Introduction
Ovarian cancer is the fifth leading cause of
cancer-related mortality in females and is a fatal disease among
all gynecologic malignancies. The main difficulty in curing ovarian
cancer is that the majority of patients are diagnosed at an
advanced stage. According to previous studies, as many as 70% of
patients have already reached stages III or IV of the disease at
the time of diagnosis (1). Due to
the latent onset of ovarian cancer, no effective medical screening
methods have yet been identified for the improved identification
and detection of the disease. In addition, the current clinical
therapy for ovarian cancer is optimal primary cytoreductive
surgery, followed by systemic chemotherapy with a combination of
paclitaxol and platinum (2).
However, due to the side effects of these medicines and the
increasing tolerance of ovarian cancer to chemotherapy, the
efficacy of chemotherapy is limited. Traditional Chinese medicine
(TCM) has been used in China for >1,000 years as an anticancer
treatment for a number of different malignancies, including liver,
lung and hematopoietic cancers (3–5). TCM
is considered to induce few side-effects and little tumor cell
resistance and has recently been recognized as a key source of
novel drugs for molecular therapies. Clinical practices have also
shown that a number of TCMs exhibit antitumor activity, which
provides a novel therapeutic strategy for cancer treatment
(6). Pien Tze Huang (PZH) is a
well-known TCM that was identified ~450 years ago and has been
applied to the treatment of liver diseases, cancer, stroke and
inflammation (7). Similar to a
number of other TCMs, PZH contains numerous ingredients, including
musk, calculus bovis, snake’s gall and tienchi. As for its
application as a cancer treatment, PZH has been widely used in
China and Southeast Asia for centuries as a folk remedy for various
types of cancer, due to its temperature reducing and detoxification
effects. Recent studies have also demonstrated that PZH exhibits
therapeutic effects in clinical trials of tumors, such as
hepatocellular carcinoma and colon cancer. In addition, it has been
reported that PZH inhibits the growth of gastric carcinoma, colon
cancer and hepatoma in vivo and in vitro (8–10).
However, the complexity of the ingredients and the obscurity of its
mechanism of action have inhibited the wider use of PZH. In the
current study, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to investigate the effects of PZH on the cell
viability of the human ovarian cancer OVCAR-3 cell line, and a
Transwell assay was conducted to analyze the effects of PZH on cell
invasiveness. Hoechst 33258 staining was also performed to detect
the apoptosis of the OVCAR-3 cells. In addition, flow cytometry was
conducted to examine the apoptosis frequency and cell cycle changes
in the OVCAR-3 cells with various PZH concentrations. Finally,
western blotting was performed to investigate the effect of PZH on
the variation of important signal transduction pathways in the
OVCAR-3 cell line. The study was approved by the ethics committee
of Sun Yat-Sen University (Guangzhou, China).
Materials and methods
Materials and reagents
PZH was purchased from Zhangzhou Pientzehuang
Pharmaceutical Co., Ltd., (Zhangzhou, China). PZH solutions were
prepared by dissolving PZH powder in double distilled water to a
final concentration of 50 mg/ml and then stored at −20°C until use.
The working concentrations of PZH were made by diluting the stock
solution in the culture medium to concentrations of 250, 500 and
1,000 μg/ml.
Cell culture
The human ovarian cancer OVCAR-3 cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPMI 1640
medium containing 10% (v/v) fetal bovine serum at a temperature of
37°C in a humidified atmosphere of 5% CO2. The cells
were then subcultured at 80–90% confluence, treated with PZH
concentrations of 250, 500 and 1,000 μg/ml for 24 h and harvested
for further study.
MTT assay
The OVCAR-3 cells were seeded into 96-well plates at
a density of 5×103 cells/well in 0.1 ml medium. The
cells were then treated with consecutive concentrations of PZH (0,
250, 500 and 1,000 μg/ml) for the same time periods. Following 24
h, 100 μl MTT [0.5 mg/ml in phosphate-buffered saline (PBS)] was
added to each well and the cells were incubated for an additional 4
h. Next, the MTT formazan precipitate was dissolved in 100 μl
dimethyl sulfoxide and the absorbance was measured at 570 nm using
an ELISA reader (ST360, Flyde, Guangzhou, China). An optical
density (OD) result of zero concentration was used to present 100%
cell viability and thus, the cell viability at other concentrations
was calculated using the following formula: Cell
viabilityx = ODx / ODo, where
ODx is the OD of cells treated with a concentration of
PZH and ODo is the OD of cells without PZH
treatment.
