1. Introduction
Osteosarcoma (OS) is the most common malignant
primary bone tumor in children and adolescents. OS has a
predilection for the metaphyseal portions of the long bone, with
the distal femur and proximal tibia accounting for ~50% of all
cases (1). OS is highly aggressive
and it metastasizes primarily to the lung (2). In ~75% of cases, patients with OS are
between 15–25 years old. The median age of an OS patient is 16
years old, with a male predominance. The high incidence of OS
during the adolescence growth spurt indicates there may be a link
between this disease and bone development. The incidence of OS is
also increased in patients with germline mutations in the
retinoblastoma and P53 genes, indicating that these genes may be
involved in the occurrence of the disease. Histologically, OS is
characterized by a proliferation of malignant spindle cells.
Although several histological subtypes may exist, including
chondroblastic and fibroblastic OS, once the osteoid directly
produced by sarcoma cells is found, OS can be diagnosed (3). With regard to the clinical features,
pain and swelling of the soft tissue are the most common symptoms
of OS patients. Up to 20–25% of recently diagnosed patients present
with metastases detectable by radiography, which mainly occur in
the lung. Prior to the use of adjuvant and neoadjuvant
chemotherapy, the long-term survival rate subsequent to surgical
resection alone was <20%. Luckily, multi-agent chemotherapy
regimens that were pioneered in the 1970s and early 1980s have
dramatically improved the survival rate by up to ~70%, and the
necessity for the chemotherapy used for OS patients has been
demonstrated by randomized controlled trials (4). The current national and international
co-operative trial for patients with recently diagnosed OS mainly
builds upon the backbone of cisplatin, doxorubicin and methotrexate
(MTX) treatment. Due to the combination of these anti-OS drugs, the
5-year survival rate in patients with localized disease is ~70%.
However, the survival rate has plateaued since the mid-1980s,
despite advances in anti-OS therapy. In addition, patients with
metastatic or recurrent diseases have a <20% chance of long-term
survival despite aggressive therapies. These figures have changed
little in the past 30 years (5). To
a certain extent, the reason behind this may be ascribed to the
chemoresistance to anti-OS therapy. The development of
chemoresistance in malignant tumors limits the effectiveness of
cytotoxic drugs, and this is particularly true in OS, which is
characterized by the frequent refractoriness to chemotherapy.
Therefore, elucidation of the mechanisms of chemoresistance and
implementation of strategies to overcome chemoresistance will
definitely play a pivotal role in improving the survival rate of OS
patients. This review mainly focuses on the recent studies on the
mechanisms of chemoresistance in OS and the methods to overcome
chemoresistance.
2. Decreased intracellular drug
accumulation
The mechanism behind how the majority of
chemotherapy drugs are absorbed by the tumor cells is unclear.
Impaired transport of MTX, an effective inhibitor of dihydrofolate
reductase, is a common mechanism of resistance in OS (6). As MTX enters cells through the reduced
folate carrier (RFC) at the cell membrane, the decreased expression
of RFC is proven to be associated with MTX-resistance (Fig. 1) (7). In one study, decreased RFC expression
was found in 65% of OS biopsy samples, and decreased RFC expression
was more commonly found in samples with a poor histological
response to chemotherapy (8).
Another study demonstrated that RFC1 expression was moderately
decreased in OS samples with a poor histological response to
pre-operative treatment, and RFC expression was also lower in
initial OS biopsy specimens compared with metastatic specimens
(9). A subsequent confirmatory
study assessing the RFC protein level found that the protein levels
of RFC were lower in primary OS biopsy samples than recurrent tumor
specimens, and tumors with poor histological responses to
pre-operative chemotherapy exhibited significantly lower RFC levels
at diagnosis than those with favorable responses. However, notably,
post-chemotherapy progression to recurrence was associated with a
significant increase in RFC expression (10). Subsequently, the functionality of
the altered RFC proteins has been studied. One of the altered RFC
proteins, Leu291Pro, has been demonstrated to confer drug
resistance since the carrier is unable to translocate the substrate
across the cell membrane, and three alterations, Ser46Asn, Ser4Pro
and Gly259Trp, confer a certain degree of resistance to MTX via a
decreased rate of transport (11).
Furthermore, studies that have focused on the RFC gene have also
been reported. Analysis of the RFC gene copy number by dot blot and
Southern blot has not identified any variation between the parental
cell lines and their MTX-resistant variants, indicating that the
reduced RFC expression was not due to gene deletion (12). Sequence alterations in the RFC have
been observed, and OS tumor samples with RFC sequence alterations
have been shown to possess significantly higher frequencies of
inferior histological response (Huvos grade I or II). However, in a
study by Yang et al (13),
it was not clear if any of these sequence alterations were
germline- or tumor-specific, as normal tissue and peripheral blood
were not obtained. Although the controversy about RFC remains,
trimetrexate, a novel antifolate that does not require the RFC for
transport into cells, was enrolled in a phase II study of relapsed
or refractory OS patients, and was demonstrated to be effective in
5 out of 38 (13%) patients. In addition, a phase I trial of a
combination of trimetrexate and high-dose MTX in patients with
recurrent OS is ongoing (14).
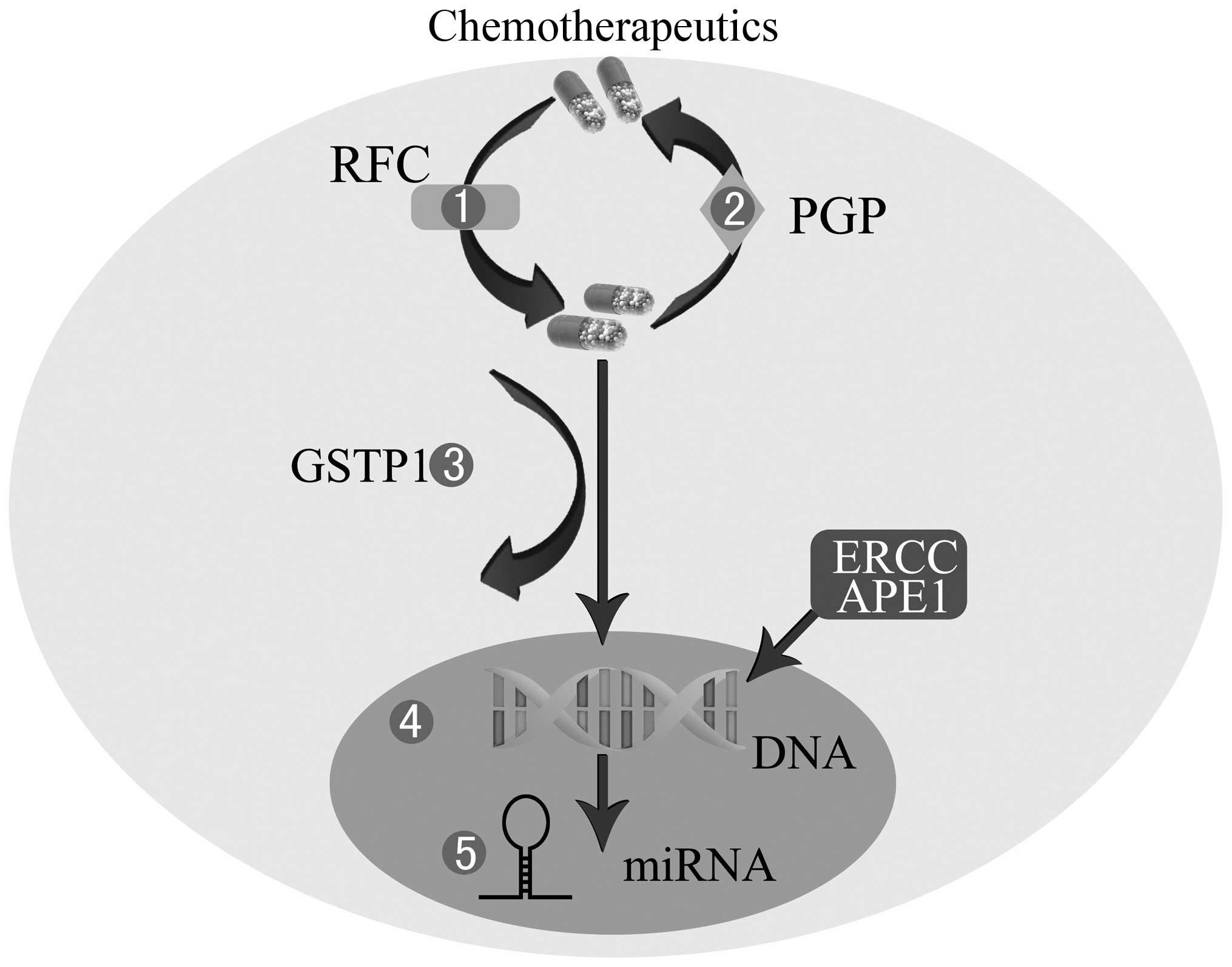 | Figure 1Mechanisms of chemoresistance in OS.
1, Decreased intracellular drug accumulation mediated by lower RFC.
2, Increased efflux of drugs through P-GP. 3, Drug inactivation by
GSTP1. 4, Enhanced DNA repair by APE1 or ERCC. 5, miRNA
dysregulation. OS, osteosarcoma; RFC, reduced folate carrier; P-GP,
P-glycoprotein; GSTP1, glutathione S-transferase P1; APE1, apurinic
endonuclease 1; ERCC, excision repair cross-complementing; miRNA,
microRNAs. |
Another mechanism leading to decreased intracellular
drug accumulation in numerous tumors is the non-specific removal of
cytotoxic drugs from tumor cells by the membrane pump
P-glycoprotein (P-GP) (15). This
membrane-associated protein, a high molecular weight protein of 170
kD coded by the multidrug-resistant (MDR) human gene known as MDR1,
belongs to the ATP-binding cassette (ABC) transporters, and is
considered to act as an efflux pump extruding drugs from the cell
(Fig. 1) (16). A series of studies has found that
the high expression of P-GP may be responsible for doxorubicin
resistance in human or canine OS cell lines (17–19).
