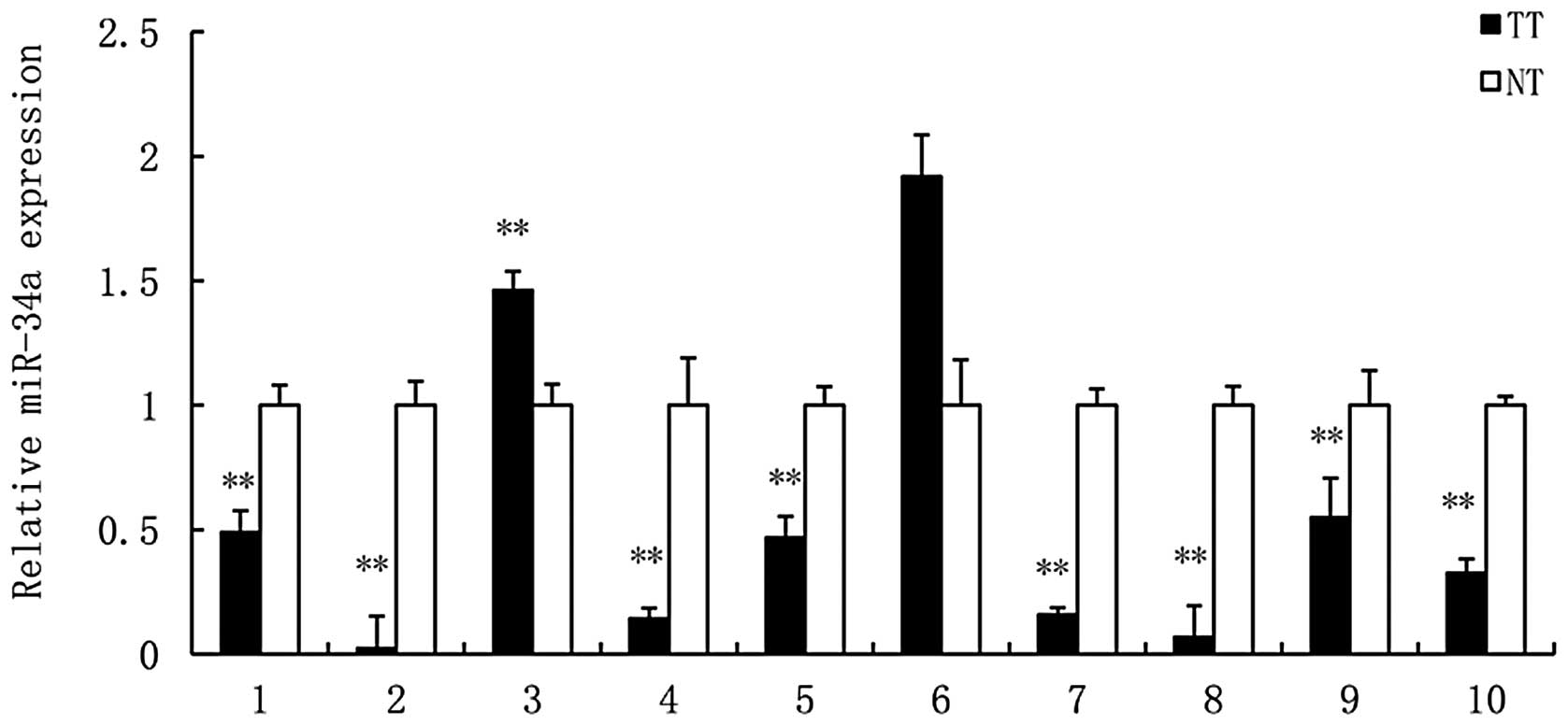
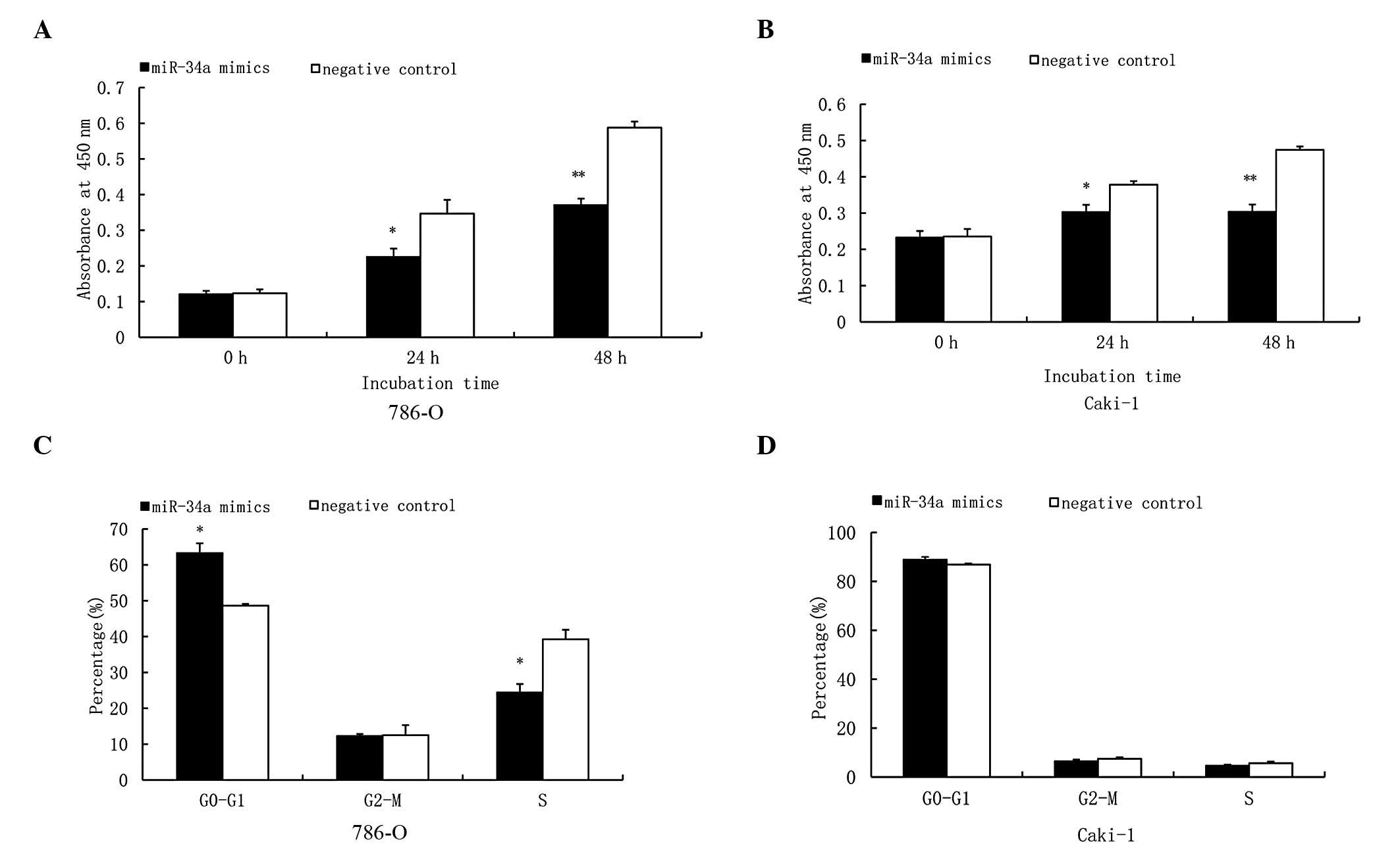
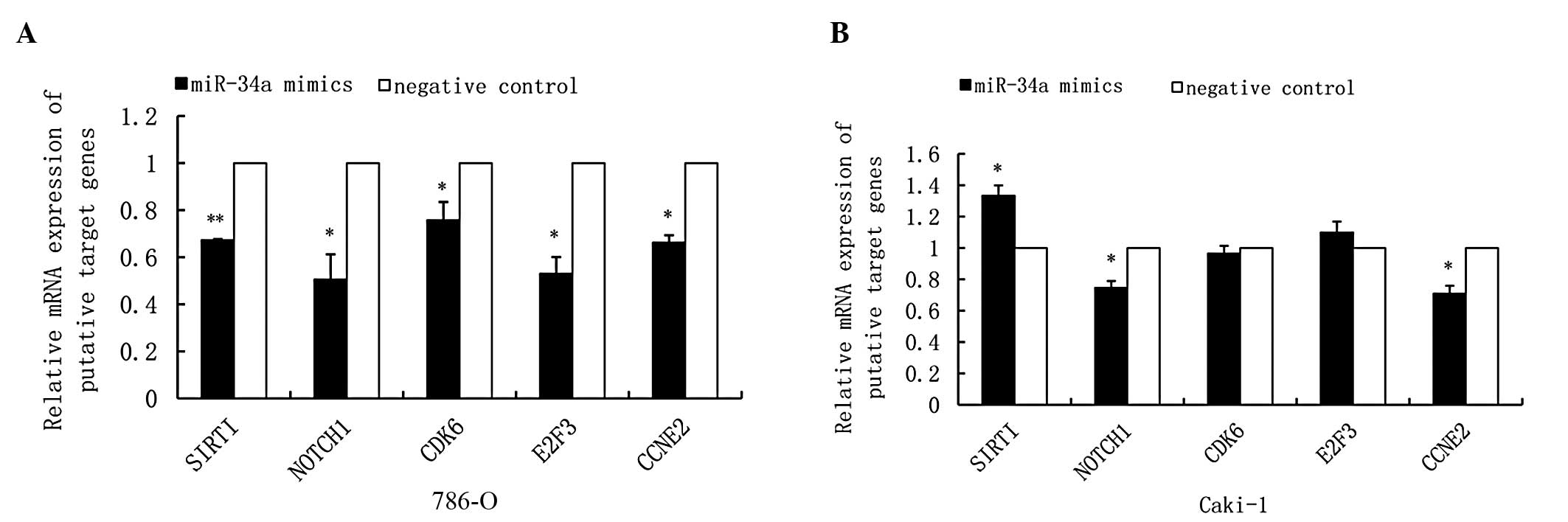
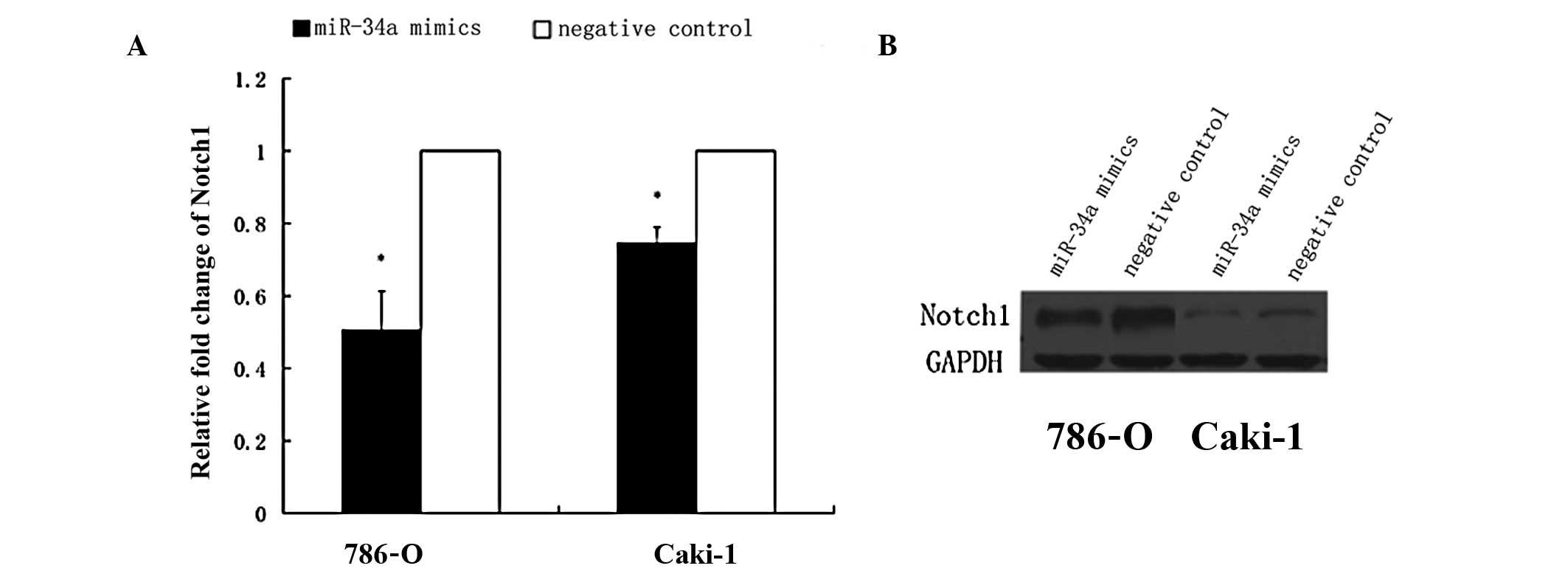
|
1
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar
|
|
3
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar
|
|
4
|
Zhou L, Yin B, Liu Y, Hong Y, Zhang C and
Fan J: Mechanism and function of decreased FOXO1 in renal cell
carcinoma. J Surg Oncol. 105:841–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Yin B, Zhang C, Zhou L and Fan J:
Hsa-let-7a functions as a tumor suppressor in renal cell carcinoma
cell lines by targeting c-myc. Biochem Biophys Res Commun.