Transwell assay
For the Transwell assay, Transwell inserts with 8-μm
pores were used. The OVCAR-3 cells were harvested and 200 μl cell
suspension (1×106 cells/ml) from each treatment was
added in triplicate wells. Following 24 h of incubation, the cells
that had migrated through the filter into the lower wells were
quantified using an MTT assay and expressed as a percentage of the
sum of the cells in the upper and lower wells.
Hoechst 33258 staining
The cells were plated in six-well plates and
incubated for 24 h. Concentrations of PZH were then added to each
well of the three experimental groups, and then incubated for 24 h
together with the negative control group. Next, the cells were
washed three times with PBS and stained with Hoechst 33258 (1 mg/l)
for 15 min at 37°C. Images of the Hoechst 33258 fluorescence were
then captured using inverted fluorescence microscopy (U-CMAD3;
Olympus, Tokyo, Japan), prior to washing the cells three times with
PBS. The percentage of positively-stained cells was calculated
according to the images.
Wound healing assay
The OVCAR-3 cells were plated in six-well plates and
incubated for 24 h. The cells were then scratched with a yellow tip
pipette to create a wound and definite scratches in the center of
the dishes with a clear field. Next, concentrations of PZH were
added to the three experimental groups, and then medium without
serum was added to these plates and that of the negative control
group. Images of the cells that had migrated from the leading edge
were captured following intervals of 0, 4, 8 and 12 h.
Apoptosis rate and flow cytometry
analysis
The OVCAR-3 cells were harvested following exposure
to various concentrations of PZH for 24 h. The cells were then
centrifuged (500 × g for 5 min), collected and washed with PBS,
followed by resuspension in binding buffer. Next, the cells were
incubated with 5 μl Annexin V in the dark for 10 min, washed with
binding buffer and resuspended in l% formaldehyde at 40°C for 30
min. The cells were then washed again and stained with 500 μl
propidium iodide (PI) for 15 min. Finally, the apoptosis rate was
determined by flow cytometry and analyzed by BD CellQuest™ software
(BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
The cell cycle analysis was performed using flow
cytometry (Beckman Coulter, Miami, FL, USA) and PI staining. The
ovarian cancer cells treated with 0, 250, 500 and 1,000 μg/ml of
PZH were dissociated from the culture plates using a solution of
0.1% trypsin in PBS for 3 min. Next, the OVCAR-3 cells were
collected, adjusted to a concentration of 1×106 cells/ml
and fixed in 70% ethanol at 4°C overnight. The fixed cells were
then washed twice with cold PBS and incubated for 30 min with RNase
(8 μg/ml), 0.1% Triton X-100 and PI (10 μg/ml). The fluorescence
signal was detected by flow cytometry (Beckman Coulter) through
fluorescence channel 2, and the proportion of DNA in each phase was
analyzed by Modfit LT version 3.0 (Verity Software House Inc.,
Topsham, ME, USA).
Western blotting
The OVCAR-3 cells were treated with various
concentrations of PZH for 24 h and then lysed with mammalian cell
lysis buffer (RIPA; Thermo Fisher Scientific, Waltham, MA, USA)
containing protease and phosphatase inhibitor cocktails (1:1,000)
for 30 min on ice. Next, the raw homogenate was centrifuged (13,000
× g) at 4°C for 20 min, and the supernatants (20 μg) with 4×
loading buffer were heated for 10 min at 100°C. The proteins were
then separated in 12% SDS-PAGE gels and transferred to
polyvinylidene fluoride membranes. Membranes were blocked with 5%
skimmed milk in 1× Tris-buffered saline with 0.1% Tween-20 for 1 h
at room temperature and then incubated overnight with monoclonal
antibodies against p53, AKT, AKT-1, phosphorylated (p)-AKT,
mammalian target of rapamycin (mTOR), p-mTOR, cyclin-dependent
kinase (CDK)4, CDK6 and GAPDH (1:1,000) at 4°C. The appropriate
horseradish peroxidase-conjugated secondary antibody was added,
followed by enhanced chemiluminescence detection.