Additionally, several retrospective studies have indicated that the
overexpression of P-GP appeared to be associated with tumor
progression, a higher relapse rate and a trend towards a worse
outcome (20,21). By contrast, other studies have found
no correlation between P-GP expression and tumor progression or
event-free survival (22,23). Similarly, a prospective, multicenter
study of 123 non-metastatic OS patients did not reveal any
correlation between P-GP mRNA expression and the risk of disease
progression or relapse (24). A
meta-analysis conclusively showed that P-GP was not associated with
the histological responses of OS patients treated with a
combination of chemotherapy regimens (25). Subsequently, a comparative clinical
pathological study examined histological biopsies from 117 patients
and found that P-GP expression could not serve as a predictor of
treatment response or survival rate of OS patients (26). Furthermore, in OS cell lines
transfected with the MDR gene, an association has been shown
between the increased expression of P-GP and a low aggressive
phenotype (27). In order to
overcome the resistance mechanism caused by P-GP, recent studies
have focused on a novel drug delivery system, consisting of a
biocompatible and lipid-modified polymeric nanoparticle. The
initial results have indicated that this nanoparticle is a
promising platform for delivering doxorubicin and small interfering
RNAs (siRNAs) to the drug-resistant OS cell lines, which may
reverse the decreased intracellular drug accumulation mediated by
P-GP (28–30).
3. Drug inactivation
Human glutathione S-transferase P1 (GSTP1), one of
the cytosolic GSTs that belong to a major group of the phase II
detoxification enzyme superfamilies, inactivates a variety of
exogenous xenobiotics, including mutagens, anticancer agents and
their metabolites (Fig. 1)
(31). It is believed that the
overexpression of GSTP1 is linked to chemoresistance in numerous
cancers (32). A study found that
OS-bearing dogs with higher GSTP1 expression had significantly
shorter median remission and survival times than dogs with a lower
expression of GSTP1 (33). In
another study of human OS specimens obtained from 60 OS patient
cases, an association was shown between the overexpression of GSTP1
at surgery and a poor histological response to pre-operative
chemotherapy (34). Similarly,
another study also found that chemotherapy can induce the
upregulation of GSTP1 protein expression, and that the high
expression of GSTP1 is associated with a poor prognosis (35). In addition, the mRNA expression
levels of GSTP1 in human OS xenografts have been assessed, and a
significant correlation was shown between a higher GSTP1 expression
and a low growth inhibition of OS cells treated with doxorubicin
(36). Furthermore, a study by
Huang et al (37) indicated
that the protective role of GSTP1 in OS cell survival may be
mediated in part by promoting the activation of extracellular
signal-regulated kinase (ERK)1/2 rather than c-Jun N-terminal
kinases (JNK) in HOS OS cells triggered by doxorubicin or
cisplatin. Windsor et al (38) investigated the association of 36
candidate genetic polymorphisms in MTX, adriamycin and cisplatin
pathway genes with the histological response and survival rate in
OS patients and found that a poor histological response was
increased in variants of GSTP1, c.313A>G p.lle(105)Val. A study by Zhang et al
(39) also showed that individuals
with the GSTP1 Val/Val genotype tended to live for less time than
those with the IIe/IIe genotype. However, a study by Yang et
al (40) found that the GSTP1
Val genotypes exhibited significantly higher rates of response to
chemotherapy. These results indicate that GSTP1 polymorphisms may
be candidate pharmacogenomic factors to be explored in the future
to benefit OS patients with chemotherapy.
To overcome GSTP1-related resistance in OS, the
in vitro effectiveness of
6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX), a highly
efficient inhibitor of GSTP1, was tested. A study found that NBDHEX
was extremely active in the resistance to doxorubicin and MTX in
the U-2OS or Saos-2 cell lines (41). A further study on NBDHEX confirmed
that the in vitro activity of NBDHEX was mostly associated
with cytostatic effects, with less evident apoptotic induction and
a positive effect against the metastasization of OS cells (42). Subsequently, a proteomic
investigation was performed and the result demonstrated that NBDHEX
was able to dissociate the GSTP1-tumor necrosis factor
receptor-associated factor (TRAF)2 complex, which restores the
TRAF2/apoptosis signal-regulating kinase 1 (Arabidopsis) signaling,
thereby leading to the simultaneous and prolonged activation of JNK
and p38 (43). These findings may
support the fact that targeting GSTP1 with NBDHEX may be a
promising novel therapeutic possibility for OS patients.
4. Enhanced DNA repair
Chemotherapeutic agents routinely used in the
therapy of OS, including cisplatin and cyclophosphamide, work by
damaging DNA. Therefore, one of the mechanisms associated with the
resistance to these drugs is the enhanced capacity of the cell to
carry out repair on damaged DNA (Fig.
1). In general, cells repair DNA damage via four main
mechanisms: Direct reversal, base excision repair, nucleotide
excision repair and mismatch repair.
Apurinic endonuclease 1 (APE1), one of the main
enzymes involved in the base excision repair pathway, has been
linked to chemosensitivity and prognosis in a number of cancers,
including glioma, melanoma and cervical, prostate and bladder
cancer (44–47). High expression levels of APE1 have
been demonstrated to significantly correlate with the reduced
survival times of OS patients, and decreased APE1 levels in
siRNA-treated human OS cells have led to enhanced cell
sensitization to the DNA damaging agents (48). Similarly, decreased APE1 levels
mediated by siRNA also enhance the sensitivity of human OS cells to
endostatin in vivo (49).
Subsequent to these findings, a study found that the APE1 gene is
amplified in siRNA and APE1 expression, and can serve as an
independent predictor of OS patients with local recurrence or
metastasis (50). Furthermore, to
overcome the increased resilience in cells to chemotherapy caused
by APE1, small molecule inhibitors of the APE1 endonuclease,
including lucanthone, 7-nitroindole-2-carboxylic acid, resveratrol
and arylstibonic acids, have been gradually reported (47,51–53).
However, these inhibitors are either fairly weak or non-specific,
or the effects in cell culture are difficult to reproduce (54). Therefore, the development of
effective small molecule inhibitors of APE1 may benefit OS
patients.
The excision repair cross-complementing (ERCC) set
of proteins, including ERCC1, 2 and 4, belongs to the nucleotide
excision repair system. A study has shown a correlation between
ERCC4 and the histological response to chemotherapy in OS patients
(55). Similarly, the expression of
ERCC4 and ERCC2 mRNA in OS cells has been shown to correlate with
the chemotherapeutic effect in OS patients (56). Subsequent to these findings, a study
found that a polymorphism in the ERCC2 gene was associated with a
positive tumor response and survival rate in cisplatin-treated OS
patients (57). Another study of
common polymorphisms also found a positive association between
ERCC2 polymorphisms and an increased event-free survival rate, and
the result indicated that the variant allele of ERCC2, rs1799793,
could serve as a marker of OS associated with an improved prognosis
following platinum therapy (58).
In addition, an association between polymorphisms in ERCC2 and an
improved cisplatin response and survival rate in OS patients was
also shown in a Chinese population (59).
5. Perturbations in signal transduction
pathways
Perturbations in signal transduction pathways are
likely to be involved in the chemoresistance of tumors. One pathway
that has been intensely studied is the mammalian target of
rapamycin (mTOR) pathway (Fig. 2).
The serine-threonine kinase, mTOR, plays a major role in the
regulation of protein translation, cell growth and metabolism.
Alterations of the mTOR signaling pathway are common in various
cancers, including OS, and the mTOR signaling pathway is being
actively pursued as a therapeutic target (60). In OS cells lines from dogs, mTOR and
its downstream product have been shown to be present and active,
and pathway inhibition by rapamycin decreased the survival rate of
the tumor cells (61). In the human
OS cell lines, HOS and KHOS/NP, the mTOR inhibitor, rapamycin,
downregulated the activity of mTOR and strongly inhibited cell
growth, as apparent by an increase in the G1 phase and a
decrease in the S-phase of the cell cycle, linked with the
downregulation of cyclin D1 (62).
Clinical studies have also been started. A correlation has been
shown between the mTOR/p70S6K signal transduction pathway and OS
patient prognosis, indicating the prognostic significance of the
mTOR/p70S6K signal transduction pathway (63). In an initial testing (phase I) of
rapamycin by the pediatric pre-clinical testing program (PPTP),
rapamycin was demonstrated to possess broad anti-tumor activity
against the PPTP tumor panels in vivo, including that of OS
(64). In a murine model of OS, the
blockade of the mTOR pathway with rapamycin or its analog, cell
cycle inhibitor-779, led to a significant inhibition of
experimental lung metastasis in vivo (65). In addition, a recent study has
revealed that rapamycin treatment reduces the gene expression of
vascular endothelial growth factor (VEGF) and bone morphogenetic
protein-2, and that it inhibits the invasion, proliferation and
migration of murine K7M2 OS cells in vitro (66). These results indicate that the mTOR
pathway may not only decrease the survival of OS tumor cells, but
that it also plays a significant role in metastasis.
