Introductıon
Giant cell tumors (GCTs) of the bone are locally
progressive and destructive borderline malignant neoplasms, which
comprise ~5% of primary bone tumors and ~20% of benign tumors
(1). The majority of these tumors
develop in patients aged ≥20 years, with a slightly higher
incidence in females. GCTs develop through endochondral
ossification, and the majority of these tumors are located in the
long bones of the extremities, however, a small proportion occur in
the pelvis, spine or skull. Only <1% of GCTs are localized in
the skull (2,3), with the most common cranial sites
being the sphenoid and temporal bones.
The majority of data on GCTs in the skull consist of
case reports. Despite reports of a number of cases localized in the
sphenoid, temporal and parietal bones, cases of occipital bone
involvement are extremely rare (1,4).
Function-preserving surgery is the standard of care
for GCTs. The achievement of local control is possible in 85–90% of
all cases subsequent to complete resection (5), however, in ≤50% of the cases,
incomplete resection and tumor recurrence are frequently
associated. Although improvements have been made to surgical
techniques, certain regions, particularly the sacral or pelvic
bones, the spine or the skull base, remain a challenge with regard
to complete tumor removal without major functional deficits
(6). As a consequence, primary
radiotherapy (RT) has been recommended as an alternative treatment
for GCTs in these regions; however, concerns have also been raised
with regard to the local side-effects of RT at the appropriate
doses (7,8).
The current study presents and discusses, with a
review of the available literature, the case of a 22-year-old
patient with occipital GCT who was referred to to the Department of
Radiation Oncology (Karadeniz Technical University, Faculty of
Medicine, Trabzon, Turkey) with complaints of neck pain and
headaches. Patient provided written informed consent.
Case report
A 22-year-old female patient was admitted to the
Department of Radiation Oncology (Karadeniz Technical University,