Statistical analysis
All data were analyzed using the SPSS package for
windows (version 13.0; SPSS, Inc., Chicago, IL, USA) and
statistical analysis of the data was performed using Student’s
t-test and a one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
PZH inhibits the proliferation of OVCAR-3
cells
The cell viability of the OVCAR-3 cells was detected
by MTT assay to compare the PZH-treated cells with the untreated
controls. As shown in Fig. 1,
treatment with 250–1,000 μg/ml PZH for 24 h was found to reduce
cell viability from 100 to 84.73, 72.67 and 67.00% compared with
the control (P=0.002; Fig. 1A).
PZH inhibits the invasion of OVCAR-3
cells
Treatment with PZH alone marginally decreased the
invasion ability of the OVCAR-3 cells. Moreover, the invasion
ability of the OVCAR-3 cells was found to markedly decrease with
increasing concentrations of PZH (Fig.
1B).
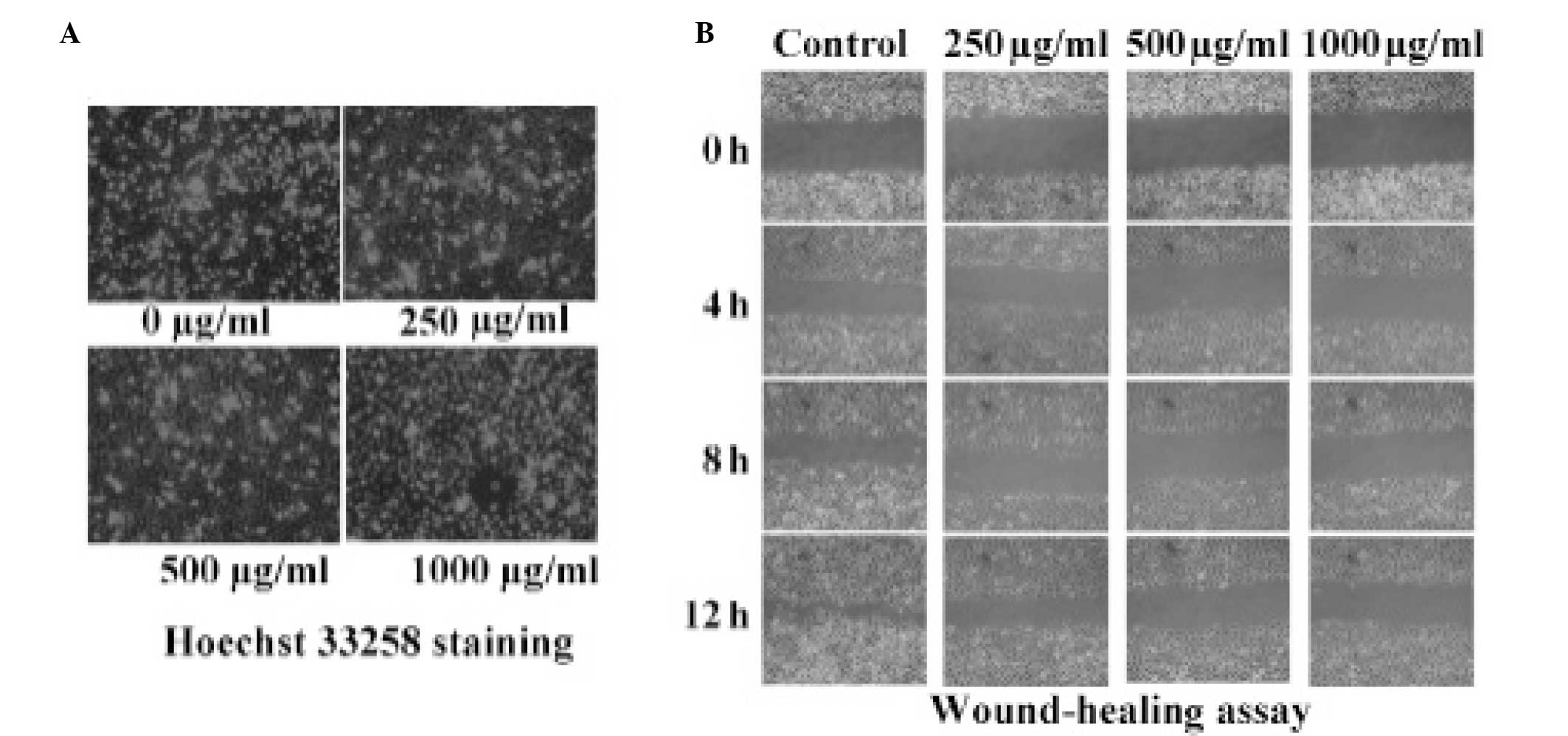
PZH does not effect the apoptosis of
OVCAR-3 cells
PZH was not found to induce the apoptosis of the
OVCAR-3 cells. The percentage of apoptotic cells was calculated
using Hoechst 33258 staining, which revealed that the apoptotic
rate of the OVCAR-3 cell line did not significantly increase with
increasing concentrations of PZH (Fig.