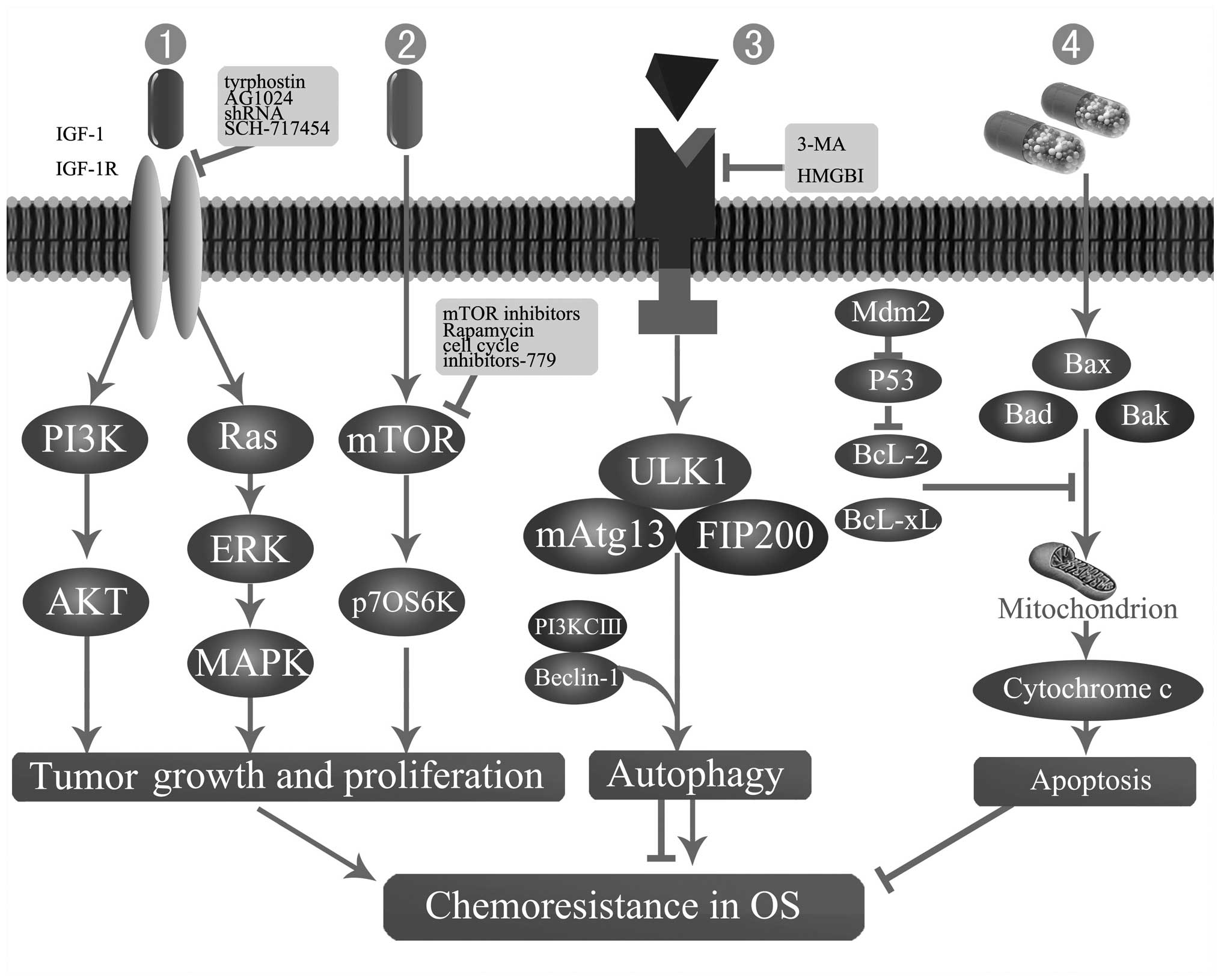 | Figure 2Mechanisms of chemoresistance in OS.
1 and 2, Perturbations in mTOR or IGF-IR signal transduction
pathways. 3 and 4, Apoptosis and autophagy-related chemoresistance.
3-MA, 3-methyladenine; AKT (PKB), protein kinase B; Bad, basal cell
lymphoma 2-associated death protein; Bak, basal cell lymphoma 2
homologous antagonist killer protein; Bax, basal cell lymphoma
2-associated X protein; Bcl-2, basal cell lymphoma 2 protein;
Bcl-xl, basal cell lymphoma extra large protein; ERK, extracellular
signal-regulated kinase; FIP200, family interacting protein of 200
kDa; HMGB1, high mobility group box 1 protein; IGF-I, insulin-like
growth factor I; IGF-IR, IGF-I receptor; MAPK, mitogen activated
protein kinase; mAtg13, mammalian autophagy-related gene 13; Mdm2,
murine double minute 2; mTOR, mammalian target of rapamycin; OS,
osteosarcoma; p70S6K, ribosomal protein S6 kinases, 70 kDa; PI3K,
phosphoinositide 3-kinase; PI3KCIII, PI3K class III; ULK1,
Unc-51-like kinase 1. |
The insulin-like growth factor I receptor (IGF-IR),
a transmembrane receptor with tyrosine kinase activity, is involved
in the initiation and progression of a variety of cancers (67). Activation of the phosphorylation of
IGF-IR leads to subsequent activation of at least two pro-survival
signaling pathways. Following IGF-1R phosphorylation, stimulation
of the phosphoinositol 3-kinase (PI3K)-protein kinase B signaling
pathway is the main event, which leads to cell survival. The second
pathway consists of Ras, Raf and ERK/mitogen-activated protein
kinase (MAPK) activation, which leads to proliferation and tumor
growth (Fig. 2) (68). These two key downstream pathways of
the IGF-IR have also been demonstrated to be activated in OS cell
lines (69). Pre-clinical data has
indicated that IGF-IR may constitute a significant therapeutic
target in a variety of pediatric solid tumors, including
neuroblastoma and musculoskeletal tumors cells (70,71).
Similarly, IGF-1R has been found to be abundantly expressed in OS,
indicating that the inhibition of IGF-IR may be effective in the
therapy of OS (72). A study by Luk
et al (73) indicated that
IGF-1R inhibition by tyrphostin AG1024 together with doxorubicin
achieves an additive anti-OS growth effect, accompanied with
increased apoptosis, cytotoxicity and dual cell cycle arrest, which
indicates that IGF-1R inhibition can enhance the effect of
doxorubicin chemotherapy in OS cell lines. Another study by Wang
et al (74) has shown that
targeting IGF-1R using lentivirus-mediated short hairpin RNA could
lead to growth suppression and the enhanced caspase-3-mediated
apoptosis of OS cells not only in vitro, but also in
vivo. In addition, a recent study indicated that IGF-1R was
involved in the in vitro behavior of metastatic OS cell
lines (75). A subsequent study
found that the expression of the IGF-1R protein was closely
associated with the surgical stage and distant metastasis of OS
patients, and that IGF-1R can be used as an independent prognostic
marker for OS patients (76).
Although the resistance mechanism of IGF-1R
inhibitors remains largely unclear, candidate drugs, including
monoclonal antibodies, small molecule tyrosine kinase inhibitors
and ligand binding antibodies, are being introduced in phase I and
II studies for a wide variety of cancers (77). Ewing sarcoma provides the most clear
evidence of clinical activity. The results of a recently published
phase II trial found that AMG 479 (a fully human monoclonal
antibody to IGF-1R) achieved a clinical benefit rate of 17% in
recurrent refractory Ewing’s family tumors (78). In addition, the efficacy of
SCH-717454 (robatumumab, a fully human neutralizing anti-IGF-1R
antibody) in OS patients is planned to be investigated in a phase
II trial, and the result of the study is eagerly awaited (79).
Additionally, other receptor tyrosine kinases,
including human epidermal growth factor receptor 2 (HER2/neu) and
VEGF have also been recognized as potential targets for the therapy
of OS, as studies have shown that HER2/neu and VEGF expression
correlate with the malignant phenotype in OS (80,81).
Cediranib (AZD-2171), a specific VEGF receptor inhibitor, has been
demonstrated to possess a growth inhibitory effect in solid tumor
xenograft models, including those of OS (82). However, in a phase II trial of the
HER2-targeted agent trastuzumab in combination with cytotoxic
chemotherapy for treatment of metastatic OS patients, no
significant difference was found between the HER2-positive and
HER2-negative groups (83).
Therefore, the therapeutic benefit of this HER2-targeted agent
remains uncertain, and a definitive assessment of the potential
role of trastuzumab in treating OS requires further studies of
patients with HER2-positive disease.
6. Apoptosis and cell cycle-related gene
expression turbulence
Apoptosis is the primary mode of cell death induced
by chemotherapy. Conversely, cell cycle arrest allows the host cell
to repair its damaged DNA prior to cell division, while cells with
excessive DNA damage undergo apoptosis. Therefore, cell cycle and
apoptosis-related gene expression may be involved in the modulation
of chemotherapeutic cytotoxicity (Fig.
2).
The P53 gene, which plays a pivotal role in cell
cycle arrest and in the regulation of apoptosis has been
demonstrated to be involved in the modulation of anticancer drug
cytotoxicity (84). Wild-type or
mutant P53 genes were shown to be associated with the
chemoresistance in OS cells (85).
However, whether the P53 gene takes part in the elevated or
decreased chemoresistance in OS has been confusing. A Study by Wong
et al (86) showed that the
transfection of a mutated form of P53, p53-R273H, can downregulate
the procaspase-3 level and induce resistance to drug toxicity in
the p53-null human Saos-2 cell line. However, the various available
studies have not yielded consistent results. A study by Fan and
Bertino revealed that the induction of p53 conferred resistance to
cisplatin when OS cells were cultured in media containing normal
serum concentrations, whereas p53 induction led to increased
cisplatin sensitivity when cells were grown in low serum media
(87). A study by Tsuchiya et
al (88) demonstrated that the
human OS cell line, Saos2, transfected with wild-type p53, was
twice as sensitive to cisplatin as the parental cells. Furthermore,
another study revealed that the enhanced expression of murine
double minute 2 (Mdm2), a downstream mediator of p53, may inhibit
p53-mediated apoptosis and endow cells with resistance to DNA
damaging agents (89). A further
study found that modified p53, particularly p53 14/19, retains the
pro-apoptotic and transcriptional activity of wide-type p53, and
augments the effectiveness of chemotherapy even in cells
overexpressing Mdm2 (90).
