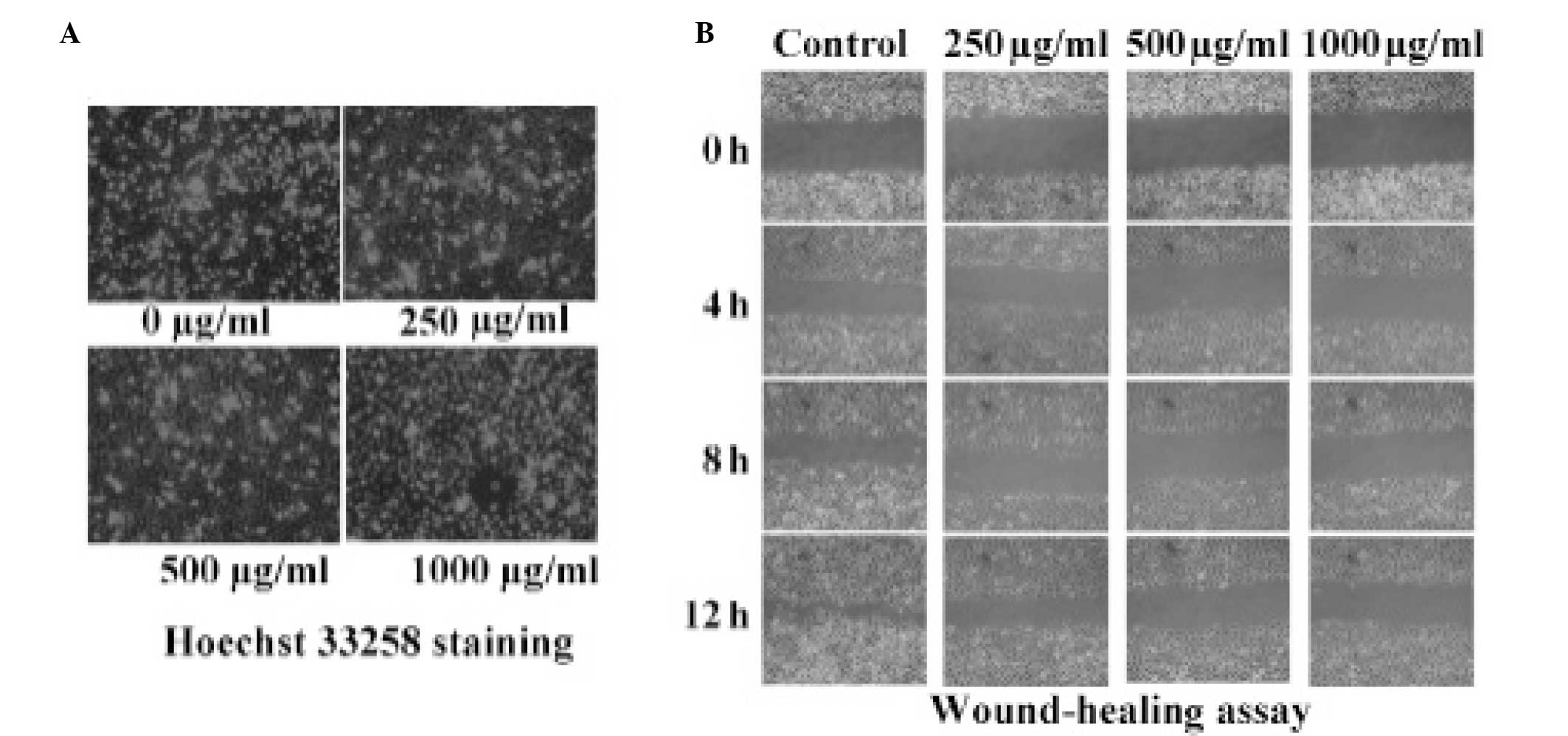
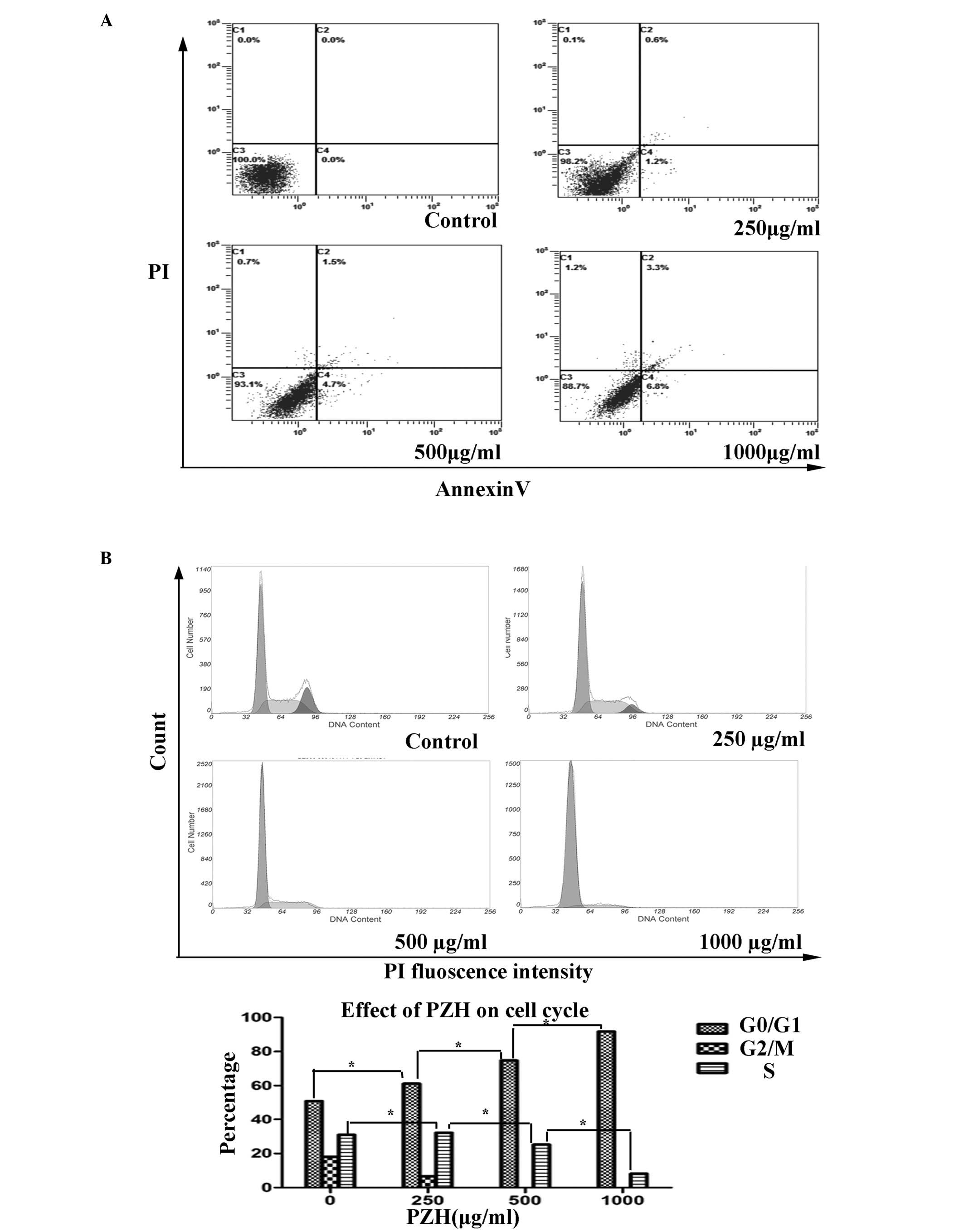
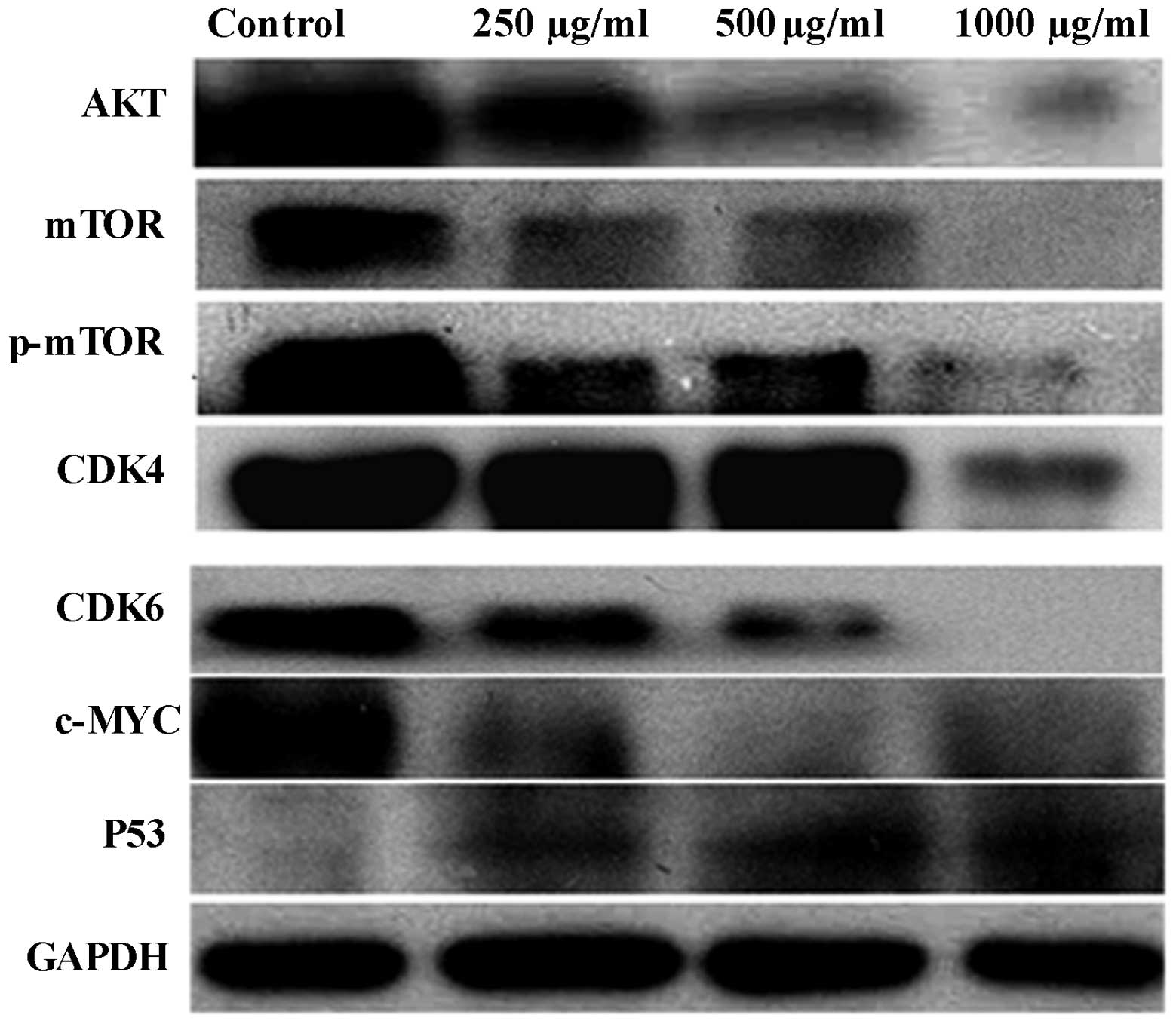
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angioli R, Palaia I, Zullo MA, et al:
Diagnostic open laparoscopy in the management of advanced ovarian
cancer. Gynecol Oncol. 100:455–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan JY, Cheung JY, Luk SC, Wu YJ, Pang SF
and Fung KP: Anti-cancer and pro-apoptotic effects of an herbal
medicine and Saccharomyces cerevisiae product (CKBM) on
human hepatocellular carcinoma HepG2 cells in vitro and in vivo.
Immunopharmacol Immunotoxicol. 26:597–609. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HJ, Lee EO, Rhee YH, et al: An
oriental herbal cocktail, ka-mi-kae-kyuk-tang, exerts anti-cancer
activities by targeting angiogenesis, apoptosis and metastasis.