2A).
PZH-induced inhibition of OVCAR-3 cell
migration
A wound-healing assay was performed to investigate
the migratory ability of cells treated with various concentrations
(0, 250, 500 and 1,000 μg/ml) of PZH. It was demonstrated that the
OVCAR-3 cells treated with PZH healed the scratch wounds more
rapidly than the negative control group in a dose-dependent manner.
As shown in Fig. 2B, increasing
concentrations of PZH reduced the migration and invasion ability of
the OVCAR-3 cells.
Effects of PZH on the apoptosis of
OVCAR-3 cells
The percentage of the OVCAR-3 cells undergoing
apoptosis was detected by PI staining and determined by
fluorescence-activated sorting (FACS) analysis. With the
consecutive treatment of the OVCAR-3 cells with concentrations of
PZH (0, 250, 500 and 1,000 μg/ml), the apoptosis rates were
observed to be 0, 6.6, 30.9 and 43.2%, respectively (P=0.141;
Fig. 3A).
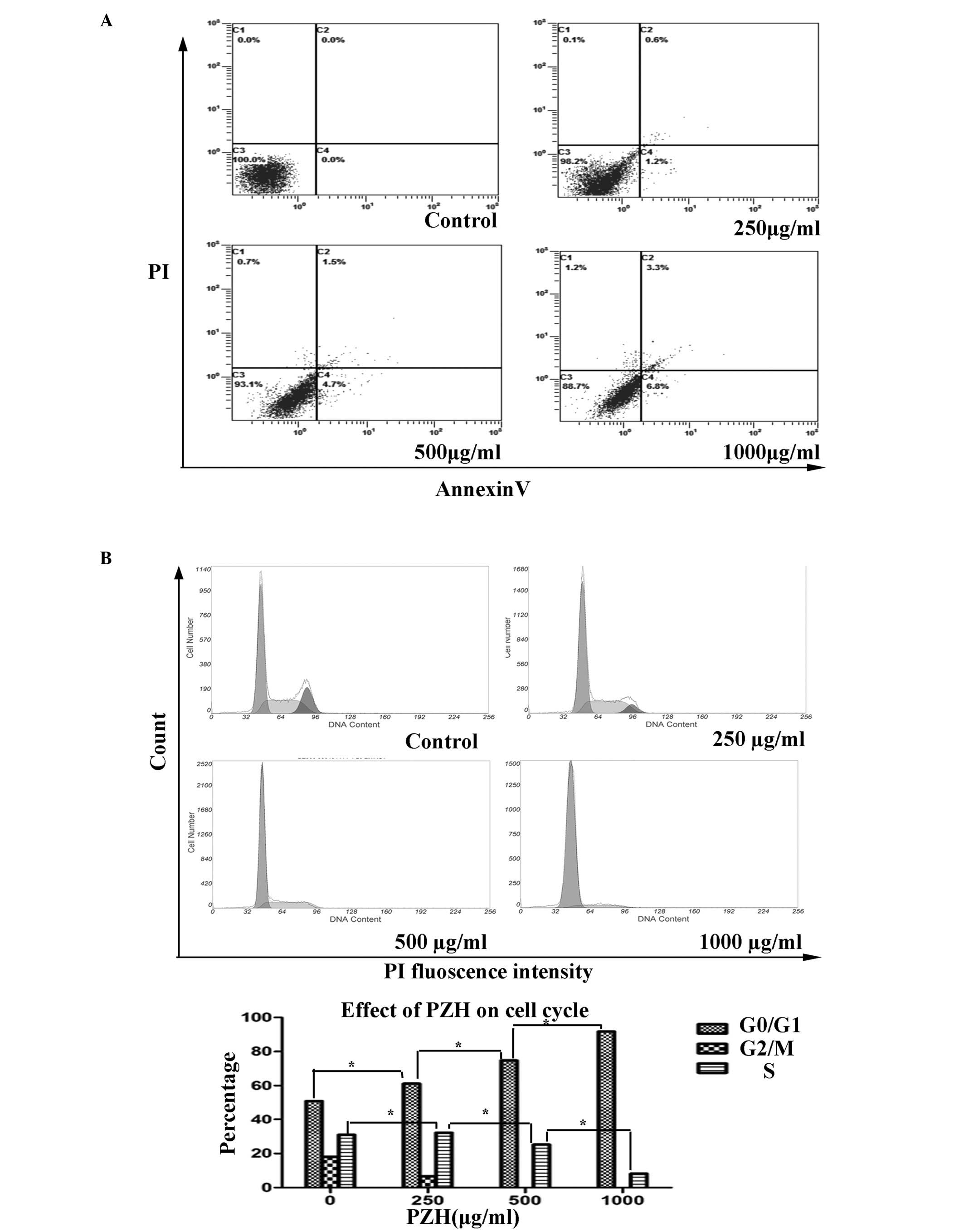 | Figure 3FACs analysis of the apoptosis and
cell cycle progression of OVCAR-3 cells. (A) PZH was not found to
induce apoptosis in the OVCAR-3 cells collected and stained with
Annexin V/PI. Annexin V-positive/PI-negative stained cells (lower
right) and Annexin V/PI double-positive stained cells (upper right)
represent early and late apoptosis, while Annexin V-negative and
PI-positive stained cells (upper left) represent dead cells. Data
are presented as the mean ± standard deviation from three
independent experiments *P<0.05 vs. control cells.
(B) PZH blocked G1/S progression in the OVCAR-3 cells
