Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common solid cancer and the third leading cause of cancer-related
mortality worldwide (1). The
occurrence of HCC is multi-factorial and it often develops under an
established background of chronic liver diseases (2–4). The
most outstanding risk factor in Eastern Asia is chronic hepatitis B
virus (HBV) infection, while in Japan, Europe and North America,
hepatitis C virus (HCV) infection is the notable risk factor,
synergistically with alcohol abuse (5). Liver resection is potentially a
curative therapy in HCC patients who are within the Milan criteria
and have an adequate liver reserve. However, tumor recurrence
occurs in >50% of cases within 5 years following surgery,
combining true recurrence of the original cancer, which usually
arises within the first 2 years, and de novo tumor formation
(6). Early recurrence is associated
with microvascular invasion, poor histological differentiation,
satellites and multifocal disease, while late recurrence is mainly
dependent on the oncogenic potential of underlying chronic liver
disease (7,8). Specific molecules have been identified
as factors for predicting postoperative survival. These include
proline-directed protein kinase FA, mitogen-activated protein
kinase phosphatase-1, vascular endothelial growth factor,
proliferating cell nuclear antigen, p53, tissue factor,
cytokeratin-19, telomerase activity and interleukin-10 (4,9–16). In
patients with HBV-related HCC, the HBV basal core promoter mutation
and HBV-DNA level in liver tissues also predict a poor
postoperative survival rate (17).
Oxidative phosphorylation (OXPHOS) in mitochondria
provides biological energy for intracellular metabolic pathways
(18). It is particularly
significant in hepatocytes as the liver is one of the most
energy-consuming organs. In 1930, Warburg proposed that cancer
originated from an irreversible injury to mitochondrial OXPHOS,
which forced cancer cells to shift to an energy-generation process
through glycolysis despite the presence of aerobic conditions. This
condition has been named as the Warburg effect (19). It renders cancer cells capable of
surviving and proliferating under adverse conditions. Mitochondrial
DNA (mtDNA) is more susceptible to oxidative damage and has a
higher mutation rate than nuclear DNA due to a lack of protective
histones, limited DNA repair activities and a proximity to the high
rate of reactive oxygen species generated in mitochondria (20–22).
As such, the accumulation of mtDNA alterations in cancer cells
leading to defects in adenosine triphosphate (ATP) generation
through the OXPHOS system is consistent with the theory of the
Warburg effect. Mutations in mtDNA have been reported in various
types of human cancers (23–25).
These findings indicate that defects of the OXPHOS complex with
mitochondrial-encoded subunits may be a decisive factor in
hepatocarcinogenesis.
The OXPHOS process consists of five complexes, which
are complex I (NADH: ubiquinone oxidoreductase), complex II
(succinate dehydrogenase), complex III (cytochrome bc1
complex), complex IV (cytochrome c oxidase) and complex V
(ATP synthase), and all are localized on the inner mitochondrial
membrane. During electron transport, complexes I, III and IV pump
protons from the mitochondrial matrix to the inter-membrane space,
resulting in an increase in the membrane potential across the inner
mitochondrial membrane. Following this, complex V actively allows
the flow of protons back to the matrix, resulting in the generation
of energy in the form of ATP from adenosine diphosphate (26). The OXPHOS system consists of 85
subunits as components of various complexes, in which 13 are
encoded in mtDNA. These 13 mitochondrial-encoded proteins
constitute various subunits that make up four OXPHOS complexes
(complex I, III, IV and V) (27).
Studies have demonstrated that the mtDNA mutations alter the
mitochondrial-encoded subunits and play a significant role in
numerous malignancies, including renal oncocytoma, thyroid
oncocytic carcinoma, bladder, prostate and colon cancer (28–32).
MtDNA mutations have also been identified in HCC
(33–35). These mutations potentially cause the
defects of mitochondrial-encoded subunits in the OXPHOS system and
result in mitochondrial dysfunction in HCC (36). It has been demonstrated that the
defects of complexes III and IV can be detected by
immunohistochemical (IHC) staining in normal human and cirrhotic
liver during aging. However, these defects were not shown in 27
sections of HCC tissues (37). The
actual defects of mitochondrial-encoded subunits in HCC and the
association with the outcome remain unclear. The present study
aimed to examine the expression of the defects of the
mitochondrial-encoded OXPHOS subunits; complex I subunit 6 (CI-6),
complex III subunit 3 (CIII-3), complex IV subunit 1 (CIV-1) and
complex V subunit 6 (CV-6), in surgically removed HCCs, and their
correlations with clinicopathological factors and prognosis.
Patients and methods
Patients and specimens
Under the approval of the Institutional Review
Board, Chang Gung Medical Center (Taoyuan, Taiwan), 102 human HCC
tissues with a clear surgical margin were randomly collected from
patients undergoing curative resection at Chang Gung Memorial
Hospital at Linkou (Taoyuan, Taiwan) between 1996 and 2006 and
stored in the Chang Gung tissue bank. The tissues were retrieved
from the tissue bank and formalin-fixed and paraffin-embedded for
the IHC study. The inclusion criteria included pathological
diagnosis of HCC, no anticancer therapy prior to the surgery,
curative resection, adequately formalin-fixed and paraffin-embedded
tissues, complete clinicopathological data, regular follow-up and
reliable medical records. The exclusion criteria included
pregnancy, other co-existing malignancies and mortality due to
unrelated diseases.
The clinical parameters included age, gender,
chronic HBV, chronic HCV, liver cirrhosis, ascites, α-fetoprotein
(AFP), albumin, bilirubin, prothrombin time, creatinine, aspartate
aminotransferase (AST), alanine aminotranferease (ALT), alcohol use
(average alcohol consumption of >210 g per week in males or
>140 per week in females over at least a 2-year period), local
recurrence, time to local recurrence, distant metastasis, time to
distant metastasis, mortality and overall survival time. The
pathological findings, including tumor grade, microvascular
invasion, macrovascular invasion, capsule, tumor number and largest
tumor diameter, were examined by two experienced pathologists
without information of the clinical data.
Curative resection was defined as a complete
resection of all tumors with the margin free from cancer invasion
by histological examination, no tumor thrombus in the main trunk,
two major portal branches, hepatic veins or bile duct and no
extrahepatic metastasis (38). HCC
diagnosis and grading were established according to World Health
Organization criteria (39).