Contradictory results also exist between studies
that focus on the expression of P53 in clinical OS patients. OS
patients with a p53 gene deletion were found to be more sensitive
to pre-operative chemotherapy compared to those without such gene
loss (91). Similarly, several
studies demonstrated a direct correlation between p53-positive
expression and the resistance to therapy or the survival of OS
patients, and concluded that p53 expression may be a useful
prognostic factor in OS patients (92,93).
However, a prospective study found no evidence that P53 mutations
can predict the development of metastases, chemotherapy response
and clinical outcome in patients with high-grade OS (94). Therefore, additional studies are
required to obtain an improved explanation.
B-cell lymphoma 2 (Bcl-2) is the founding member of
a family of proteins associated with cell death signaling, and was
first isolated as the product of an oncogene (95). The Bcl-2 protein family is comprised
of anti-apoptotic proteins, including Bcl-2 and Bcl-xL, and
pro-apoptotic proteins, including Bax, Bak and Bad (96). These proteins mainly regulate
apoptosis at the mitochondrial outer membrane and control the
initiation of mitochondrial outer membrane permeabilization
(97). Studies have shown that
Bcl-2 and Bax have a role in affecting drug-induced apoptosis and
regulating the resistance to chemotherapy in various tumor cells,
including hepatocellular carcinoma and bladder, lung and ovarian
cancer (98–101).
In vitro studies of anti-apoptotic proteins,
the downregulation of Bcl-2 and Bcl-xL by lentivirus-mediated
Bcl-2-knockdown or stable transfection with Bcl-xL cDNA could
significantly enhance the in vitro chemosensitivity of OS
cells to doxorubicin and cisplatin (93,102,103). A synergistic effect, created by
co-silencing Bcl-2 and cyclin D1, was also found to enhance the
chemosensitivity of OS cells (104). Subsequently, a study revealed that
Bcl-xL may exert an anti-apoptotic effect by stimulating oxidative
phosphorylation or inhibiting caspase activation (105). Conversely, downregulation of a
pro-apoptotic protein, Bax, by lentivirus-mediated knockdown
decreases the in vitro chemosensitivity of OS cells
(106,107). In addition, upregulated Bax gene
expression by runt-related transcription factor 2 (Runx2), which
can directly bind to two Runx-specific regulatory elements on the
human Bax promoter, could increase the apoptosis and drug
sensitivity of OS cells (108).
Additionally, another study has demonstrated that although Bax
expression is not affected by the knockdown of c-Myc or caspase-2,
since caspase-2 is important for cytosolic Bax to integrate into
the outer mitochondrial membrane, and as c-Myc is critical for the
oligomerization of Bax, that during cytotoxic drug-induced
apoptosis, c-Myc and caspase-2 remain involved in activating Bax
(109).
In clinical trials, a higher cellular expression
level of Bcl-2 has been shown in high-grade OS patients with
recurrent pulmonary metastases compared with those with primary
tumors, and the expression of Bcl-2 was also shown to be closely
associated with the prognosis of OS patients (110,111). Subsequently, a higher mRNA
expression level of Bcl-xL was found to significantly correlate
with an advanced clinical stage and a poorer survival rate in OS
patients (112). However, although
Bc1–2 is highly expressed in the specimens of OS patients, no
correlation between the expression of Bc1–2 and chemosensitivity
and the overall survival of high-grade OS patients was shown in the
study by Nedelcu et al (113). Similarly, Bax and Bcl-2 protein
expression was observed in OS patients, but proteins were found to
be unable to predict the overall or disease-free survival rate.
Nevertheless, an increased Bax/Bcl-2 protein expression ratio was
associated with a decreased 4-year survival and disease-free
survival rate of OS patients (114,115).
7. Autophagy-related chemoresistance
Autophagy is a homeostatic and evolutionarily
conserved process that degrades cellular organelles and proteins
and maintains cellular biosynthesis (116). This process can be triggered under
physiological conditions, including nutrient starvation and growth
factor deprivation, or in response to a variety of stress stimuli,
including hypoxia and the exposition to cytotoxic compounds
(117). Autophagy has been
referred to as a double-edged sword. On one hand, it allows tumor
cells to survive bioenergetic stress via clearance of damaged
organelles and proteins under adverse conditions (116). On the other hand, in certain
cellular contexts, sustained or excessive tumor cell autophagy
promotes programmed cell death, particularly in apoptosis-defective
cells, although certain studies have considered autophagic cell
death to be a misnomer (118,119). In recent years, numerous studies
have focused on the association between autophagy and the
chemoresistance of tumor cells. In leukemia and colon cancer cell
lines, the inhibition of autophagy was shown to sensitize resistant
cells to tumor necrosis factor-related apoptosis-inducing
ligand-mediated apoptosis (120).
In addition, the ability of autophagy inhibition to enhance
chemosensitivity and tumor regression was confirmed in various
animal models. Firstly, the inhibition of autophagy by chloroquine
was shown to increase apoptosis and enhance tumor cell death in
lymphoma, colon cancer and prostate cancer xenograft mouse models
(121–123). Secondly, autophagy inhibition
triggered by 3-methyladenine (3-MA) increased apoptotic induction
by 5-fluorouracil in association with tumor regression in colon
cancer xenografts (124). Thirdly,
multiple studies have revealed that the inhibition of autophagy by
the knockdown of autophagy-related genes can effectively enhance
tumor cell death induced by diverse anti-cancer drugs in
pre-clinical models (125,126).
Subsequent studies on the dual role of autophagy in
OS have been published. A study by Lambert et al (128) found that induction of autophagy
was shown in U2OS cells treated with doxorubicin and roscovitine,
and it was considered that autophagy may be the cause of increased
cytotoxicity. One study by Meschini et al (129) demonstrated that autophagy induced
by a natural product, bisindolic alkaloid voacamine, showed a
lethal effect, which is effective against drug-resistant OS cell
lines either used alone or in association with conventional
chemotherapeutics. A study by Kim et al (130) found that in the Saos-2 cell line,
the inhibition of autophagy along with 3-MA significantly increased
paclitaxel (PCX)-induced apoptotic cell death. It was indicated
that a combination of treatment involving autophagy inhibitor
therapy and low-dose PCX therapy could be an effective and potent
strategy for improving the chemotherapy for OS, although PCX has
not been incorporated in the current protocols for OS treatment. By
contrast, a study by Zhang et al (131) found that following the
downregulation of autophagy in the MG63 cells by the autophagy
inhibitor, 3-MA, the chemotherapeutic sensitivity of MG63 cells
treated with cisplatin was enhanced, which indicated that autophagy
may have a protective effect on OS cells. Similarly, a study by
Coupienne et al (132)
found that autophagy protected OS cells against photodynamic
therapy-induced cell death and thus provided an improved survival
rate for the OS cells. The protective role of OS cells mediated by
autophagy was also shown in studies from the Central Laboratory of
the Second Xiangya Hospital (Changsha, China). The results
demonstrated that autophagy induced by the high mobility group box
1 protein (HMGB1), a highly conserved nuclear protein, increased
chemoresistance to conventional anti-OS agents, including
doxorubicin, cisplatin and MTX. Subsequently, our further studies
identified that HMGB1 bound to the autophagy regulator Beclin1, and
the interaction between HMGB1 and Beclin1 was dependent upon the
autophagic complex, ULK1-mAtg13-FIP200. The formation of the
Beclin1-PI3K class 3 complex that facilitates autophagic
progression was also regulated by HMGB1 (Fig. 2) (133,134).
8. microRNA (miRNA) dysregulation
miRNAs are a class of small non-coding regulatory
RNA molecule that have recently been shown to be involved in a wide
array of biological processes (135). The abnormal expression of miRNA
has been indicated to be associated with various cancers (136,137). When miR-34a expression was
enforced, as shown by the functional analysis of miR-34a in Ewing’s
sarcoma cell lines, this indicated that the cells were sensitized
to doxorubicin and vincristine (138). A study by Gougelet et al
(139) found that OS of rat and
human origins showed an miRNA signature that could discriminate
promising from unpromising responders for ifosfamide treatment. The
study also identified five discriminating miRNAs (miR-92a, miR-99b,
miR-132, miR-193a-5p and miR-422a) in tumors of OS patients, which
could be used as a potent diagnostic tool to predict tumor
sensitivity to ifosfamide. In addition, a study by Song et
al (140) found that the
expression of miR-140 was involved in the chemoresistance to OS
xenografts by reduced cell proliferation via G1- and
G2-phase arrest. Their subsequent study indicated that
G2 arrest was induced by a decrease in cell
proliferation stimulated by miR-215 via the suppression of
denticleless protein homolog expression, which resulted in an
increase in MTX-chemoresistance in the human OS cell lines, U-2 and
MG63 (141). Furthermore, a study
by Cai et al (142) found
that miR-15a and miR-16-1 downregulated cyclin D1 and induced
apoptosis and cell cycle arrest in OS, indicating that miR-15a and
miR-16-1 may be used for OS therapy (Fig. 1).
9. Cancer stem cells (CSCs) and drug
resistance
The CSC hypothesis, first proposed ~50 years ago,
postulates that a small subpopulation of cancer cells with an
unlimited proliferative capacity drives tumor self-renewal and
differentiation (143). However,
no substantial progress was made in the CSC hypothesis until Bonnet
and Dick (144) first isolated a
subpopulation of human acute myeloid leukemia cells with a
CD34++/CD38− phenotype, where
CD34++ has a stronger affinity to the antigen.