that were collected and stained with Annexin V/PI and analyzed by
FACS. Following treatment with consecutive concentrations of PZH
(0, 250, 500 and 1,000 μg/ml), the number of cells in the
G1 phase increased by 50.99, 61.23, 74.76 and 91.81%,
respectively (P=0.004), while the percentage of cells in S-phase
was 30.98, 32.11, 25.243 and 8.19%, respectively (P=0.022). PZH,
Pien Tze Huang; FACS, fluorescence-activated cell sorting; PI,
propidium iodide. |
PZH regulates the cell cycle in OVCAR-3
cells
PZH was observed to block G1/S phase
progression in the OVCAR-3 cells, as determined by PI staining and
FACS analysis. With the consecutive treatment of the OVCAR-3 cells
with the various concentrations of PZH (0, 250, 500 and 1,000
μg/ml), the number of cells in the G1 phase was found to
increase, with percentages of 50.99, 61.23, 74.76 and 91.81%,
respectively (P=0.004). By contrast, the percentage of cells in the
S phase were found to be 30.98, 32.11, 25.24 and 8.19%,
respectively (P=0.022; Fig. 3B).
These results indicated that PZH may inhibit OVCAR-3 cell
proliferation by blocking cell cycle progression from the
G1 to S phases.
PZH regulates protein expression in
OVCAR-3 cells
PZH regulates the expression of AKT, p-AKT, mTOR,
p-mTOR, CDK4, CDK6, p53 and c-Myc in OVCAR-3 cells, and following
PZH treatment for 24 h in the present study, the expression levels
of these proteins were found to decrease compared with those in the
control group. In addition, a marked increase in p53 protein
expression was observed (Fig.
4).