Macrovascular invasion was defined as the invasion of the tumor
into the vessels that can be identified during macroscopic
examination or radiological imaging. The definition of
microvascular invasion included: i) Presence of tumor cells forming
a plug or polyp in a subendothelial location, partially or totally
covered by endothelial cells; ii) presence of tumor thrombus,
partially or totally covered by the endothelium; iii) vascular
structures involved can be portal vein branches, hepatic vein
branches or capsule vessels, inside the tumor or closely situated
to the tumor edge; and iv) invasion of arteries and lymphatic
vessels (40). Local recurrence was
defined as intrahepatic recurrence and distant metastasis was equal
to extrahepatic metastasis.
Ethics statement
This study was approved by Chang Gung Medical
Foundation Institutional Review Board (no. 100-1728B, between
01/08/2011 and 31/07/2014; Taoyuan, Taiwan). The Institutional
Review Board waived the requirement for informed consent from the
participants as the present study was a retrospectively
observational analysis and the information identifying patients was
not included in the collected data. Tissue samples were obtained
from the Tissue Bank of Linkou Chang Gung Memorial Hospital
(Taoyuan, Taiwan) through approval of the committee.
IHC analysis
The IHC stains were performed for detection of the
expression defects of CI-6, CIII-3, CIV-1 and CV-6 with rabbit
anti-nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit
6 polyclonal antibody (Abcam, Cambridge, MA, USA), goat
anti-cytochrome b polyclonal antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), mouse anti-OXPHOS
complex IV subunit I monoclonal antibody (Invitrogen Life
Technologies, Carlsbad, CA, USA) and rabbit anti-mt-ATP6 polyclonal
antibody (Abcam), respectively. Deparaffinized rehydrated sections
were treated with H2O2 (3% in distilled
water) for 15 min, pre-incubated with normal serum (goat serum for
rabbit anti-NADH dehydrogenase subunit 6 antibody and anti-mt-ATP6
antibody; horse serum for anti-OXPHOS complex IV subunit I
monoclonal antibody; and rabbit serum for anti-cytochrome b)
with phosphate-buffered saline in the proportion of 1:10 for 40
min. Following this, the sections were then incubated with the
specific primary antibody (1 h at 37°C for anti-NADH dehydrogenase
subunit 6 antibody and anti-OXPHOS complex IV subunit I monoclonal
antibody; and overnight at 4°C for anti-cytochrome b and
anti-mt-ATP6 antibody) and the secondary antibody of the VECTASTIN
Elite ABC kit [anti-rabbit immunoglobulin G (IgG) for rabbit
anti-NADH dehydrogenase subunit 6 antibody and anti-mt-ATP6
antibody; anti-mouse IgG for anti-OXPHOS complex IV subunit I
monoclonal antibody; and anti-goat IgG for anti-cytochrome
b; Vector Laboratories, Inc., Burlingame, CA, USA] for 40
min at room temperature. Visualization was performed with the
3,3′-diaminobenzidine substrate kit, SK-4100 (Vector Laboratories,
Inc.). The stained sections were examined separately by two
experienced pathologists, who were blinded to the clinical
information. The defect areas (absence of reactivity) in the tumor
were estimated and recorded as percentages. If there was a
discrepancy in the interpretation, a consensus was reached between
the two pathologists by reviewing slides simultaneously. Tissues
were classified into two groups based on the percentages of the
defect areas in a single cross-section of the HCC samples (major
defect, >25% HCC area; and minor defect, ≤25% of the HCC area)
for further evaluation.
Statistical analysis
Numerical data are presented as mean ± standard
deviation, while categorical data were expressed as absolute number
and percentages. The χ2 test was used for group
comparisons involving binary data and independent samples.
Mann-Whitney U test was used for the largest tumor size, AFP, AST,
ALT, local recurrent time, distal metastatic time and disease-free
survival time, due to a skewed distribution. Other numerical data
were evaluated by Student’s t-test. The results were considered to
indicate a statistically significant difference when P<0.05.
Univariate and multivariate analyses were performed by Cox
proportional hazards regression to identify independent risk
factors for mortality. The results were presented with hazard ratio
(HR), 95% confidence interval (CI) and P-value. Survival
curves were also analyzed by the Kaplan-Meier curve and log-rank
test. All statistical calculations were performed using SPSS, 18.0
software (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The age of the patients at the time of surgery
ranged between 25 and 89 years, and the male to female ratio was
3.64. The tumor-node-metastasis stage was between stages I and
IIIA, according to the American Joint Committee on Cancer Cancer
Staging Manual, seventh edition (2010) (41). The cohort of the present study
included 51 liver cirrhosis patients, 38 alcohol users, 70 chronic
HBV carriers, 17 chronic HCV carriers and 3 HBV and HCV
co-infection patients. The average follow-up duration was
50.29±43.50 months. The rates of mortality, local recurrence,
distal metastasis, 5-year survival and 5-year disease-free survival
were 36.27, 64.71, 39.22, 37.25 and 22.55%, respectively.