Subsequently, CSCs have been shown to be indicated in the
pathogenesis of numerous tumors, including leukemia, brain tumors
and cortical glial tumors (144–146). Studies have also found that CSCs
may be involved in the mechanisms of chemoresistance (147–149).
Although the specific role that CSCs play in the
chemoresistance of OS cells has not been clearly elucidated,
several of the aforementioned mechanisms could mediate the
intrinsic chemoresistance in CSCs. A study by Di Fiore et al
(150) found that a novel CSC cell
line, 3AB-OS, irreversibly selected from human OS MG-63 cells by
long-term treatment with 3-aminobenzamide (3AB), expressed higher
levels of the ABC transporter, ABCG2 (a drug resistance marker),
with a high-drug efflux capacity and anti-apoptosis genes,
including FADD-like apoptosis regulating protein-L, Bcl-2, X-linked
inhibitor of apoptosis protein, inhibitor of apoptosis proteins and
survivin. A study by Fujii et al (151) found that the MG63 OS cell line
possessed an ability to form clonal expanding colonies
(sarcospheres), which show a strong resistance to doxorubicin and
cisplatin due to the increased expression of the DNA repair enzyme
genes, MutL homolog 1 and MutS protein homolog 2. Additionally,
caffeine, a DNA repair inhibitor, enhanced the efficacy of these
drugs, indicating that the drug resistance in sarcosphere cells was
partly associated with the efficient DNA repair ability. Their
subsequent study indicated that CSCs and the sarcosphere cells from
the MG63 cell line showed a strong chemoresistance against
doxorubicin and cisplatin, which may be attributed to the efficient
detoxification by elevated aldehyde dehydrogenase 1 mRNA expression
(152). In addition, a study by
Martins-Neves et al (153)
showed that OS cells contained a CSC population relatively
resistant to doxorubicin and MTX, and this resistant phenotype
appeared to be associated with the high expression of the drug
efflux transporter, P-GP.
10. Conclusions
Although great progress has been made by combination
chemotherapy and aggressive surgical resection in the treatment of
OS, the survival rate of OS patients with localized disease at
diagnosis has plateaued at ~70% since the mid-1980s, and the
long-term survival rate of patients with metastatic or recurrent
disease remains at <20% (154).
Accordingly, an improved understanding of the molecular mechanisms
of chemoresistance and the identification of novel strategies to
circumvent the resistance mechanisms are desperately required. As
mentioned in the present review, chemoresistance in OS has been
shown to occur by a variety of mechanisms, including decreased
intracellular drug accumulation mediated by RFC or P-GP, drug
inactivation by GSTP1, enhanced DNA repair by APE1 or ERCC,
perturbations in mTOR or IGF-IR signal transduction pathways,
apoptosis and autophagy-related chemoresistance, miRNA
dysregulation and CSC-mediated drug resistance. In addition, the
interaction between OS cells and their micro-environment has also
been shown to be involved in the chemoresistance in OS, and
therapies disrupting this interaction have been demonstrated to be
efficacious in OS treatment in pre-clinical studies. However,
almost all these studies on the mechanisms of chemoresistance in OS
are at an early stage, and further studies are eagerly anticipated
on the following aspects.
On the basis of the current understanding of the
mechanism of resistance mediated by RFC, a novel antifolate,
trimetrexate, which does not require the RFC for transport into
cells, has been already demonstrated to be effective in OS patients
in a phase II study (14). Prior to
its use in clinical trials, further clinical studies are required
to assess the effect of trimetrexate in OS patients either used
alone or in combination with other anti-OS drugs. To circumvent the
mechanism of resistance mediated by P-GP and to improve
intracellular drug accumulation, novel delivery patterns, including
biocompatible nanoparticles and liposomal encapsulation, have
emerged and have been shown to improve delivery efficacy in several
studies (155). Further studies
should be focused on the co-administration of nanoparticles
combined with conventional chemotherapy and an efflux pump
inhibitor, and the precise mechanism of the interaction between
these drugs also deserves further investigation.
In the past two decades, studies about the signal
transduction pathways and targets involved in the malignant
behavior of OS led to the development of a variety of novel
targeted therapeutic agents for OS, including IGF-1R antibodies and
mTOR inhibitors (65,70). The following challenge is to
identify promising agents in the treatment of OS, which requires
more trials for successful design and completion. In addition, an
improved understanding of the targeted molecules of signal
transduction pathways that regulate cell proliferation and growth
and the interaction between these pathways will lead to the
development of numerous novel targeted agents.
The association between autophagy and
chemoresistance in tumors attracts more and more attention in
studies. However, the exact role that autophagy plays in cancer
drug-resistance remains controversial, and studies on the autophagy
and chemoresistance of OS remain rare (156). Similarly, little is known about
the autophagy-related pathways and the association with apoptosis.
Therefore, elucidating the signaling pathways of autophagy and the
association with apoptosis in OS cells is definitely of great
significance, and will bring a novel perspective on the therapy of
OS.
Recently, miRNA has become a hot spot in the area of
molecular biology. The majority of studies are focusing on
elucidating the impact of miRNAs in the chemoresistance of a
variety of tumors, including OS. However, almost all these studies
are immature (140,141). In the future, utilizing
high-throughput miRNA expression analysis to identify miRNAs
associated with chemoresistance should be continued. Meanwhile,
further studies are required to define chemoresistance-related
molecular pathways mediated by miRNA.
Following a period of silence, CSCs have returned to
the study horizons again. An increase in CSC studies has revealed
implications for CSCs in the drug resistance and tumor metastasis
of OS. However, numerous problems remain. For instance, methods
used for the isolation and identification of CSCs require a degree
of improvement, and the role that CSCs play in OS metastasis and
the in-depth mechanism of CSC-mediated drug resistance in OS
require further systemic study (146–153).
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81272947).
References
|
1
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: current challenges and future
directions. Expert Rev Anticancer There. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: state
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar
|
|
4
|
Eilber FR and Rosen G: Adjuvant
chemotherapy for osteosarcoma. Semin Oncol. 16:312–322.
1989.PubMed/NCBI
|
|
5
|
Sakamoto A and Iwamoto Y: Current status
and perspectives regarding the treatment of osteo-sarcoma:
chemotherapy. Rev Recent Clin Trials. 3:228–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertino JR: Karnofsky memorial lecture.
Ode to methotrexate. J Clin Oncol. 11:5–14. 1993.PubMed/NCBI
|
|
7
|
Hattinger CM, Reverter-Branchat G,
Remondini D, et al: Genomic imbalances associated with methotrexate
resistance in human osteosarcoma cell lines detected by comparative
genomic hybridization-based techniques. Eur J Cell Biol.
82:483–493. 2003. View Article : Google Scholar
|
|
8
|
Guo W, Healey JH, Meyers PA, Ladanyi M,
Huvos AG, Bertino JR and Gorlick R: Mechanisms of methotrexate
resistance in osteosarcoma. Clin Cancer Res. 5:621–627. 1999.
|
|
9
|
Patiño-García A, Zalacaín M, Marrodán L,
San-Julián M and Sierrasesúmaga L: Methotrexate in pediatric
osteosarcoma: response and toxicity in relation to genetic
polymorphisms and dihydrofolate reductase and reduced folate
carrier 1 expression. J Pediatr. 154:688–693. 2009.
|
|
10
|
Ifergan I, Meller I, Issakov J and Assaraf
YG: Reduced folate carrier protein expression in osteosarcoma:
implications for the prediction of tumor chemosensitivity. Cancer.
98:1958–1966. 2003. View Article : Google Scholar
|
|
11
|
Flintoff WF, Sadlish H, Gorlick R, Yang R
and Williams FM: Functional analysis of altered reduced folate
carrier sequence changes identified in osteosarcomas. Biochim
Biophys Acta. 1690:110–117. 2004. View Article : Google Scholar
|
|
12
|
Serra M, Reverter-Branchat G, Maurici D,
et al: Analysis of dihydrofolate reductase and reduced folate
carrier gene status in relation to methotrexate resistance in
osteosarcoma cells. Ann Oncol. 15:151–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang R, Sowers R, Mazza BA, et al:
Sequence alterations in the reduced folate carrier are observed in
osteosarcoma tumor samples. Clin Cancer Res. 9:837–844. 2003.
|
|
14
|
Trippett T, Meyers P, Gorlick R, et al:
High dose trimetrexate with leucovorin protection in recurrent
childhood malignancies: a phase II trial. J Clin Oncol (ASCO Annual
Meeting Abstracts). 9:8891999.
|
|
15
|
Weinstein RS, Kuszak JR, Kluskens LF and
Coon JS: P-glycoproteins in pathology: the multidrug resistance
gene family in humans. Hum Pathol. 21:34–48. 1990. View Article : Google Scholar
|
|
16
|
Safa AR, Stern RK, Choi K, et al:
Molecular basis of preferential resistance to colchicine in
multidrug-resistant human cells conferred by Gly-185→Val-185
substitution in P-glycoprotein. Proc Natl Acad Sci USA.
87:7225–7229. 1990.PubMed/NCBI
|
|
17
|
Bramwell VH: osteosarcomas and other
cancers of bone. Curr Opin Oncol. 12:330–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park YB, Kim HS, Oh JH and Lee SH: The
co-expression of p53 protein and P-glycoprotein is correlated to a
poor prognosis in osteosarcoma. Int Orthop. 24:307–310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomes CM, van Paassen H, Romeo S, et al:
Multidrug resistance mediated by ABC transporters in osteosarcoma
cell lines: mRNA analysis and functional radiotracer studies. Nucl
Med Biol. 33:831–840. 2006. View Article : Google Scholar
|
|
20
|
Serra M, Pasello M, Manara MC, et al: May
P-glycoprotein status be used to stratify high-grade osteosarcoma
patients? Results from the Italian/Scandinavian Sarcoma Group 1
treatment protocol. Int J Oncol. 29:1459–1468. 2006.
|
|
21
|
Baldini N, Scotlandi K, Serra M, Picci P,
Bacci G, Sottili S and Campanacci M: P-glycoprotein expression in
osteosarcoma: a basis for risk-adapted adjuvant chemotherapy. J
Orthop Res. 17:629–632. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kusuzaki K, Hirata M, Takeshita H, Murata
H, Hashiguchi S, Ashihara T and Hirasawa Y: Relationship between
P-glycoprotein positivity, doxorubicin binding ability and
histologic response to chemotherapy in osteosarcomas. Cancer Lett.