Carcinogenesis. 27:2455–2463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumagai T, Müller CI, Desmond JC, Imai Y,
Heber D and Koeffler HP: Scutellaria baicalensis, a herbal
medicine: anti-proliferative and apoptotic activity against acute
lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res.
31:523–530. 2007. View Article : Google Scholar
|
|
6
|
Sha O, Niu J, Ng TB, Cho EY, Fu X and
Jiang W: Anti-tumor action of trichosanthin, a type 1
ribosome-inactivating protein, employed in traditional Chinese
medicine: a mini review. Cancer Chemother Pharmacol. 71:1387–1393.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KK, Kwong WH, Chau FT, Yew DT and Chan
WY: Pien Tze Huang protects the liver against carbon
tetrachloride-induced damage. Pharmacol Toxicol. 91:185–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin JM, Wei LH, Chen YQ, et al: Pien Tze
Huang induced apoptosis in human colon cancer HT-29 cells is
associated with regulation of the Bcl-2 family and activation of
caspase 3. Chin J Integr Med. 17:685–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang Q, Hong F, Shen A, et al: Pien Tze
Huang inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in a colorectal cancer mouse model.
Int J Oncol. 40:1569–1574. 2012.PubMed/NCBI
|
|
10
|
Shen AL, Hong F, Liu LY, et al: Effects of
Pien Tze Huang on angiogenesis in vivo and in vitro. Chin J Integr
Med. 18:431–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kigawa J: New strategy for overcoming
resistance to chemotherapy of ovarian cancer. Yonago Acta Med.
56:43–50. 2013.PubMed/NCBI
|
|
12
|
Li X, Yang G, Li X, et al: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shima H, Pende M, Chen Y, Fumagalli S,
Thomas G and Kozma SC: Disruption of the p70(s6k)/p85(s6k) gene
reveals a small mouse phenotype and a new functional S6 kinase.
EMBO J. 17:6649–6659. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montagne J, Stewart MJ, Stocker H, Hafen
E, Kozma SC and Thomas G: Drosophila S6 kinase: a regulator of cell
size. Science. 285:2126–2129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oldham S, Montagne J, Radimerski T, Thomas
G and Hafen E: Genetic and biochemical characterization of dTOR,
the Drosophila homolog of the target of rapamycin. Genes Dev.
14:2689–2694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Stallock JP, Ng JC, Reinhard C
and Neufeld TP: Regulation of cellular growth by the Drosophila
target of rapamycin dTOR. Genes Dev. 14:2712–2724. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fingar DC and Blenis J: Target of
rapamycin (TOR): an integrator of nutrient and growth factor
signals and coordinator of cell growth and cell cycle progression.
Oncogene. 23:3151–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
21
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhutani J, Sheikh A and Niazi AK: Akt
inhibitors: mechanism of action and implications for anticancer
therapeutics. Infect Agent Cancer. 8:492013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madan E, Gogna R, Bhatt M, Pati U,
Kuppusamy P and Mahdi AA: Regulation of glucose metabolism by p53:
emerging new roles for the tumor suppressor. Oncotarget. 2:948–957.
2011.PubMed/NCBI
|
|
24
|
Marx J: Oncology. Recruiting the cell’s
own guardian for cancer therapy. Science. 315:1211–1213. 2007.
|
|
25
|
Bartek J and Lukas J: Mammalian G1- and
S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol.
13:738–747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedberg Y, Ljungberg B, Roos G and
Landberg G: Expression of cyclin D1, D3, E, and p27 in human renal
cell carcinoma analysed by tissue microarray. Br J Cancer.
88:1417–1423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baker SJ and Reddy EP: CDK4: A key player
in the cell cycle, development, and cancer. Genes Cancer.
3:658–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hleb M, Murphy S, Wagner EF, et al:
Evidence for cyclin D3 as a novel target of rapamycin in human T
lymphocytes. J Biol Chem. 279:31948–31955. 2004. View Article : Google Scholar : PubMed/NCBI
|