Clinicopathological characteristics
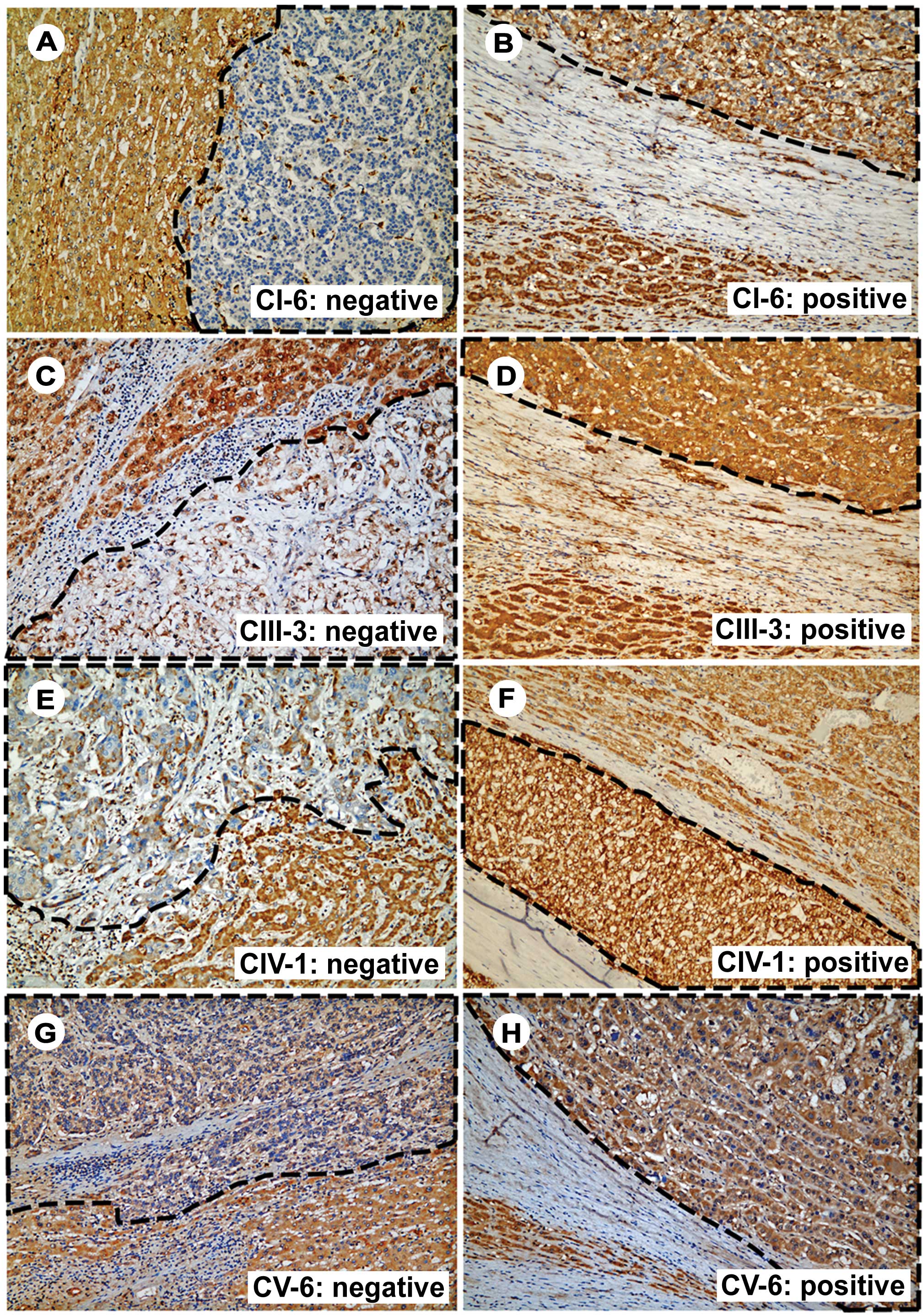
The majority of the HCC tissues contained various
degrees of mitochondrial-encoded OXPHOS enzyme defects. The
expression defects of CI-6, CIII-3, CIV-1 and CV-6 were present in
100, 98, 98 and 93% of the tissues assessed, respectively. The
major and minor defects were defined as IHC staining defect area of
>25% and ≤25%, respectively (Fig.
1). According to this definition, the frequency of the major
defect for CI-6, CIV-1, CIII-3 and CV-6 was 67.7, 50.0, 31.3 and
24.5%, respectively. The CI-6 major-defect group had lower ALT
levels compared with the minor-defect group [35 (10-280) and 59
(9-371) U/l, P=0.038]. The CIII-3 major-defect group had fewer
encapsulated tumors (56.25 and 75.71%, P=0.047) and higher albumin
levels (4.03±0.44 and 3.67±0.68 g/dl, P=0.002), compared with the
minor-defect group. The CIV-1 major-defect group had higher AFP
levels [204.08 (0.9-89637.7) and 21.92 (2-286980) ng/ml, P=0.021]
and higher AST levels [63 (12-351) and 36 (11-278) U/l, P=0.018]
compared with the minor-defect group. The CV-6 major-defect group
was older (63.12±9.80 and 53.86±15.53 years, P=0.001) compared with
the minor-defect group. There was no other significant difference
in clinicopathological parameters between the major- and
minor-defect groups (Table I).
 | Table ICorrelation between CI-6, CIII-3,
CIV-1 and CV-6 and clinicopathological characteristics in 102 human
HCC tissues. |
Table I
Correlation between CI-6, CIII-3,
CIV-1 and CV-6 and clinicopathological characteristics in 102 human
HCC tissues.
| CI-6 defect | CIII-3 defect | CIV-1 defect | CV-6 defect |
|---|
|
|
|
|
|
|---|
| Parameter | Minor | Major | P-value | Minor | Major | P-value | Minor | Major | P-value | Minor | Major | P-value |
|---|
| Case number, n
(%) | 33 (32.35) | 69 (67.65) | | 70 (68.63) | 32 (31.37) | | 51 (50) | 51 (50) | | 77 (75.49) | 25 (24.51) | |
| Gender, n
(M/F) | 23/10 | 57/12 | 0.138 | 53/17 | 27/5 | 0.324 | 42/9 | 38/13 | 0.336 | 58/19 | 22/3 | 0.181 |
| Age, years | 58.48±14.22 | 55.00±15.10 | 0.269 | 57.90±15.64 | 52.25±12.27 | 0.052 | 55.63±16.30 | 56.63±13.37 | 0.736 | 53.86±15.53 | 63.12±9.80 | 0.001a |
| HBsAg, n (%) | 21 (63.64) | 52 (75.36) | 0.219 | 47 (67.14) | 26 (81.25) | 0.143 | 35 (68.63) | 38 (74.51) | 0.510 | 57 (74.03) | 16 (64.00) | 0.334 |
| HCV, n (%) | 9 (27.27) | 11 (15.94) | 0.178 | 15 (21.43) | 5 (15.63) | 0.493 | 7 (13.73) | 13 (25.49) | 0.135 | 14 (18.18) | 6 (24.00) | 0.524 |
| Liver cirrhosis, n
(%) | 16 (48.48) | 35 (50.72) | 0.832 | 37 (52.86) | 14 (43.75) | 0.393 | 23 (45.10) | 28 (54.90) | 0.322 | 37 (48.05) | 14 (56.00) | 0.490 |
| Tumor grade | 2.50±0.70 | 2.42±0.61 | 0.547 | 2.47±0.66 | 2.38±0.60 | 0.535 | 2.48±0.66 | 2.41±0.62 | 0.582 | 2.47±0.62 | 2.36±0.70 | 0.449 |
| Microvascular
invasion, n (%) | 12 (36.36) | 25 (36.23) | 0.990 | 23 (32.86) | 14 (43.75) | 0.288 | 19 (37.25) | 18 (35.29) | 0.837 | 32 (41.56) | 5 (20.00) | 0.051 |