138:203–208. 1999. View Article : Google Scholar
|
|
23
|
Trammell RA, Johnson CB, Barker JR, Bell
RS and Allan DG: Multidrug resistance-1 gene expression does not
increase during tumor progression in the MGH-OGS murine
osteosarcoma tumor model. J Orthop Res. 18:449–455. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wunder JS, Bull SB, Aneliunas V, et al:
MDR1 gene expression and outcome in osteosarcoma: a prospective,
multicenter study. J Clin Oncol. 18:2685–2694. 2000.PubMed/NCBI
|
|
25
|
Pakos EE and Ioannidis JP: The association
of P-glycoprotein with response to chemotherapy and clinical
outcome in patients with osteosarcoma. A meta-analysis. Cancer.
98:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sorensen FB, Jensen K, Vaeth M, et al:
Immunohistochemical estimates of angiogenesis, proliferative
activity, p53 expression, and multiple drug resistance have no
prognostic impact in osteosarcoma: A comparative
clinicopathological investigation. Sarcoma. 2008:8740752008.
View Article : Google Scholar
|
|
27
|
Takeshita H, Kusuzaki K, Murata H, et al:
Osteoblastic differentiation and P-glycoprotein multidrug
resistance in a murine osteosarcoma model. Br J Cancer.
82:1327–1331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Susa M, Iyer AK, Ryu K, Choy E, Hornicek
FJ, Mankin H, Milane L, Amiji MM and Duan Z: Inhibition of ABCB1
(MDR1) expression by an siRNA nanoparticulate delivery system to
overcome drug resistance in osteosarcoma. PLoS One. 5:e107642010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Susa M, Iyer AK, Ryu K, Hornicek FJ,
Mankin H, Amiji MM and Duan Z: Doxorubicin loaded polymeric
nanoparticulate delivery system to overcome drug resistance in
osteosarcoma. BMC Cancer. 9:3992009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi E, Iyer AK, Hornicek FJ, Amiji
MM and Duan Z: Lipid-functionalized dextran nanosystems to overcome
multidrug resistance in cancer: a pilot study. Clin Orthop Relat
Res. 471:915–925. 2013. View Article : Google Scholar
|
|
31
|
Townsend DM and Tew KD: The role of
glutathione-S-transferase in anti-cancer drug resistance. Oncogene.
22:7369–7375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tew KD: Glutathione-associated enzymes in
anticancer drug resistance. Cancer Res. 54:4313–4320.
1994.PubMed/NCBI
|
|
33
|
Shoieb A and Hahn K: Detection and
significance of glutathione-S-transferase pi in osteogenic tumors
of dogs. Int J Oncol. 10:635–639. 1997.
|
|
34
|
Uozaki H, Horiuchi H, Ishida T, Iijima T,
Imamura T and Machinami R: Overexpression of resistance-related
proteins (metallothioneins, glutathione-S-transferase pi, heat
shock protein 27, and lung resistance-related protein) in
osteosarcoma. Relationship with poor prognosis. Cancer.
79:2336–2344. 1997. View Article : Google Scholar
|
|
35
|
Wei L, Song XR, Wang XW, Li M and Zuo WS:
Expression of MDR1 and GST-pi in osteosarcoma and soft tissue
sarcoma and their correlation with chemotherapy resistance.
Zhonghua Zhong Liu Za Zhi. 28:445–448. 2006.(In Chinese).
|
|
36
|
Bruheim S, Bruland OS, Breistol K,
Maelandsmo GM and Fodstad O: Human osteosarcoma xenografts and
their sensitivity to chemotherapy. Pathol Oncol Res. 10:133–141.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang G, Mills L and Worth LL: Expression
of human glutathione S-transferase P1 mediates the chemosensitivity
of osteosarcoma cells. Mol Cancer Ther. 6:1610–1619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Windsor RE, Strauss SJ, Kallis C, Wood NE
and Whelan JS: Germline genetic polymorphisms may influence
chemotherapy response and disease outcome in osteosarcoma: a pilot
study. Cancer. 118:1856–1867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang SL, Mao NF, Sun JY, Shi ZC, Wang B
and Sun YJ: Predictive potential of glutathione S-transferase
polymorphisms for prognosis of osteosarcoma patients on
chemotherapy. Asian Pac J Cancer Prev. 13:2705–2709. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang LM, Li XH and Bao CF: Glutathione
S-transferase P1 and DNA polymorphisms influence response to
chemotherapy and prognosis of bone tumors. Asian Pac J Cancer Prev.
13:5883–5886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pasello M, Michelacci F, Scionti I,
Hattinger CM, Zuntini M, Caccuri AM, Scotlandi K, Picci P and Serra
M: Overcoming glutathione S-transferase P1-related cisplatin
resistance in osteosarcoma. Cancer Res. 68:6661–6668. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pasello M, Manara MC, Michelacci F, et al:
Targeting glutathione-S transferase enzymes in musculoskeletal
sarcomas: a promising therapeutic strategy. Anal Cell Pathol
(Amst). 34:131–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sau A, Filomeni G, Pezzola S, et al:
Targeting GSTP1-1 induces JNK activation and leads to apoptosis in
cisplatin-sensitive and -resistant human osteosarcoma cell lines.
Mol Biosyst. 8:994–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sak SC, Harnden P, Johnston CF, Paul AB
and Kiltie AE: APE1 and XRCC1 protein expression levels predict
cancer-specific survival following radical radiotherapy in bladder
cancer. Clin Cancer Res. 11:6205–6211. 2005. View Article : Google Scholar
|
|
45
|
Evans AR, Limp-Foster M and Kelley MR:
Going APE over ref-1. Mutat Res. 461:83–108. 2000. View Article : Google Scholar
|
|
46
|
Silber JR, Bobola MS, Blank A, Schoeler
KD, Haroldson PD, Huynh MB and Kolstoe DD: The
apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1
contributes to human glioma cell resistance to alkylating agents
and is elevated by oxidative stress. Clin Cancer Res. 8:3008–3018.
2002.
|
|
47
|
Yang S, Irani K, Heffron SE, Jurnak F and
Meyskens FL Jr: Alterations in the expression of the
apurinic/apyrimidinic endonuclease-1/redox factor-1 (Ape/Ref-1) in
human melanoma and identification of the therapeutic potential of
resveratrol as an Ape1/Ref-1 inhibitor. Mol Cancer Ther.
4:1923–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang D, Luo M and Kelley MR: Human
apurinic endonuclease 1 (APE1) expression and prognostic
significance in osteosarcoma: enhanced sensitivity of osteosarcoma
to DNA damaging agents using silencing RNA APE1 expression
inhibition. Mol Cancer Ther. 3:679–686. 2004.
|
|
49
|
Wang D, Zhong ZY, Li MX, Xiang DB and Li
ZP: Vector-based Ape1 small interfering RNA enhances the
sensitivity of human osteosarcoma cells to endostatin in
vivo. Cancer Sci. 98:1993–2001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang JL, Yang D, Cogdell D, et al: APEX1
gene amplification and its protein overexpression in osteosarcoma:
correlation with recurrence, metastasis, and survival. Technol
Cancer Res Treat. 9:161–169. 2010. View Article : Google Scholar
|
|
51
|
Luo M and Kelley MR: Inhibition of the
human apurinic/apyrimidinic endonuclease (APE1) repair activity and
sensitization of breast cancer cells to DNA alkylating agents with
lucanthone. Anticancer Res. 24:2127–2134. 2004.PubMed/NCBI
|
|
52
|
Madhusudan S, Smart F, Shrimpton P, et al:
Isolation of a small molecule inhibitor of base excision repair.
Nucleic Acids Res. 33:4711–4724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seiple LA, Cardellina JH II, Akee R and
Stivers JT: Potent inhibition of human apurinic/apyrimidinic
endonuclease 1 by arylstibonic acids. Mol Pharmacol. 73:669–677.
2008. View Article : Google Scholar
|
|
54
|
Fishel ML and Kelley MR: The DNA base
excision repair protein Ape1/Ref-1 as a therapeutic and
chemopreventive target. Mol Aspects Med. 28:375–395. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nathrath M, Kremer M, Letzel H, Remberger
K, Höfler H and Ulle T: Expression of genes of potential importance
in the response to chemotherapy in osteosarcoma patients. Klin
Padiatr. 214:230–235. 2002.(In German).
|
|
56
|
Li X, Guo W, Shen DH, Yang RL, Liu J and
Zhao H: Expressions of ERCC2 and ERCC4 genes in osteosarcoma and
peripheral blood lymphocytes and their clinical significance.
Beijing Da Xue Xue Bao. 39:467–471. 2007.(In Chinese).
|
|
57
|
Caronia D, Patiño-García A, Milne RL,
Zalacain-Díez M, Pita G, Alonso MR, Moreno LT, et al: Common
variations in ERCC2 are associated with response to cisplatin
chemotherapy and clinical outcome in osteosarcoma patients.