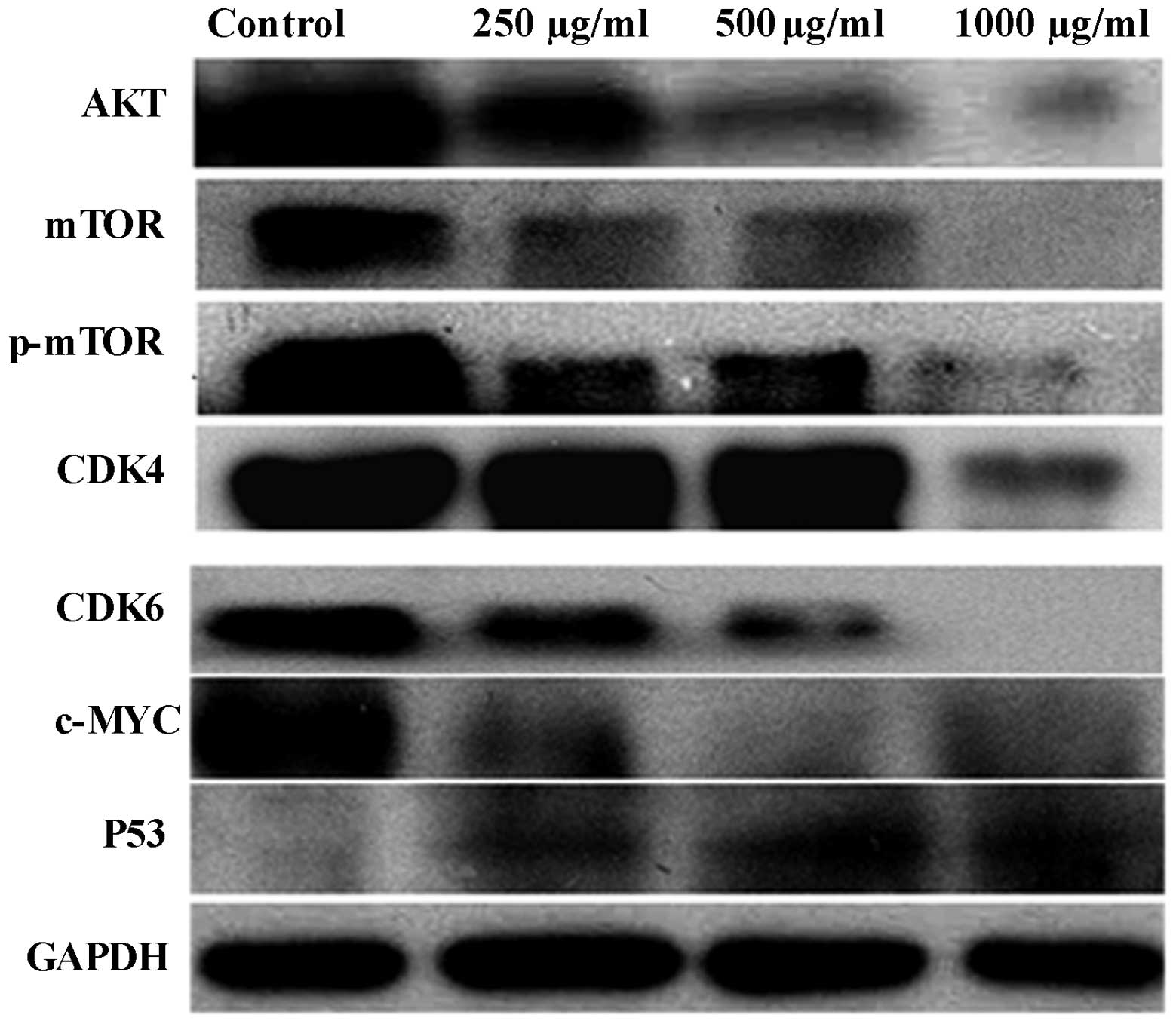 | Figure 4Effect of PZH on the expression of
AKT, p-AKT, AKT-1, mTOR, p-mTOR, CDK4, CDK6, p53 and c-Myc in
OVCAR-3 cells. Cells were treated with the indicated concentrations
of PZH for 24 h and analyzed by western blotting. GAPDH was used as
the internal control. Data are representative of three independent
experiments. The expression levels of AKT, p-AKT, AKT-1, mTOR,
p-mTOR, CDK4, CDK6 and c-Myc were found to decrease compared with
the control group. Furthermore, a marked increase in the protein
expression of p53 was observed. PZH, Pien Tze Huang; mTOR,
mammalian target of rapamycin; CDK, cyclin-dependent kinase; p−,
phosphorylated. |
Discussion
The main disadvantage of current cancer chemotherapy
is drug resistance in tumor cells and toxicity against normal
cells, which largely limits the efficacy of chemotherapy treatments
(11). However, it is well
acknowledged that TCM, which consists of several herbs, is
characterized by few side-effects and limited drug tolerance, thus
indicating the potential use of TCM in cancer treatment (12). Previous studies and clinical
practice have shown that PZH exhibits an anticancer capacity and
has the ability to induce cell apoptosis. However, its effects on
cell proliferation and the cell cycle and its underlying molecular
mechanism remain unclear. Therefore, the full elucidation of the
effect and molecular mechanisms of PZH with regard to its specific
anticancer function is required.