| Macrovascular
invasion, n (%) | 4 (12.12) | 3 (4.35) | 0.146 | 5 (7.14) | 2 (6.25) | 0.869 | 1 (1.96) | 6 (11.76) | 0.050 | 7 (9.09) | 0 (0.00) | 0.118 |
| Capsule, n (%) | 25 (75.76) | 46 (66.67) | 0.350 | 53 (75.71) | 18(56.25) | 0.047a | 33 (64.71) | 38 (74.51) | 0.282 | 53 (68.83) | 18 (72.00) | 0.765 |
| Tumor number | 1.58±0.97 | 1.59±0.85 | 0.922 | 1.46±0.74 | 1.88±1.10 | 0.057 | 1.55±0.86 | 1.63±0.92 | 0.656 | 1.55±0.82 | 1.72±1.06 | 0.393 |
| Largest tumor size
(diameter, cm) | 5.3 (2–20) | 5 (1–77.5) | 0.429 | 5 (1–20) | 6.5 (2–77.5) | 0.323 | 4.8 (1.8–20.5) | 6 (1–77.5) | 0.254 | 5.5 (2–20.5) | 4 (1–77.5) | 0.190 |
| Ascites, n (%) | 3 (9.09) | 6 (8.70) | 0.911 | 8 (11.43) | 1 (3.13) | 0.165 | 3 (5.88) | 6 (11.76) | 0.309 | 7 (9.09) | 2 (8.00) | 0.854 |
| AFP, ng/ml | 38 (2-44890.2) | 59
(0.9-286980) | 0.468 | 35.5
(1.47-286980) | 133.5
(0.9-89637.7) | 0.070 | 21.92
(2-286980) | 204.08
(0.9-89637.7) | 0.021a | 50.22
(1.47-286980) | 61 (0.9-4585) | 0.531 |
| Albumin, g/dl | 3.70±0.63 | 3.83±0.64 | 0.356 | 3.67±0.68 | 4.03±0.44 | 0.002a | 3.87±0.62 | 3.70±0.65 | 0.201 | 3.76±0.67 | 3.85±0.50 | 0.564 |
| Billirubin,
mg/dl | 0.98±0.48 | 1.04±0.92 | 0.736 | 1.10±0.92 | 0.85±0.38 | 0.153 | 0.91±0.42 | 1.13±1.04 | 0.170 | 1.04±0.90 | 0.98±0.36 | 0.750 |
| Prothrombin time,
sec | 12.4±1.13 | 12.55±1.67 | 0.651 | 12.65±1.67 | 12.15±1.01 | 0.125 | 12.44±1.78 | 12.55±1.20 | 0.722 | 12.61±1.61 | 12.18±1.13 | 0.220 |
| Creatinine,
mg/dl | 1.22±0.76 | 1.25±1.26 | 0.911 | 1.24±0.95 | 1.25±1.45 | 0.963 | 1.15±0.61 | 1.325±1.47 | 0.439 | 1.10±0.52 | 1.68±2.04 | 0.172 |
| AST, U/l | 52 (11-278) | 36 (12-351) | 0.520 | 40.5 (11-351) | 39 (15-312) | 0.908 | 36 (11-278) | 63 (12-351) | 0.018a | 45 (11-351) | 35 (12-260) | 0.361 |
| ALT, U/l | 59 (9-371) | 35 (10-280) | 0.038a | 41.5 (9-371) | 41.5 (13-280) | 0.905 | 40 (9-371) | 47 (13-280) | 0.322 | 45 (9-371) | 33 (13-279) | 0.800 |
| Alcohol use, n
(%) | 14 (42.42) | 24 (34.78) | 0.455 | 26 (37.14) | 12 (37.50) | 0.972 | 15 (29.41) | 23 (45.10) | 0.101 | 28 (36.36) | 10 (40.00) | 0.744 |
Clinicopathological parameters and OXPHOS
expression defects associated with post-operative survival in
HCC
Univariate analysis revealed that ascites (HR,
6.016; 95% CI, 2.364–15.309; P<0.001), albumin levels (HR,
0.524; 95% CI, 0.304–0.905; P=0.020), time to local recurrence (HR,
0.948; 95% CI, 0.927–0.970, P<0.001), time to distant metastasis
(HR, 0.934; 95% CI, 0.915–0.953; P<0.001), disease-free survival
time (HR, 0.948; 95% CI, 0.927–0.969; P<0.001) and major CIV-1
defect (HR, 3.050; 95% CI, 1.471–6.324; P=0.003) were associated
with the overall survival time (Table
II). These six factors were further analyzed by multivariate
analysis. It was found that the major CIV-1 defect (HR, 5.676; 95%
CI, 2.243–14.360; P<0.001) and the time to distant metastasis
(HR, 0.924; 95% CI, 0.894–0.955; P<0.001) were significant
independent predictive factors for overall survival time (Table III). Kaplan-Meier survival