Pharmacogenomics J. 9:347–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Biason P, Hattinger CM, Innocenti F,
Talamini R, Alberghini M, Scotlandi K, Zanusso C, Serra M and
Toffoli G: Nucleotide excision repair gene variants and association
with survival in osteosarcoma patients treated with neoadjuvant
chemotherapy. Pharmacogenomics J. 12:476–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hao T, Feng W, Zhang J, Sun YJ and Wang G:
Association of four ERCC1 and ERCC2 SNPs with survival of bone
tumour patients. Asian Pac J Cancer Prev. 13:3821–3824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meric-Bernstam F and Gonzalez-Angulo AM:
Targeting the mTOR signaling network for cancer therapy. J Clin
Oncol. 27:2278–2287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gordon IK, Ye F and Kent MS: Evaluation of
the mammalian target of rapamycin pathway and the effect of
rapamycin on target expression and cellular proliferation in
osteosarcoma cells from dogs. Am J Vet Res. 69:1079–1084. 2008.
View Article : Google Scholar
|
|
62
|
Gazitt Y, Kolapathi V, Moncada K, Thomas C
and Freeman J: Targeted therapy of human osteosarcoma with 17AAG or
rapamycin: characterization of induced apoptosis and inhibition of
mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 34:551–561. 2009.
|
|
63
|
Zhou Q, Deng Z, Zhu Y, Long H, Zhang S and
Zhao J: mTOR/p70S6K signal transduction pathway contributes to
osteosarcoma progression and patients’ prognosis. Med Oncol.
27:1239–1245. 2010.
|
|
64
|
Houghton PJ, Morton CL, Kolb EA, et al:
Initial testing (stage 1) of the mTOR inhibitor rapamycin by the
pediatric preclinical testing program. Pediatr Blood Cancer.
50:799–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wan X, Mendoza A, Khanna C and Helman LJ:
Rapamycin inhibits ezrin-mediated metastatic behavior in a murine
model of osteosarcoma. Cancer Res. 65:2406–2411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mu X, Isaac C, Schott T, Huard J and Weiss
K: Rapamycin inhibits ALDH activity, resistance to oxidative
stress, and metastatic potential in murine osteosarcoma cells.
Sarcoma. 2013:4807132013.
|
|
67
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chitnis MM, Yuen JS, Protheroe AS, Pollak
M and Macaulay VM: The type 1 insulin-like growth factor receptor
pathway. Clin Cancer Res. 14:6364–6370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chou AJ, Merola PR, Sowers R, et al:
Analysis of aberrant signal transduction pathways in osteosarcoma
cell lines. Proc Amer Assoc Cancer Res. 46:45512005.
|
|
70
|
Scotlandi K, Manara MC, Nicoletti G, et
al: Antitumor activity of the insulin-like growth factor-I receptor
kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res.
65:3868–3876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tanno B, Mancini C, Vitali R, et al:
Down-regulation of insulin-like growth factor I receptor activity
by NVP-AEW541 has an antitumor effect on neuroblastoma cells in
vitro and in vivo. Clin Cancer Res. 12:6772–6780. 2006. View Article : Google Scholar
|
|
72
|
Hassan SE, Bekarev M, Kim MY, Lin J,
Piperdi S, Gorlick R and Geller DS: Cell surface receptor
expression patterns in osteosarcoma. Cancer. 118:740–749. 2012.
View Article : Google Scholar
|
|
73
|
Luk F, Yu Y, Walsh WR and Yang JL:
IGF1R-targeted therapy and its enhancement of doxorubicin
chemosensitivity in human osteosarcoma cell lines. Cancer Invest.
29:521–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang YH, Xiong J, Wang SF, Yu Y, Wang B,
Chen YX, Shi HF and Qiu Y: Lentivirus-mediated shRNA targeting
insulin-like growth factor-1 receptor (IGF-1R) enhances
chemosensitivity of osteosarcoma cells in vitro and in vivo. Mol
Cell Biochem. 341:225–233. 2010. View Article : Google Scholar
|
|
75
|
Rettew AN, Young ED, Lev DC, Kleinerman
ES, Abdul-Karim FW, Getty PJ and Greenfield EM: Multiple receptor
tyrosine kinases promote the in vitro phenotype of metastatic human
osteosarcoma cell lines. Oncogenesis. 1:e342012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang YH, Han XD, Qiu Y, et al: Increased
expression of insulin-like growth factor-1 receptor is correlated
with tumor metastasis and prognosis in patients with osteosarcoma.
J Surg Oncol. 105:235–243. 2012. View Article : Google Scholar
|
|
77
|
Gombos A, Metzger-Filho O, Dal Lago L and
Awada-Hussein A: Clinical development of insulin-like growth factor
receptor-1 (IGF-1R) inhibitors: at the crossroad? Invest New Drugs.
30:2433–2442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tap WD, Demetri GD, Barnette P, et al: AMG
479 in relapsed or refractory Ewing’s family tumors (EFT) or
desmoplastic small round cell tumors (DSRCT): Phase II results. J
Clin Oncol. 28(15 Suppl): 100012010.
|
|
79
|
Natinoal Institutes of Health. A Study to
Determine the Activity of SCH 717454 in Subjects with Relapsed
Osteosarcoma or Ewing’s Sarcoma (Study P04720AM3). http://clinicaltrials.gov/ct2/show/NCT00617890?term=sch-717454&rank=2.
Accessed April 6, 2011
|
|
80
|
Akatsuka T, Wada T, Kokai Y, et al: ErbB2
expression is correlated with increased survival of patients with
osteosarcoma. Cancer. 94:1397–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S and
Chen X: VEGF and EMMPRIN expression correlates with survival of
patients with osteosarcoma. Surg Oncol. 20:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Maris JM, Courtright J, Houghton PJ, et
al: Initial testing of the VEGFR inhibitor AZD2171 by the pediatric
preclinical testing program. Pediatr Blood Cancer. 50:581–587.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ebb D, Meyers P, Grier H, et al: Phase II
trial of trastuzumab in combination with cytotoxic chemotherapy for
treatment of metastatic osteosarcoma with human epidermal growth
factor receptor 2 overexpression: a report from the children’s
oncology group. J Clin Oncol. 30:2545–2551. 2012.PubMed/NCBI
|
|
84
|
Liebermann DA, Hoffman B and Steinman RA:
Molecular controls of growth arrest and apoptosis: p53-dependent
and independent pathways. Oncogene. 11:199–210. 1995.
|
|
85
|
Asada N, Tsuchiya H and Tomita K: De novo
deletions of p53 gene and wild-type p53 correlate with acquired
cisplatin-resistance in human osteosarcoma OST cell line.
Anticancer Res. 19:5131–5137. 1999.PubMed/NCBI
|
|
86
|
Wong RP, Tsang WP, Chau PY, Co NN, Tsang
TY and Kwok TT: p53-R273H gains new function in induction of drug
resistance through down-regulation of procaspase-3. Mol Cancer
Ther. 6:1054–1061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fan J and Bertino JR: Modulation of
cisplatinum cytotoxicity by p53: effect of p53-mediated apoptosis
and DNA repair. Mol Pharmacol. 56:966–972. 1999.PubMed/NCBI
|
|
88
|
Tsuchiya H, Mori Y, Ueda Y, Okada G and
Tomita K: Sensitization and caffeine potentiation of cisplatin
cytotoxicity resulting from introduction of wild-type p53 gene in
human osteosarcoma. Anticancer Res. 20:235–242. 2000.PubMed/NCBI
|
|
89
|
Sato N, Mizumoto K, Maehara N, Kusumoto M,
Nishio S, Urashima T, Ogawa T and Tanaka M: Enhancement of
drug-induced apoptosis by antisense oligodeoxynucleotides targeted
against Mdm2 and p21WAF1/CIP1. Anticancer Res. 20:837–842.
2000.PubMed/NCBI
|
|
90
|
Tang HJ, Qian D, Sondak VK, Stachura S and
Lin J: A modified p53 enhances apoptosis in sarcoma cell lines
mediated by doxorubicin. Br J Cancer. 90:1285–1292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Goto A, Kanda H, Ishikawa Y, et al:
Association of loss of heterozygosity at the p53 locus with
chemoresistance in osteosarcomas. Jpn J Cancer Res. 89:539–547.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pápai Z, Féja CN, Hanna EN, Sztán M, Oláh
E and Szendrôi M: P53 overexpression as an indicator of overall
survival and response to treatment in osteosarcomas. Pathol Oncol
Res. 3:15–19. 1997.
|
|
93
|
Ozger H, Eralp L, Atalar AC, et al: The
effect of resistance-related proteins on the prognosis and survival
of patients with osteosarcoma: an immunohistochemical analysis.
Acta Orthop Traumatol Turc. 43:28–34. 2009.(In Turkish).
|
|
94
|
Wunder JS, Gokgoz N, Parkes R, et al: TP53
mutations and outcome in osteosarcoma: a prospective, multicenter
study. J Clin Oncol. 23:1483–1490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Ann Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
96
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59(7 Suppl):
1693s–1700s. 1999.PubMed/NCBI
|
|
98
|
Ye D, Li H, Qian S, Sun Y, Zheng J and Ma
Y: bcl-2/bax expression and p53 gene status in human bladder
cancer: relationship to early recurrence with intravesical
chemotherapy after resection. J Urol. 160:2025–2029. 1998.
View Article : Google Scholar
|
|
99
|
Han JY, Chung YJ, Park SW, Kim JS, Rhyu
MG, Kim HK and Lee KS: The relationship between cisplatin-induced
apoptosis and p53, bcl-2 and bax expression in human lung cancer
cells. Korean J Intern Med. 14:42–52. 1999.PubMed/NCBI
|
|
100
|
Luo D, Cheng SC, Xie H and Xie Y:
Chemosensitivity of human hepatocellular carcinoma cell line
QGY-7703 is related to bcl-2 protein levels. Tumour Biol.