The aim of the present study was to investigate the
underlying pathway and effect of PZH on the proliferation and cell
cycle of OVCAR-3 cells. The FACS analysis revealed that the
percentage of OVCAR-3 cells in the S and G2 phases of
the cell cycle decreased significantly with increasing
concentrations of PZH, which indicated that a high concentration of
PZH may exhibit a significant anticancer effect via regulation of
the cell cycle. In addition, PZH was found to inhibit the migration
and invasion ability of the OVCAR-3 cells.
It is well known that cellular growth, proliferation
and survival are regulated by a complex network of intracellular
and extracellular signal transduction cascades, and that the growth
factor-responsive receptor tyrosine kinase
(RTK)-phosphatidylinositol 3-kinase (PI3K) pathway is an important
mediator of these processes (13).
The serine/threonine kinase AKT functions as a central integrator
of the RTK-PI3K signaling cascade, which modulates downstream
effectors and most notably the tuberous sclerosis complex 1/2-mTOR
pathway. Furthermore, mTOR is a serine/threonine protein kinase,
termed the ‘target of rapamycin’, which serves as a primary
regulator of protein synthesis and cell growth (14). Genetic studies in Drosophila and
mice (15–18) have also shown that mTOR activity
affects cell size, which is a key parameter that governs entry into
the cell cycle (19). mTOR also
integrates diverse upstream signals, including amino acid- and
energy stress-sensing, to regulate cell proliferation, growth and
survival (20,21). Notably, it has also been confirmed
that the aberrant stimulation of the PI3K-AKT-mTOR pathway is
associated with a poor prognosis in ovarian cancer patients
(22). In terms of cell survival
rate and cell cycle changes, to improve our understanding of the
potential molecular mechanism of PZH, the current study detected
the expression levels of total AKT, mTOR and p-mTOR proteins
following treatment with various concentrations of PZH. The results
revealed that the expression levels of the AKT, mTOR and p-mTOR
proteins evidently decreased with increasing concentrations of PZH,
which indicated that the AKT-mTOR pathway may be inhibited by
PZH.
In addition, the expression of certain downstream
proteins of the AKT-mTOR pathway was investigated, and the
expression of p53, termed as the ‘guardian of the genome’ (23,24),
was evidently upregulated with the increasing concentrations of
PZH. It is well known that the p53 protein functions as a cancer
suppressor by inhibiting cell growth through cell cycle arrest at
the G1/S regulation point and initiating apoptosis if
the cell is damaged (25). The
present results revealed that the expression of specific proteins
associated with G1/S transition, including cyclin D3,
CDK4 and CDK6 (26–28), decreased with the dose-dependent
repression of the G1/S transition by PHZ, as determined
by flow cytometry. Based on the aforementioned PZH-induced
apoptosis and reduced cell viability, we propose that inhibition of
the PZH-induced OVCAR-3 cell proliferation and G1/S
transition may be involved in the AKT-mTOR pathway.
In conclusion, the present study indicates that PZH
may inhibit the cell proliferation, migration and G1/S
transition of OVCAR-3 cells via the AKT-mTOR pathway.
Acknowledgements
The authors would like to thank Dr Tian Yun for
providing useful advice and Professor Cheng and Dr Tang for their
support with the study.
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angioli R, Palaia I, Zullo MA, et al:
Diagnostic open laparoscopy in the management of advanced ovarian
cancer. Gynecol Oncol. 100:455–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JY, Cheung JY, Luk SC, Wu YJ, Pang SF
and Fung KP: Anti-cancer and pro-apoptotic effects of an herbal
medicine and Saccharomyces cerevisiae product (CKBM) on
human hepatocellular carcinoma HepG2 cells in vitro and in vivo.