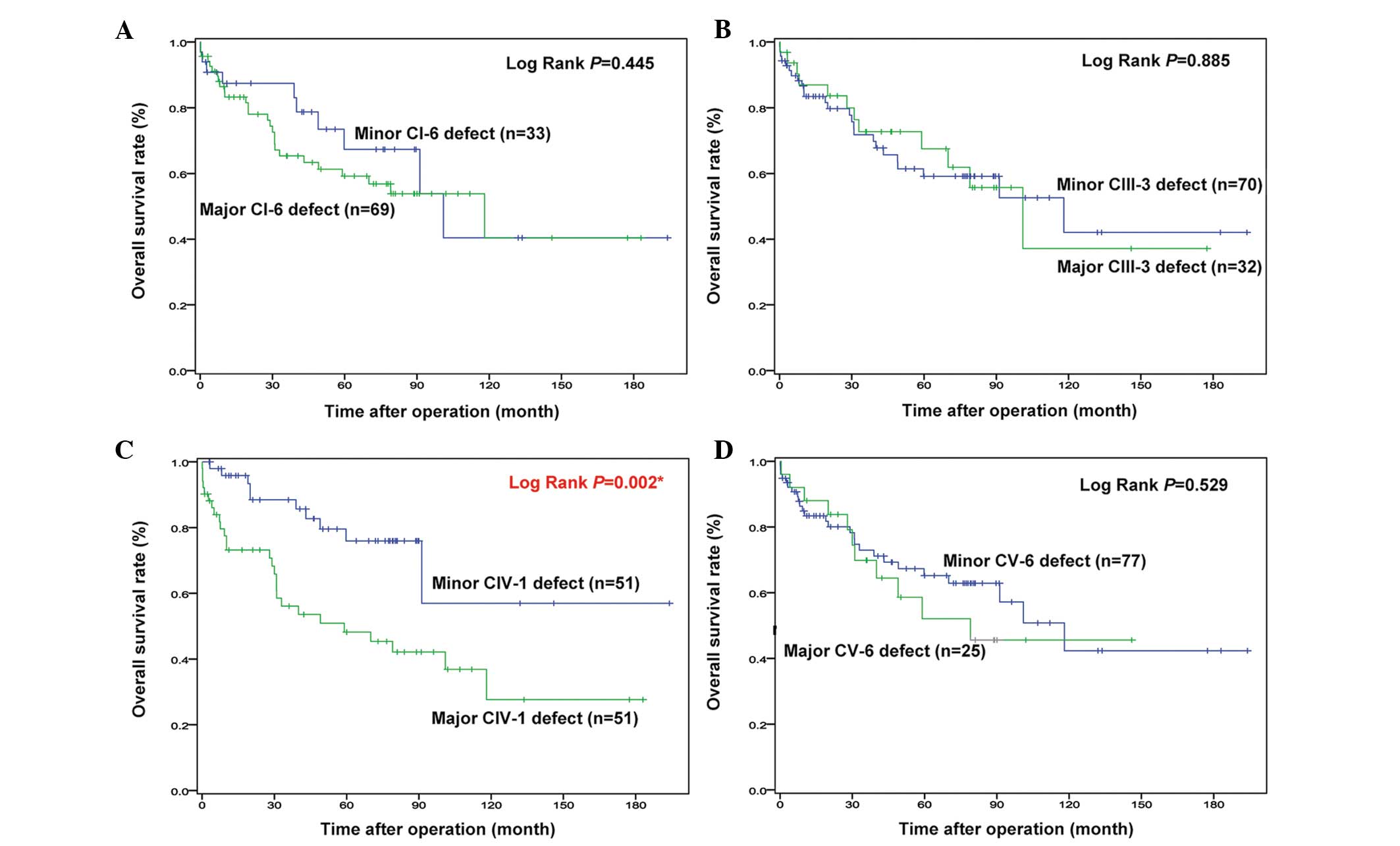
analysis using the log-rank test (Fig.
2) also showed that the CIV-1 major-defect group had an
unfavorable survival time compared with that of the minor-defect
group (P=0.002). Additionally, the major CI-6 and CV-6 defect
groups appeared to have a poor survival rate within 8 years, but it
did not reach statistical significance. There was no significant
difference between major- and minor-defect groups of CIII-3,
according to the Kaplan-Meier survival curve.
 | Table IIUnivariate analysis of parameters
associated with overall survival time. |
Table II
Univariate analysis of parameters
associated with overall survival time.
| Parameter | Patient data | SE | HR | 95% CI | P-value |
|---|
| Gender, male | 80 (78.4%)a | 0.384 | 0.964 | 0.454~2.046 | 0.924 |
| Age, years | 56.13±14.84 | 0.011 | 1.012 | 0.990–1.034 | 0.292 |
| HBsAg | 73 (71.6%)a | 0.391 | 0.999 | 0.464~2.150 | 0.999 |
| HCV | 20 (19.6%)a | 0.485 | 0.727 | 0.281~1.879 | 0.511 |
| Liver
cirrhosis | 51 (50.0%)a | 0.332 | 0.919 | 0.479~1.762 | 0.798 |
| Tumor grade | 2.44±0.64 | 0.255 | 1.001 | 0.608–1.649 | 0.996 |
| Microvascular
invasion | 37 (36.3%)a | 0.366 | 1.181 | 0.577~2.418 | 0.649 |
| Macrovascular
invasion | 7 (6.9%)a | 0.535 | 1.799 | 0.630~5.139 | 0.273 |
| Capsule | 71 (69.6%)a | 0.386 | 1.272 | 0.597~2.709 | 0.533 |
| Tumor number | 1.59±0.88 | 0.181 | 1.136 | 0.797–1.619 | 0.481 |
| Largest tumor size
(diameter, cm) | 5.1
(1.0–77.5)b | 0.012 | 1.012 | 0.989–1.037 | 0.313 |
| Ascites | 9 (8.8%)a | 0.477 | 6.016 | 2.364~15.309 | <0.001c |
| AFP (ng/ml) | 50.26
(0.9-286980)b | 0.000 | 1.000 | 1.000~1.000 | 0.585 |
| Albumin (g/dl) | 3.78±0.64 | 0.278 | 0.524 | 0.304–0.905 | 0.020c |
| Billirubin
(mg/d) | 1.02±0.80 | 0.219 | 1.163 | 0.758–1.785 | 0.490 |
| Prothrombin time
(sec) | 12.50±1.51 | 0.083 | 1.159 | 0.985–1.365 | 0.076 |
| Creatinine
(mg/dl) | 1.24±1.12 | 0.111 | 1.171 | 0.942–1.456 | 0.155 |
| AST (U/l) | 39.5
(11-351)b | 0.002 | 1.004 | 1.000–1.009 | 0.073 |
| ALT (U/l) | 41.5
(9-371)b | 0.003 | 0.999 | 0.993~1.005 | 0.692 |
| Alcohol use | 38 (37.3%)a | 0.332 | 1.387 | 0.723~2.660 | 0.325 |
| Local recurrent
time (month) | 14
(0.07-194)b | 0.011 | 0.948 | 0.927–0.970 | <0.001c |
| Distal metastatic
time (month) | 34.5
(0.07–194)b | 0.010 | 0.934 | 0.915–0.953 | <0.001c |
| Disease free
survival time (month) | 14
(0.07–194)b | 0.011 | 0.948 | 0.927–0.969 | <0.001c |
| Major complex I
subunit 6 defect | 69 (67.6%)a | 0.371 | 1.326 | 0.641–2.742 | 0.446 |
| Major complex III
subunit 3 defect | 32 (31.4%)a | 0.352 | 0.950 | 0.477–1.893 | 0.885 |
| Major complex IV
subunit 1 defect | 51 (50.0%)a | 0.372 | 3.050 | 1.471~6.324 | 0.003c |
| Major complex V
subunit 6 defect | 25 (24.5%)a | 0.361 | 1.255 | 0.618–2.546 | 0.530 |
 | Table IIIMultivariate analysis of parameters
associated with overall survival time. |
Table III
Multivariate analysis of parameters
associated with overall survival time.