417:371–375. 2012. View Article : Google Scholar
|
|
6
|
Chang TC, Wentzel EA, Kent OA, et al:
Transactivation of miR-34a by p53 broadly influences gene
expression and promotes apoptosis. Mol Cell. 26:745–752. 2007.
View Article : Google Scholar
|
|
7
|
Yamamura S, Saini S, Majid S, et al:
MicroRNA-34a modulates c-Myc transcriptional complexes to suppress
malignancy in human prostate cancer cells. PLoS One. 7:e297222012.
View Article : Google Scholar
|
|
8
|
Gallardo E, Navarro A, Viñolas N, et al:
miR-34a as a prognostic marker of relapse in surgically resected
non-small-cell lung cancer. Carcinogenesis. 30:1903–1909. 2009.
View Article : Google Scholar
|
|
9
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar
|
|
10
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar
|
|
11
|
Sun F, Fu H, Liu Q, et al: Downregulation
of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett.
582:1564–1568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar
|
|
13
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive miR-34a induces senescence-like
growth arrest through modulation of the E2F pathway in human colon
cancer cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007.
View Article : Google Scholar
|
|
14
|
Pang RT, Leung CO, Ye TM, et al:
MicroRNA-34a suppresses invasion through downregulation of Notch1
and Jagged1 in cervical carcinoma and choriocarcinoma cells.
Carcinogenesis. 31:1037–1044. 2010. View Article : Google Scholar
|
|
15
|
Li WB, Ma MW, Dong LJ, Wang F, Chen LX and
Li XR: MicroRNA-34a targets notch1 and inhibits cell proliferation
in glioblastoma multiforme. Cancer Biol Ther. 12:477–483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar
|
|
17
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar
|
|
18
|
Bolós V, Grego-Bessa J and de la Pompa JL:
Notch signaling in development and cancer. Endocr Rev. 28:339–363.
2007.
|
|
19
|
Tohda S and Nara N: Expression of Notch1
and Jagged1 proteins in acute myeloid leukemia cells. Leuk
Lymphoma. 42:467–472. 2001. View Article : Google Scholar
|
|
20
|
Reedijk M, Odorcic S, Chang L, et al:
High-level coexpression of JAG1 and NOTCH1 is observed in human
breast cancer and is associated with poor overall survival. Cancer
Res. 65:8530–8537. 2005. View Article : Google Scholar
|
|
21
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar
|
|
22
|
Ai Q, Ma X, Huang Q, et al: High-level
expression of Notch1 increased the risk of metastasis in T1 stage
clear cell renal cell carcinoma. PLoS One. 7:e350222012. View Article : Google Scholar
|
|
23
|
Sjölund J, Boström AK, Lindgren D, et al:
The notch and TGF-β signaling pathways contribute to the
aggressiveness of clear cell renal cell carcinoma. PLoS One.
6:e230572011.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
25
|
Kaghad M, Bonnet H, Yang A, et al:
Monoallelically expressed gene related to p53 at 1p36, a region
frequently deleted in neuroblastoma and other human cancers. Cell.
90:809–819. 1997. View Article : Google Scholar
|
|
26
|
Wei JS, Song YK, Durinck S, et al: The
MYCN oncogene is a direct target of miR-34a. Oncogene.
27:5204–5213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reiter RE, Anglard P, Liu S, Gnarra JR and
Linehan WM: Chromosome 17p deletions and p53 mutations in renal
cell carcinoma. Cancer Res. 53:3092–3097. 1993.
|
|
28
|
Lodygin D, Tarasov V, Epanchintsev A, et
al: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Zhu Y, Xu J, et al: Notch1
activation promotes renal cell carcinoma growth via PI3K/Akt
signaling. Cancer Sci. 103:1253–1258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du R, Sun W, Xia L, et al: Hypoxia-induced
down-regulation of microRNA-34a promotes EMT by targeting the Notch
signaling pathway in tubular epithelial cells. PLoS One.
7:e307712012. View Article : Google Scholar
|
|
31
|
Bueno MJ and Malumbres M: MicroRNAs and
the cell cycle. Biochim Biophys Acta. 1812:592–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nana-Sinkam SP and Croce CM: MicroRNAs as
therapeutic targets in cancer. Transl Res. 157:216–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|