Faculty of Medicine, Trabzon, Turkey) with complaints of neck pain
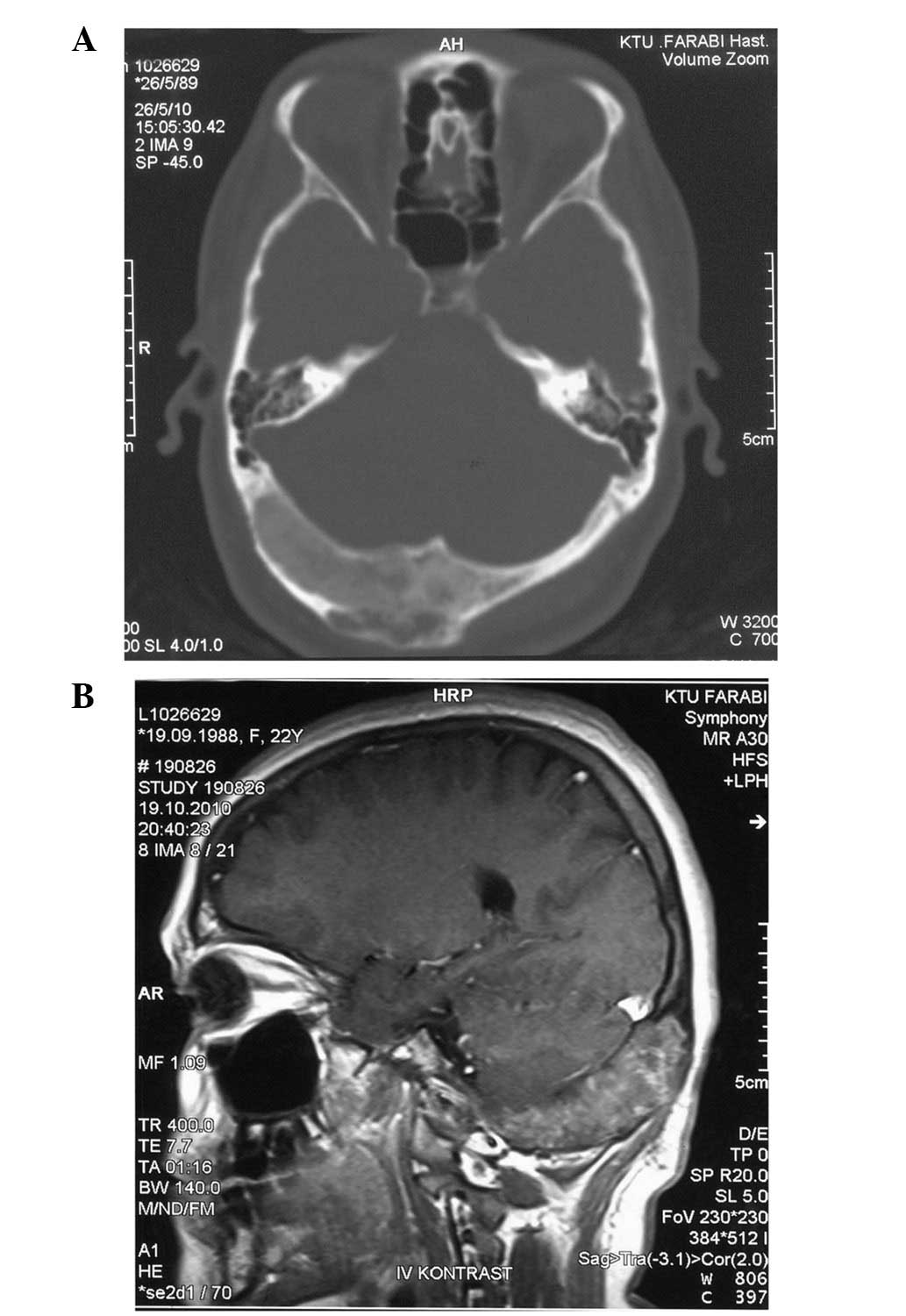
and headaches. The cranial computed tomography images images showed
a ground-glass appearance with lytic areas of 2.5×6 cm in the
occipital bone (Fig. 1A). The
cranial magnetic resonance images showed a 2.5×6-cm mass in the
occipital bone, with dural sinuses on the right side, and with the
middle line slightly extending to the left. A mass that was causing
expansion of the bone and that was of equal intensity with the
muscle tissue in T1-weighted magnetic resonance imaging (MRI) was
observed (Fig. 1B). In addition,
the mass showed slightly hyperintense contrast staining in
T2-weighted MRI.
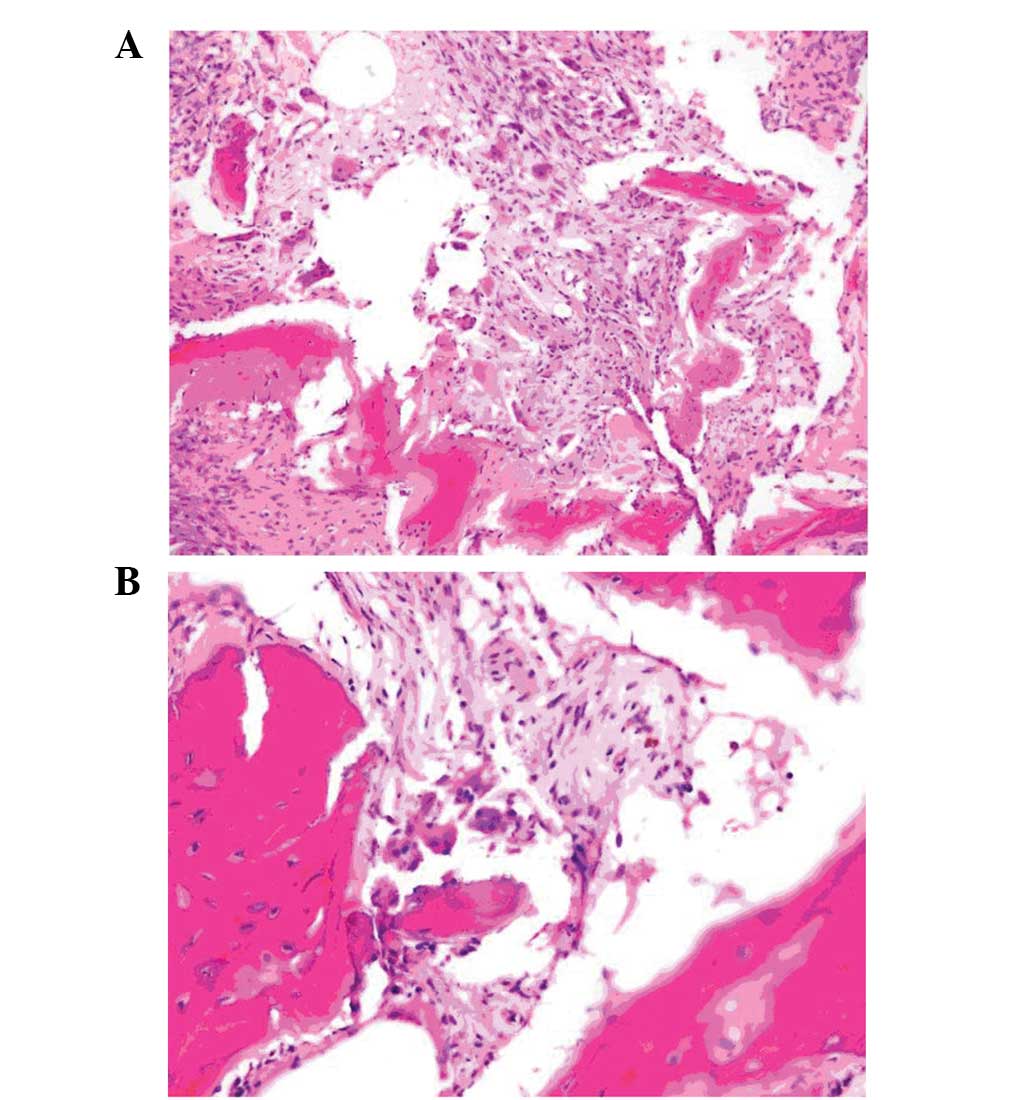
The mass was subtotally excised, and the
post-operative pathological examination showed a neoplasm
characterized with dispersed osteoclast-like nuclear giant cells in
the fibrohistiocytic stroma between the osseous spicules. A number
of foreign body-like giant cells were also detected in the
neoplasm. In the immunohistochemical study, histiocytes and
multinuclear giant cells were possitively stained for CD68. As a
result, a GCT was diagnosed (Fig.
2).
The patient was referred to the Department of
General Surgery (Karadeniz Technical University, Faculty of
Medicine, Trabzon, Turkey), and post-operative RT was delivered
using a 6-MV linear accelerator (Varian Clinac®, Varian
Medical Systems, Inc., Palo Alto, CA, USA), with a 2-cm safety
margin, and a dose of 50 Gy by external radiotherapy, with 200
cGy/fraction on the gross tumor volume of the subtotally resected
mass. At present, the patient is being followed up and no
progression has been observed for 20 months.
Discussion
GCTs are benign, but locally aggressive, primary
osseous tumors usually found in the epiphysis of the long bones
(1,9), particularly involving sites such as
the distal femur, proximal tibia and distal radius (10). In total, <1% of GCTs are found in
the cranial bones, and typically, GCTs are observed in adults aged
between 20 and 40 years (11). In