| Parameter | SE | HR | 95% CI | P-value |
|---|
| Ascites | 0.500 | 1.697 | 0.637~4.521 | 0.290 |
| Albumin, g/dl | 0.341 | 1.012 | 0.519~1.975 | 0.971 |
| Local recurrent
time, month | 0.607 | 0.791 | 0.241~2.598 | 0.699 |
| Distal metastatic
time, month | 0.017 | 0.924 | 0.894~0.955 | <0.001a |
| Disease-free
survival time, month | 0.609 | 1.262 | 0.383~4.159 | 0.702 |
| Major complex IV
subunit 1 defect | 0.474 | 5.676 | 2.243~14.360 | <0.001a |
Discussion
According to the present study, the majority of the
HCC tissues contained various degrees of mitochondrial-encoded
OXPHOS complex defects, indicating the impairment of ATP production
by pyruvate oxidation in mitochondria. This result is compatible
with the phenomenon of the Warburg effect, indicating that energy
is mainly supplied by glycolysis in cancer cells (19). To understand whether the degree of
OXPHOS defects correlate with clinicopathological presentation and
prognosis, the major and minor defects were defined as IHC staining
defect area of >25% (major) and ≤25% (minor). The frequencies of
the major defect were as follows: CI-6, 67.65%; CIV-1, 50.00%;
CIII-3, 31.37%; and CV-6, 24.51%). These results indicate that HCC
tissues tend to have a larger portion of CI-6 and CIV-1 defects,
rather than CIII-3 and CV-6. It has been indicated that the OXPHOS
enzyme defects are associated with aging (37). However, in the present study, only
the major CV-6 defect was correlated with age (63.12±9.80 and
53.86±15.53 years, P=0.001), indicating that other mechanisms are
responsible for the generation of these defects in HCC, rather than
aging. Through univariate and multivariate analyses, the major
CIV-1 defect was found to be a negative predictive factor for HCC
patients following curative resection.
Complex IV is the terminal oxidase of the
respiratory chain in the mitochondria. In mammals, it contains 13
subunits, of which 3 catalytic subunits (subunit 1, 2 and 3) are
encoded by the mitochondrial genes. The remaining 10 subunits are
encoded by nuclear DNA and are suspected to be involved in the
regulation and/or assembly of the complex (41). Complex IV represents the
rate-limiting enzyme of the mitochondrial respiratory chain and its
activity is an indicator of the oxidative capacity of the cells. It
is therefore fated to be a central site of regulation of oxidative
phosphorylation, proton pumping efficiency, ATP and reactive oxygen
species production, which in turn affect cell signaling and
survival (42,43). Complex IV can also be incorporated
into larger structures containing complex I, II and III, and the
mobile electron carriers, cytochrome c and ubiquinol, to
form functional supercomplexes; respirasomes (44,45).
These supercomplexes may stabilize the individual complexes
(46) to enhance respiration due to
coordinated channeling of electrons (47). Due to the significance of complex
IV, organisms have evolved various levels of regulation for its
activity. A defect of complex IV has been proved to result in
numerous diseases, including Leber hereditary optic neuropathy,
Leigh syndrome, recurrent myoglobinuria mitochondrial disorder,
deafness sensorineural mitochondrial disorder and colorectal cancer
(48–54). However, their roles in HCC are not
clear. The present study firstly revealed the potential
significance of the CIV-I defects in HCC. Further experiments
involving knocking out the CIV-I gene by small hairpin RNA in the
Huh7 HCC cell line will be performed to demonstrate their effects
on liver tumor.
It has been demonstrated that OXPHOS protein defects
can be found in normal and cirrhotic human liver. A study by
Müller-Höcker et al (37)
enrolled 107 normal livers (including 11 HCC cases) and 64
cirrhotic livers (including 16 HCC cases) and aimed to detect the
respiratory chain protein (complex II, III and IV) and complex V
defects in normal and cirrhotic liver during aging. Enzyme
histochemistry was performed to detect complex II, IV and V, and
immunohistochemistry with polyclonal antibody (against both nuclear
and mitochondrial subunits) was conducted for detection of total
complex III and complex IV subunits 2, 3 and 4. In normal livers,
the respiratory chain defects were detected in 57% cases, and 87%
in advanced age (>50 years old). In cirrhotic liver, the overall
frequency of defects was higher (78%) compared with normal liver,
but was inverse (60%) in advanced age. However, there were no
defects of complex II, III, IV and V observed in 27 HCC tissues in
their study. On the contrary, the present study showed that the
majority of resected HCC tissues contain various degrees of
mitochondrial-encoded subunits defects. The conflicting results may
be due to the varied HCC tissue origins (metastatic and primary),
various OXPHOS enzymes examined and different methods performed.
Nevertheless, the results of the present study firstly demonstrated
that the majority of HCCs contain mitochondrial-encoded subunit
defects, indicating a potential role of these defects in
hepatocarcinogenesis.
MtDNA mutations have been detected in HCC. A study
by Lee et al (33) examined
the D-loop mutations and mtDNA numbers in 61 HCCs and the
corresponding non-tumor sections. The results showed that 39.3% of
HCCs carried somatic mutations in the D-loop of mtDNA. A
significant decrease in the copy number of mtDNA was also detected
in 60.5% of patients with HCC (33). A small-scale study examining 18 HCC
patients also demonstrated a significant decrease of mtDNA copy
number, particularly in females but not in males with HCC (34). Another study examined 44 HCCs and in
total 13 somatic mtDNA mutations were found in 11 HCC samples
(35). Among these mutations, the
T6768C (CIV-1), G7976A (complex IV subunit II), G9267 (complex IV
subunit 3) and A111708 (complex I subunit 4) mutations could result
in amino acid substitutions in the highly conserved regions and
have the potential to cause mitochondrial dysfunction in HCC.
Although the mutation of mtDNA was not examined in the present
study, the mitochondrial-encoded OXPHOS subunit defects shown may
have at least partially resulted from mtDNA mutations and the
decrease of mtDNA copy number.
