YG and Jin J:
Casticin induces leukemic cell death through apoptosis and mitotic
catastrophe. Ann Hematol. 88:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen D, Cao J, Tian L, Liu F and Sheng X:
Induction of apoptosis by casticin in cervical cancer cells through
reactive oxygen species-mediated mitochondrial signaling pathways.
Oncol Rep. 26:1287–1294. 2011.
|
|
15
|
Zeng F, Tian L, Liu F, Cao J, Quan M and
Sheng X: Induction of apoptosis by casticin in cervical cancer
cells: reactive oxygen species-dependent sustained activation of
Jun N-terminal kinase. Acta Biochim Biophys Sin (Shanghai).
44:442–449. 2012. View Article : Google Scholar
|
|
16
|
Yang J, Yang Y, Tian L, Sheng XF, Liu F
and Cao JG: Casticin-induced apoptosis involves death receptor 5
upregulation in hepatocellular carcinoma cells. World J
Gastroenterol. 17:4298–4307. 2011. View Article : Google Scholar
|
|
17
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kops GJ, Medema RH, Glassford J, et al:
Control of cell cycle exit and entry by protein kinase B-regulated
forkhead transcription factors. Mol Cell Biol. 22:2025–2036. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sunters A, Fernández de Mattos S, Stahl M,
et al: FoxO3a transcriptional regulation of Bim controls apoptosis
in paclitaxel-treated breast cancer cell lines. J Biol Chem.
278:49795–49805. 2003. View Article : Google Scholar
|
|
20
|
Krol J, Francis RE, Albergaria A, et al:
The transcription factor FOXO3a is a crucial cellular target of
gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther.
6:3169–3179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Essafi A, Fernández de Mattos S, Hassen
YA, et al: Direct transcriptional regulation of Bim by FoxO3a
mediates STI571-induced apoptosis in Bcr-Abl-expressing cells.
Oncogene. 24:2317–2329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hollestelle A, Elstrodt F, Nagel JH,
Kallemeijn WW and Schutte M: Phosphatidylinositol-3-OH kinase or
RAS pathway mutations in human breast cancer cell lines. Mol Cancer
Res. 5:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: a novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Wang Z, Kong D, Li R, Sarkar SH and
Sarkar FH: Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network
by isoflavone in prostate cancer cells. J Biol Chem.
283:27707–27716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Ganapathy S, Singh KP, Shankar S
and Srivastava RK: Resveratrol induces growth arrest and apoptosis
through activation of FOXO transcription factors in prostate cancer
cells. PloS One. 5:e152882010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roy SK, Chen Q, Fu J, Shankar S and
Srivastava RK: Resveratrol inhibits growth of orthotopic pancreatic
tumors through activation of FOXO transcription factors. PloS One.
6:e251662011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalinichenko VV, Major ML, Wang X, et al:
Foxm1b transcription factor is essential for development of
hepatocellular carcinomas and is negatively regulated by the p19ARF
tumor suppressor. Genes Dev. 18:830–850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zanella F, Link W and Carnero A:
Understanding FOXO, new views on old transcription factors. Curr
Cancer Drug Targets. 10:135–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Zhao S, Tian H, et al: Depletion of
PI3K p85alpha induces cell cycle arrest and apoptosis in colorectal
cancer cells. Oncol Rep. 22:1435–1441. 2009.PubMed/NCBI
|
|
32
|
Barreyro FJ, Kobayashi S, Bronk SF,
Werneburg NW, Malhi H and Gores GJ: Transcriptional regulation of
Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem.
282:27141–27154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lynch RL, Konicek BW, McNulty AM, et al:
The progression of LNCaP human prostate cancer cells to androgen
independence involves decreased FOXO3a expression and reduced
p27KIP1 promoter transactivation. Mol Cancer Res. 3:163–169. 2005.
View Article : Google Scholar
|
|
34
|
Davis R, Singh KP, Kurzrock R and Shankar
S: Sulforaphane inhibits angiogenesis through activation of FOXO
transcription factors. Oncol Rep. 22:1473–1478. 2009.PubMed/NCBI
|
|
35
|
Shankar S, Chen Q and Srivastava RK:
Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to
enhance antiangiogenic effects of EGCG through activation of FOXO
transcription factor. J Mol Signal. 3:72008. View Article : Google Scholar
|
|
36
|
Zhao X, Ogunwobi OO and Liu C: Survivin
inhibition is critical for Bcl-2 inhibitor-induced apoptosis in
hepatocellular carcinoma cells. PloS One. 6:e219802011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|