an analysis across a series of GCT patients, cranial bone
involvement was identified in only 24 out of 2,404 cases, the
majority of which was observed in the sphenoid and temporal bones
(1,7,12). In
the available literature, 115 cases of GCT of the cranium have
previously been reported (Table I)
and a number of the case studies have reported temporal, sphenoid,
frontal and parietal bone involvement (13–14).
The present case is the third case of GCT of the occipital bone to
be reported in the literature and the only case to undergo
postoperative radiotherapy. The first case underwent excision of
the mass and received no treatment following surgery. Treatment
information concerning the second case was not available (Table II).
 | Table INumber of cranial region GCT cases in
the literature. |
Table I
Number of cranial region GCT cases in
the literature.
| Localization | Cases, n | % |
|---|
| Temporal bone | 38 | 33.0 |
| Sphenoid bone | 60 | 52.2 |
| Parietal bone | 5 | 4.4 |
| Frontal bone | 9 | 7.8 |
| Occipital bone | 3a | 2.6 |
| Total | 115 | 100.0 |
 | Table IIData on three patients with giant cell
tumor of the occipital bone. |
Table II
Data on three patients with giant cell
tumor of the occipital bone.
| Case no. (ref.) | Age, years | Presenting
symptom | Surgical
treatment | Ancillary
treatment | Outcome | Follow-up,
months |
|---|
| 1 (15) | 19 | Headache | Total resection | Unknown | Unknown | Unknown |
| 2 (16) | Unknown |
Neurofibromatosis | Unknown | Unknown | Unknown | Unknown |
| 3 (Present case) | 22 | Neck pain and
headache | Subtotal
resection | Radiotherapy | Good; no radiographic
progression | 20 |
Females are affected more frequently in all age
groups. The clinical presentation depends on the site of origin,
however, pain and swelling in the region of the affected bone are
the most common symptoms; the current patient presented with neck
pain and headaches. A differential diagnosis must also consider
chondroblastomas, chondrosarcomas, aneurismal bone cysts, dermoid
cysts, eosinophilic granulomas and pigmented villonodular synovitis
(17).