There were limitations in the present study
regarding the case number, the choice of specimens and the lack of
western blotting, reverse transcription polymerase chain reaction
and mtDNA analysis. In total, 102 HCC tissues were collected with
an average follow-up duration of 50.29 months (range, 0.07–194
months). The case number appeared to not be enough for subgroup
analyses. Due to the strength of the IHC staining, it was more
difficult to evaluate objectively and it was not associated with
prognosis (data not shown); the percentages of IHC stain defect
areas were chosen in a single cross-section of HCC without
significant necrosis to evaluate the degree of the OXPHOS complex
defects. The same scoring system read by the pathologists is also
widely used in clinical practice, including HER2 in breast cancer
and Ki-67 in neuroendocrine tumor (55,56).
Although it was easy for clinical applications, it may have bias
between different pathologists when scoring the defects areas. We
hypothesize that this bias can be reduced by repeated reading and
experienced examiners.
To the best of our knowledge, the present study was
the first to demonstrate the correlation between OXPHOS subunit
defects and overall survival time in HCC patients. The results
showed that the majority of HCCs contained mitochondrial-encoded
OXPHOS subunit defects. Among these, the major CIV-1 defect was a
negative predictor for post-operative survival in HCC. It may
provide a simple way to predict the outcome in this group of
patients.
Acknowledgements
This study was supported by the grants from the
National Science Council (NSC100-2314-B-182A-056) and Chang Gung
Medical Research Council (CMRPG3A0921), Taiwan, Republic of
China.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar
|
|
2
|
Sherman M: Hepatocellular carcinoma:
epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar
|
|
3
|
Marra M, Sordelli IM, Lombardi A, et al:
Molecular targets and oxidative stress biomarkers in hepatocellular
carcinoma: an overview. J Transl Med. 9:1712011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen HM and Ong CN: Mutations of the p53
tumor suppressor gene and ras oncogenes in aflatoxin
hepatocarcinogenesis. Mut Res. 366:23–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshida Y, Villanueva A, Kobayashi M, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imamura H, Matsuyama Y, Tanaka E, et al:
Risk factors contributing to early and late phase intrahepatic
recurrence of hepatocellular carcinoma after hepatectomy. J
Hepatol. 38:200–207. 2003. View Article : Google Scholar
|
|
9
|
Chau GY, Wu CW, Lui WY, et al: Serum
interleukin-10 but not interleukin-6 is related to clinical outcome
in patients with resectable hepatocellular carcinoma. Ann Surg.
231:552–558. 2000. View Article : Google Scholar
|
|
10
|
Hsu YC, Fu HH, Jeng YM, Lee PH and Yang
SD: Proline-directed protein kinase FA is a powerful and
independent prognostic predictor for progression and patient
survival of hepatocellular carcinoma. J Clin Oncol. 24:3780–3788.
2006. View Article : Google Scholar
|
|
11
|
Kitamoto M, Nakanishi T, Kira S, et al:
The assessment of proliferating cell nuclear antigen
immunohistochemical staining in small hepatocellular carcinoma and
its relationship to histologic characteristics and prognosis.
Cancer. 72:1859–1865. 1993. View Article : Google Scholar
|
|
12
|
Kobayashi T, Kubota K, Takayama T and
Makuuchi M: Telomerase activity as a predictive marker for
recurrence of hepatocellular carcinoma after hepatectomy. Am J
Surg. 181:284–288. 2001. View Article : Google Scholar
|
|
13
|
Poon RT, Lau CP, Ho JW, Yu WC, Fan ST and
Wong J: Tissue factor expression correlates with tumor angiogenesis
and invasiveness in human hepatocellular carcinoma. Clin Cancer
Res. 9:5339–5345. 2003.
|
|
14
|
Poon RT, Ng IO, Lau C, et al: Serum
vascular endothelial growth factor predicts venous invasion in
hepatocellular carcinoma: a prospective study. Ann Surg.
233:227–235. 2001. View Article : Google Scholar
|
|
15
|
Tsujita E, Taketomi A, Gion T, et al:
Suppressed MKP-1 is an independent predictor of outcome in patients
with hepatocellular carcinoma. Oncology. 69:342–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uenishi T, Kubo S, Yamamoto T, et al:
Cytokeratin 19 expression in hepatocellular carcinoma predicts
early postoperative recurrence. Cancer Sci. 94:851–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh CT, So M, Ng J, et al: Hepatitis B
virus-DNA level and basal core promoter A1762T/G1764A mutation in
liver tissue independently predict postoperative survival in
hepatocellular carcinoma. Hepatology. 52:1922–1933. 2010.
View Article : Google Scholar
|
|
18
|
Schapira AH: Mitochondrial disease.
Lancet. 368:70–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warburg O: The Metabolism of Tumors.
Arnold Constable; London: pp. 254–270. 1930
|
|
20
|
Maynard S, Schurman SH, Harboe C, de
Souza-Pinto NC and Bohr VA: Base excision repair of oxidative DNA
damage and association with cancer and aging. Carcinogenesis.
30:2–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Croteau DL and Bohr VA: Repair of
oxidative damage to nuclear and mitochondrial DNA in mammalian
cells. J Biol Chem. 272:25409–25412. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallace DC: A mitochondrial paradigm of
metabolic and degenerative diseases, aging, and cancer: a dawn for
evolutionary medicine. Annu Rev Genet. 39:359–407. 2005. View Article : Google Scholar
|
|
23
|
Brandon M, Baldi P and Wallace DC:
Mitochondrial mutations in cancer. Oncogene. 25:4647–4662. 2006.
View Article : Google Scholar
|
|
24
|
Chatterjee A, Mambo E and Sidransky D:
Mitochondrial DNA mutations in human cancer. Oncogene.
25:4663–4674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HC and Wei YH: Mitochondrial DNA
instability and metabolic shift in human cancers. Int J Mol Sci.
10:674–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poyton RO and McEwen JE: Crosstalk between
nuclear and mitochondrial genomes. Annu Rev Biochem. 65:563–607.
1996. View Article : Google Scholar
|
|
27
|
Chandra D and Singh KK: Genetic insights
into OXPHOS defect and its role in cancer. Biochim Biophys Acta.
1807:620–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mayr JA, Meierhofer D, Zimmermann F, et
al: Loss of complex I due to mitochondrial DNA mutations in renal
oncocytoma. Clin Cancer Res. 14:2270–2275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dasgupta S, Hoque MO, Upadhyay S and
Sidransky D: Mitochondrial cytochrome B gene mutation promotes
tumor growth in bladder cancer. Cancer Res. 68:700–706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonora E, Porcelli AM, Gasparre G, et al:
Defective oxidative phosphorylation in thyroid oncocytic carcinoma
is associated with pathogenic mitochondrial DNA mutations affecting
complexes I and III. Cancer Res. 66:6087–6096. 2006. View Article : Google Scholar
|
|
31
|
Namslauer I, Dietz MS and Brzezinski P:
Functional effects of mutations in cytochrome c oxidase related to
prostate cancer. Biochim Biophys Acta. 1807:1336–1341. 2011.