Immunopharmacol Immunotoxicol. 26:597–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HJ, Lee EO, Rhee YH, et al: An
oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer
activities by targeting angiogenesis, apoptosis and metastasis.
Carcinogenesis. 27:2455–2463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumagai T, Müller CI, Desmond JC, Imai Y,
Heber D and Koeffler HP: Scutellaria baicalensis, a herbal
medicine: anti-proliferative and apoptotic activity against acute
lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res.
31:523–530. 2007. View Article : Google Scholar
|
|
6
|
Sha O, Niu J, Ng TB, Cho EY, Fu X and
Jiang W: Anti-tumor action of trichosanthin, a type 1
ribosome-inactivating protein, employed in traditional Chinese
medicine: a mini review. Cancer Chemother Pharmacol. 71:1387–1393.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KK, Kwong WH, Chau FT, Yew DT and Chan
WY: Pien Tze Huang protects the liver against carbon
tetrachloride-induced damage. Pharmacol Toxicol. 91:185–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin JM, Wei LH, Chen YQ, et al: Pien Tze
Huang induced apoptosis in human colon cancer HT-29 cells is
associated with regulation of the Bcl-2 family and activation of
caspase 3. Chin J Integr Med. 17:685–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang Q, Hong F, Shen A, et al: Pien Tze
Huang inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
10
|
Shen AL, Hong F, Liu LY, et al: Effects of
Pien Tze Huang on angiogenesis in vivo and in vitro. Chin J Integr
Med. 18:431–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kigawa J: New strategy for overcoming
resistance to chemotherapy of ovarian cancer. Yonago Acta Med.
56:43–50. 2013.PubMed/NCBI
|
|
12
|
Li X, Yang G, Li X, et al: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shima H, Pende M, Chen Y, Fumagalli S,
Thomas G and Kozma SC: Disruption of the p70(s6k)/p85(s6k) gene
reveals a small mouse phenotype and a new functional S6 kinase.
EMBO J. 17:6649–6659. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montagne J, Stewart MJ, Stocker H, Hafen
E, Kozma SC and Thomas G: Drosophila S6 kinase: a regulator of cell
size. Science. 285:2126–2129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oldham S, Montagne J, Radimerski T, Thomas
G and Hafen E: Genetic and biochemical characterization of dTOR,
the Drosophila homolog of the target of rapamycin. Genes Dev.
14:2689–2694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Stallock JP, Ng JC, Reinhard C
and Neufeld TP: Regulation of cellular growth by the Drosophila
target of rapamycin dTOR. Genes Dev. 14:2712–2724. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fingar DC and Blenis J: Target of
rapamycin (TOR): an integrator of nutrient and growth factor
signals and coordinator of cell growth and cell cycle progression.
Oncogene. 23:3151–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
21
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhutani J, Sheikh A and Niazi AK: Akt
inhibitors: mechanism of action and implications for anticancer
therapeutics. Infect Agent Cancer. 8:492013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madan E, Gogna R, Bhatt M, Pati U,
Kuppusamy P and Mahdi AA: Regulation of glucose metabolism by p53:
emerging new roles for the tumor suppressor. Oncotarget. 2:948–957.
2011.PubMed/NCBI
|
|
24
|
Marx J: Oncology. Recruiting the cell’s
own guardian for cancer therapy. Science. 315:1211–1213. 2007.
|
|
25
|
Bartek J and Lukas J: Mammalian G1- and
S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol.
13:738–747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedberg Y, Ljungberg B, Roos G and
Landberg G: Expression of cyclin D1, D3, E, and p27 in human renal
cell carcinoma analysed by tissue microarray. Br J Cancer.
88:1417–1423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baker SJ and Reddy EP: CDK4: A key player
in the cell cycle, development, and cancer. Genes Cancer.
3:658–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hleb M, Murphy S, Wagner EF, et al:
Evidence for cyclin D3 as a novel target of rapamycin in human T
lymphocytes. J Biol Chem. 279:31948–31955. 2004. View Article : Google Scholar : PubMed/NCBI
|