Total surgical resection is the treatment of choice
for GCT, however, recurrence rates have been found to correlate
with the width of the surgical excision. GCT is locally aggressive,
with a recurrence rate of 40–60% (18) and the prognosis largely depends on
the width of the surgical excision, as well as the radiographic and
histological grading (12).
Furthermore, limited evidence exists regarding the effects of
chemotherapy.
The role of RT in treatment is a controversial
issue. RT is an easy, safe and effective method of treatment and
although no clear dose response has been identified, the literature
indicates that total RT doses ranging between 35 and 45 Gy and
single doses between 1.8 and 2 Gy are extremely safe and effective
in controlling GCT at any location. Furthermore, total doses of
>42 Gy may result in an improved outcome. RT is also effective
in unresectable cases and provides a satisfactory outcome (8,19).
For those patients with GCTs that are not suitable
for complete resection, primary RT must be considered as an
alternative treatment method. However, this conclusion is based on
data collected from small patient series over long time periods,
with wide variations in radiation techniques, fractionation and
total dosage. Although this treatment approach has limited
available data, RT has also previously been criticized due to the
low local control rates found in certain series and concerns with
regard to the side-effects and induction of malignant
transformation (6–8). In a series by Chakravarti et al
(20), a 9.3-year follow-up of the
patients who underwent RT was performed, and radiation-induced
tumors were not observed. However, another study has argued that
GCT is not radiosensitive and that it causes sarcomatous
degeneration in the residual tumor tissue (21). By contrast, in two additional
series, 11 out of 15 patients and 9 out of 10 patients,
respectively, received adjuvant RT, and none of the patients showed
sarcomatous degeneration (4,22).
Bertoni et al (1) showed that the use of treatment
strategies involving surgical resection and RT could provide
satisfactory treatment efficacy. However, in this series, total
surgical excision was was the initial tratment modality and it is
therefore unclear whether the RT was ultimately necessary.
Coumbaras et al (23) and
Ulu et al (24) each
reported a case involving the cranial vault. In these cases no
post-operative RT was employed and there were no signs of
recurrence during the follow-up period.
In a case series by Roeder et al (25) concerning five patients treated with
intensity-modulated RT to a median dose of 64 Gy, a local control
rate of 80% was achieved. Although all primary tumors were
localized in regions with directly adjacent organs at risk,
including the rectum, small bowel or the optic nervous system, no
severe acute or late toxicity attributable to radiation treatment
has yet been observed. Furthermore, in a series of 26 lesions
treated by RT at doses of 35–55 Gy, Feigenberg et al
(26) achieved a local control rate
of 77%, with three severe and four minor associated complications.
Seider et al (27) also presented a series from the MD
Anderson Cancer Center, which observed a local control rate of 70%
when using doses of 36–66 Gy. The results of these series do not
differ significantly, even when all patients with non-extremity
tumors and those who have undergone gross total resection prior to
RT have been excluded. As a consequence, the possibility of high
tumor control rates can be offered by modern imaging and radiation
techniques, without major side-effects.
GCTs are generally benign, locally aggressive
lesions, with pain and swelling as the most common symptoms. The
preferred treatment of GCT is radical surgery, and RT is restricted
to inoperable cases or those not undergoing radical surgery. In the
current case, RT with a total dose of 50 Gy was delivered with 200
cGy/fraction post-operatively following subtotal excision, without
chemotherapy. At present, the patient is being followed up and no
recurrence or symptoms have been observed for 20 months since the
RT.
References
|
1
|
Bertoni F, Unni KK, Beabout JW and
Ebersold MJ: Giant cell tumor of the skull. Cancer. 70:1124–1132.
1992.
|
|
2
|
Murphey MD, Nomikos GC, Flemming DJ,
Gannon FH, Temple HT and Kransdorf MJ: From the archives of AFIP.
Imaging of giant cell tumor and giant cell reparative granuloma of
bone: radiologic-pathologic correlation. Radiographics.
21:1283–1309. 2001.
|
|
3
|
Seider MJ, Rich TA, Ayala AG and Murray J:
Giant cell Tumor of bone: treatment with radiation therapy.
Radiology. 16:537–540. 1986.
|
|
4
|
Reed L, Willison CD, Schochet SS Jr and
Voelker JL: Giant cell tumor of the calvaria in a child: Case
report. J Neurosurg. 80:148–151. 1994.
|
|
5
|
Leggon RE, Zlotecki R, Reith J and
Scarborough MT: Giant cell tumor of the pelvis and sacrum. Clin
Orthop Relat Res. 423:196–207. 2004.
|
|
6
|
McGrath PJ: Giant-cell tumor of bone: an
analysis of fifty-two cases. J Bone Joint Surg Br. 54:216–229.