View Article : Google Scholar
|
|
32
|
Bernstein C, Facista A, Nguyen H, et al:
Cancer and age related colonic crypt deficiencies in cytochrome c
oxidase I. World J Gastrointest Oncol. 2:429–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HC, Li SH, Lin JC, Wu CC, Yeh DC and
Wei YH: Somatic mutations in the D-loop and decrease in the copy
number of mitochondrial DNA in human hepatocellular carcinoma.
Mutat Res. 547:71–78. 2004. View Article : Google Scholar
|
|
34
|
Yin PH, Lee HC, Chau GY, et al: Alteration
of the copy number and deletion of mitochondrial DNA in human
hepatocellular carcinoma. Br J Cancer. 90:2390–2396.
2004.PubMed/NCBI
|
|
35
|
Yin PH, Wu CC, Lin JC, Chi CW, Wei YH and
Lee HC: Somatic mutations of mitochondrial genome in hepatocellular
carcinoma. Mitochondrion. 10:174–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greaves LC, Reeve AK, Taylor RW and
Turnbull DM: Mitochondrial DNA and disease. J Pathol. 226:274–286.
2012. View Article : Google Scholar
|
|
37
|
Müller-Höcker J, Aust D, Rohrbach H, et
al: Defects of the respiratory chain in the normal human liver and
in cirrhosis during aging. Hepatology. 26:709–719. 1997.
|
|
38
|
Shah SA, Cleary SP, Wei AC, et al:
Recurrence after liver resection for hepatocellular carcinoma: risk
factors, treatment, and outcomes. Surgery. 141:330–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Theise ND, Curado MP, Franceschi S, et al:
WHO classification of tumours of the digestive system.
Hepatocellular Carcinoma. Bosman FT, Carneiro F, Hruban RH and
Theise ND: 4th edition. IARC Press; Lyon: pp. 205–216. 2010
|
|
40
|
Rodríguez-Perálvarez M, Luong TV, Andreana
L, Meyer T, Dhillon AP and Burroughs AK: A systematic review of
microvascular invasion in hepatocellular carcinoma: diagnostic and
prognostic variability. Ann Surg Oncol. 20:325–339. 2013.PubMed/NCBI
|
|
41
|
Edge SE, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual and Handbook. 7th ed. Springer; New York, NY,
USA: 2010
|
|
42
|
Srinivasan S and Avadhani NG: Cytochrome c
oxidase dysfunction in oxidative stress. Free Radic Biol Med.
53:1252–1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arnold S: The power of life - cytochrome c
oxidase takes center stage in metabolic control, cell signalling
and survival. Mitochondrion. 12:46–56. 2012. View Article : Google Scholar
|
|
44
|
Schägger H and Pfeiffer K: The ratio of
oxidative phosphorylation complexes I–V in bovine heart
mitochondria and the composition of respiratory chain
supercomplexes. J Biol Chem. 276:37861–37867. 2001.
|
|
45
|
Acín-Pérez R, Fernández-Silva P, Peleato
ML, Pérez-Martos A and Enriquez JA: Respiratory active
mitochondrial supercomplexes. Mol Cell. 32:529–539. 2008.
|
|
46
|
Acín-Pérez R, Bayona-Bafaluy MP,
Fernández-Silva P, et al: Respiratory complex III is required to
maintain complex I in mammalian mitochondria. Mol Cell. 13:805–815.
2004.PubMed/NCBI
|
|
47
|
Schäfer E, Seelert H, Reifschneider NH,
Krause F, Dencher NA and Vonck J: Architecture of active mammalian
respiratory chain supercomplexes. J Biol Chem. 281:15370–15375.
2006.
|
|
48
|
Brown MD, Yang CC, Trounce I, Torroni A,
Lott MT and Wallace DC: A mitochondrial DNA variant, identified in
Leber hereditary optic neuropathy patients, which extends the amino
acid sequence of cytochrome c oxidase subunit I. Am J Hum Genet.
51:378–385. 1992.
|
|
49
|
Varlamov DA, Kudin AP, Vielhaber S, et al:
Metabolic consequences of a novel missense mutation of the mtDNA CO
I gene. Hum Mol Genet. 11:1797–1805. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lucioli S, Hoffmeier K, Carrozzo R, Tessa
A, Ludwig B and Santorelli FM: Introducing a novel human mtDNA
mutation into the Paracoccus denitrificans COX I gene explains
functional deficits in a patient. Neurogenetics. 7:51–57. 2006.
View Article : Google Scholar
|
|
51
|
Karadimas CL, Greenstein P, Sue CM, et al:
Recurrent myoglobinuria due to a nonsense mutation in the COX I
gene of mitochondrial DNA. Neurology. 55:644–649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pandya A, Xia XJ, Erdenetungalag R, et al:
Heterogenous point mutations in the mitochondrial tRNA Ser(UCN)
precursor coexisting with the A1555G mutation in deaf students from
Mongolia. Am J Hum Genet. 65:1803–1806. 1999. View Article : Google Scholar
|
|
53
|
Greaves LC, Preston SL, Tadrous PJ, et al:
Mitochondrial DNA mutations are established in human colonic stem
cells, and mutated clones expand by crypt fission. Proc Natl Acad
Sci USA. 103:714–719. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Namslauer I and Brzezinski P: A
mitochondrial DNA mutation linked to colon cancer results in proton
leaks in cytochrome c oxidase. Proc Natl Acad Sci USA.
106:3402–3407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wolff AC, Hammond ME, Hicks DG, et al:
Recommendations for human epidermal growth factor receptor 2
testing in breast cancer: American Society of Clinical
Oncology/College of American Pathologists clinical practice
guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar
|
|
56
|
Rindi G, Arnold R, Bosman FT, et al:
Nomenclature and classification of neuroendocrine neoplasms of the
digestive system. WHO Classification of Tumours of the Digestive
System. Bosman TF, Carneiro F, Hruban RH and Theise ND: 4th ed.
International Agency for Research on Cancer (IARC); Lyon, France:
pp. 132010
|