20:331–340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Murata T, Haisa M, Uetsuka H, et al:
Molecular mechanism of chemoresistance to cisplatin in ovarian
cancer cell lines. Int J Mol Med. 13:865–868. 2004.PubMed/NCBI
|
|
102
|
Perego P, Righetti SC, Supino R, et al:
Role of apoptosis and apoptosis-related proteins in the
cisplatin-resistant phenotype of human tumor cell lines. Apoptosis.
2:540–548. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT
and Oda Y: Enhanced chemosensitivity of drug-resistant osteosarcoma
cells by lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res
Commun. 390:642–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang C, Zhao Y and Zeng B: Enhanced
chemosensitivity by simultaneously inhibiting cell cycle
progression and promoting apoptosis of drug-resistant osteosarcoma
MG63/DXR cells by targeting Cyclin D1 and Bcl-2. Cancer Biomark.
12:155–167. 2012.
|
|
105
|
Dey R and Moraes CT: Lack of oxidative
phosphorylation and low mitochondrial membrane potential decrease
susceptibility to apoptosis and do not modulate the protective
effect of Bcl-x(L) in osteosarcoma cells. J Biol Chem.
275:7087–7094. 2000. View Article : Google Scholar
|
|
106
|
Zangemeister-Wittke U: Antisense to
apoptosis inhibitors facilitates chemotherapy and TRAIL-induced
death signaling. Ann NY Acad Sci. 1002:90–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang L, Yu J, Park BH, et al: Role of BAX
in the apoptotic response to anticancer agents. Science.
290:989–992. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Eliseev RA, Dong YF, Sampson E, et al:
Runx2-mediated activation of the Bax gene increases osteosarcoma
cell sensitivity to apoptosis. Oncogene. 27:3605–3614. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cao X, Bennett RL and May WS: c-Myc and
caspase-2 are involved in activating Bax during cytotoxic
drug-induced apoptosis. J Biol Chem. 283:14490–14496. 2008.
View Article : Google Scholar
|
|
110
|
Ferrari S, Bertoni F, Zanella L, et al:
Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in
primary tumor and metachronous lung metastases in patients with
high-grade osteosarcoma. Cancer. 100:1936–1942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu X, Cai ZD, Lou LM and Zhu YB:
Expressions of p53, c-MYC, BCL-2 and apoptotic index in human
osteosarcoma and their correlations with prognosis of patients.
Cancer Epidemiol. 36:212–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang ZX, Yang JS, Pan X, Wang JR, Li J,
Yin YM and De W: Functional and biological analysis of Bcl-xL
expression in human osteosarcoma. Bone. 47:445–454. 2010.
View Article : Google Scholar
|
|
113
|
Nedelcu T, Kubista B, Koller A, et al:
Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res
Clin Oncol. 134:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kaseta MK, Khaldi L, Gomatos IP, et al:
Prognostic value of bax, bcl-2, and p53 staining in primary
osteosarcoma. J Surg Oncol. 97:259–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kaseta MK, Gomatos IP, Khaldi L, et al:
Prognostic value of bax, cytochrome C, and caspase-8 protein
expression in primary osteosarcoma. Hybridoma (Larchmt).
26:355–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar
|
|
118
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kroemer G and Levine B: Autophagic cell
death: the story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008. View Article : Google Scholar
|
|
120
|
Han J, Hou W, Goldstein LA, et al:
Involvement of protective autophagy in TRAIL resistance of
apoptosis-defective tumor cells. J Biol Chem. 283:19665–19677.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Amaravadi RK, Yu D, Lum JJ, et al:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Carew JS, Medina EC, Esquivel JA II, et
al: Autophagy inhibition enhances vorinostat-induced apoptosis via
ubiquitinated protein accumulation. J Cell Mol Med. 14:2448–2459.
2010. View Article : Google Scholar
|
|
123
|
Wu Z, Chang PC, Yang JC, et al: Autophagy
blockade sensitizes prostate cancer cells towards Src family kinase
inhibitors. Genes Cancer. 1:40–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar
|
|
126
|
Katayama M, Kawaguchi T, Berger MS and
Pieper RO: DNA damaging agent-induced autophagy produces a
cytoprotective adenosine triphosphate surge in malignant glioma
cells. Cell Death Differ. 14:548–558. 2007. View Article : Google Scholar
|
|
127
|
Carew JS, Nawrocki ST, Kahue CN, et al:
Targeting autophagy augments the anticancer activity of the histone
deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug
resistance. Blood. 110:313–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lambert LA, Qiao N, Hunt KK, Lambert DH,
Mills GB, Meijer L and Keyomarsi K: Autophagy: a novel mechanism of
synergistic cytotoxicity between doxorubicin and roscovitine in a
sarcoma model. Cancer Res. 68:7966–7974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Meschini S, Condello M, Calcabrini A, et
al: The plant alkaloid voacamine induces apoptosis-independent
autophagic cell death on both sensitive and multidrug resistant
human osteosarcoma cells. Autophagy. 4:1020–1033. 2008. View Article : Google Scholar
|
|
130
|
Kim HJ, Lee SG, Kim YJ, Park JE, Lee KY,
Yoo YH and Kim JM: Cytoprotective role of autophagy during
paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int J
Oncol. 42:1985–1992. 2013.PubMed/NCBI
|
|
131
|
Zhang Z, Shao Z, Xiong L, Che B, Deng C
and Xu W: Expression of Beclin1 in osteosarcoma and the effects of
down-regulation of autophagy on the chemotherapeutic sensitivity. J
Huazhong Univ Sci Technolog Med Sci. 29:737–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Coupienne I, Fettweis G and Piette J: RIP3
expression induces a death profile change in U2OS osteosarcoma
cells after 5-ALA-PDT. Lasers Surg Med. 43:557–564. 2011.
|
|
133
|
Huang J, Ni J, Liu K, et al: HMGB1
promotes drug resistance in osteosarcoma. Cancer Res. 72:230–238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Huang J, Liu K, Yu Y, et al: Targeting
HMGB1-mediated autophagy as a novel therapeutic strategy for
osteosarcoma. Autophagy. 8:275–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Pillai RS, Bhattacharyya SN and
Fillipowicz W: Repression of protein synthesis by miRNAs: how many
mechanisms? Trends Cell Biol. 17:118–126. 2007. View Article : Google Scholar
|
|
136
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar
|
|
137
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Nakatani F, Ferracin M, Manara MC, et al:
miR-34a predicts survival of Ewing’s sarcoma patients and directly
influences cell chemo-sensitivity and malignancy. J Pathol.
226:796–805. 2012.PubMed/NCBI
|
|
139
|
Gougelet A, Pissaloux D, Besse A, et al:
Micro-RNA profiles in osteosarcoma as a predictive tool for
ifosfamide response. Int J Cancer. 129:680–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Song B, Wang Y, Xi Y, et al: Mechanism of
chemoresistance mediated by miR-140 in human osteosarcoma and colon
cancer cells. Oncogene. 28:4065–4074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Song B, Wang Y, Titmus MA, Botchkina G,
Formentini A, Kornmann M and Ju J: Molecular mechanism of
chemoresistance by miR-215 in osteosarcoma and colon cancer cells.
Mol Cancer. 9:962010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cai CK, Zhao GY, Tian LY, et al: miR-15a
and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle
arrest in osteosarcoma. Oncol Rep. 28:1764–1770. 2012.PubMed/NCBI
|
|
143
|
Makino S: The role of tumor stem-cells in
regrowth of the tumor following drastic applications. Acta Unio Int
Contra Cancrum. 15(Suppl 1): 196–198. 1959.PubMed/NCBI
|
|
144
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar
|
|
146
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
147
|
Liu B, Ma W, Jha RK and Gurung K: Cancer
stem cells in osteosarcoma: recent progress and perspective. Acta
Oncol. 50:1142–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Woodward WA and Sulman EP: Cancer stem
cells: markers or biomarkers? Cancer Metastasis Rev. 27:459–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Vangipuram SD, Wang ZJ and Lyman WD:
Resistance of stem-like cells from neuroblastoma cell lines to
commonly used chemotherapeutic agents. Pediatr Blood Cancer.
54:361–368. 2010. View Article : Google Scholar
|
|
150
|
Di Fiore R, Santulli A, Ferrante RD, et
al: Identification and expansion of human osteosarcoma-cancer-stem
cells by long-term 3-aminobenzamide treatment. J Cell Physiol.
219:301–313. 2009.PubMed/NCBI
|
|
151
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
152
|
Honoki K, Fujii H, Kubo A, Kido A, Mori T,
Tanaka Y and Tsujiuchi T: Possible involvement of stem-like
populations with elevated ALDH1 in sarcomas for chemotherapeutic
drug resistance. Oncol Rep. 24:501–505. 2010. View Article : Google Scholar
|
|
153
|
Martins-Neves SR, Lopes ÁO, do Carmo A, et
al: Therapeutic implications of an enriched cancer stem-like cell
population in a human osteosarcoma cell line. BMC Cancer.
12:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chou AJ, Merola PR, Wexler LH, et al:
Treatment of osteosarcoma at first recurrence after contemporary
therapy: the Memorial Sloan-Kettering Cancer Center experience.
Cancer. 104:2214–2221. 2005. View Article : Google Scholar
|
|
155
|
Alberts DS, Muggia FM, Carmichael J, et
al: Efficacy and safety of liposomal anthracyclines in phase I/II
clinical trials. Semin Oncol. 31(Suppl 13): S53–S90. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Maes H, Rubio N, Garg AD and Agostinis P:
Autophagy: shaping the tumor microenvironment and therapeutic
response. Trends Mol Med. 19:428–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
