1972.
|
|
7
|
Goldenberg RR, Campbell CJ and Bonfiglio
M: Giant-cell tumor of bone: An analysis of two hundred and
eighteen cases. J Bone Joint Surg Am. 52:619–664. 1970.
|
|
8
|
Malone S, O’Sullivan B, Catton C, Bell R,
Fornasier V and Davis A: Long-term follow up of efficacy and safety
of megavoltage radiotherapy in high risk giant cell tumors of bone.
Int J Radiat Biol Oncol Phys. 33:689–694. 1995.
|
|
9
|
Elder JB, Berry C, Gonzalez-Gomez I,
Kreger MD and McComb JG: Giant cell tumor of the skull in pediatric
patients. Report of two cases. J Neurosurg. 107:69–74. 2007.
|
|
10
|
Yamamoto M, Fukushima T, Sakamoto S and
Tomonaga M: Giant cell tumor of sphenoid bone: long-term follow-up
of two cases after chemotherapy. Surg Neurol. 49:547–552. 1998.
|
|
11
|
Germanò A, Caruso G, Caffo M, Galatioto S,
Belvedere M and Cardia E: Temporal osteoclastoma: an exceptional
lesion in infancy. Childs Nerv Syst. 14:213–217. 1998.
|
|
12
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.
|
|
13
|
Arseni C, Horvath L, Maretsis M and Carp
N: Giant cell tumors of the calvaria. J Neurosurg. 42:535–540.
1975.
|
|
14
|
Gebhart M, Vandeweyer E and Nemec E:
Paget’s disease of bone complicated by giant cell tumor. Clin
Orthop Relat Res. 352:187–193. 1998.
|
|
15
|
Lu ZH and Yao ZW: Giant-cell tumour of the
posterior cranial fossa: a case report. Br J Radiol. 84:e206–e209.
2011.
|
|
16
|
Opitz H, Petersen D, Heiss E, Duffner F
and Meyermann R: Giant cell tumor of the occipital bone in a case
of von Recklinghausen neurofibromatosis. Clin Neuropathol.
15:226–230. 1996.
|
|
17
|
Usul H, Kuzeyli K, Çakir E, Karaarslan G,
Arslan E, Yazar U and Arslan S: Dumbbell shaped giant cell tumor of
the temporal bone: case report and literature review. Turkish
Neurosurgery. 14:112–116. 2004.
|
|
18
|
Rosen MP: General diagnosis case of the
day. Giant cell tumor of the temporal bone. AJR Am J Roentgenol.
156:1290–1292. 1991.
|
|
19
|
Mendenhall WM, Zlotecki RA, Scarborough
MT, Gibbs CP and Mendenhall NP: giant cell tumor of bone. Am J Clin
Oncol. 29:96–99. 2006.
|
|
20
|
Chakravarti A, Spiro IJ, Hug EB, Mankin
HJ, Efird JT and Suit HD: Megavoltage radiation therapy for axial
and inoperable giant-cell tumor of bone. J Bone Joint Surg Am.
81:1566–1573. 1999.
|
|
21
|
Carrasco CH and Murray JA: Giant cell
tumors. Orthop Clin North Am. 20:395–405. 1989.
|
|
22
|
Wolfe JT III, Scheithauer BW and Dahlin
CD: Giant-cell tumor of the sphenoid bone. J Neurosurg. 59:322–327.
1983.
|
|
23
|
Coumbaras M, Pierot L, Felgeres AA, Boulin
A, Gaillard S and Derome PJ: Giant-cell tumour involving the
cranial vault: imaging and treatment. Neuroradiology. 41:826–828.
1999.
|
|
24
|
Ulu MO, Biceroglu H, Ozlen F, Oz B and
Gazioglu N: Giant cell tumor of the frontal bone in an 18-month-old
girl: a case report. Cent Eur Neurosurg. 71:104–107. 2010.
|
|
25
|
Roeder F, Timke C, Zwicker F, Thieke C,
Bischof M, Debus J and Huber PE: Intensity modulated radiotherapy
(IMRT) in benign giant cell tumors-a single institution case series
and a short review of the literature. Radiat Oncol. 26:1–7.
2010.
|
|
26
|
Feigenberg SJ, Marcus RB, Zlotecki RA,
Scarborough MT, Berrey BH and Enneking WF: Radiation Therapy for
Giant cell tumors of bone. Int J Radiat Oncol Biol Phys.
49:1243–1247. 2001.
|