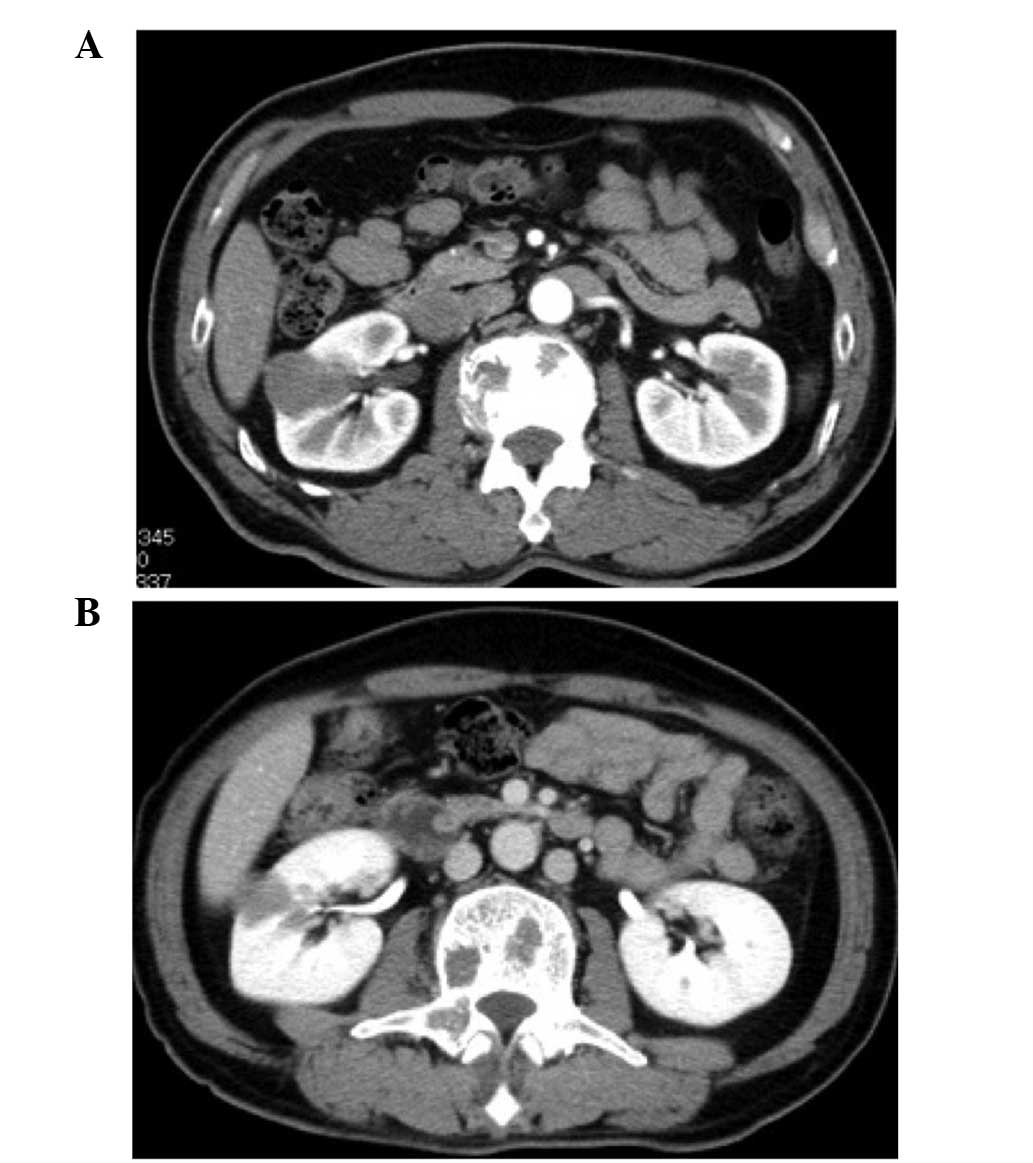
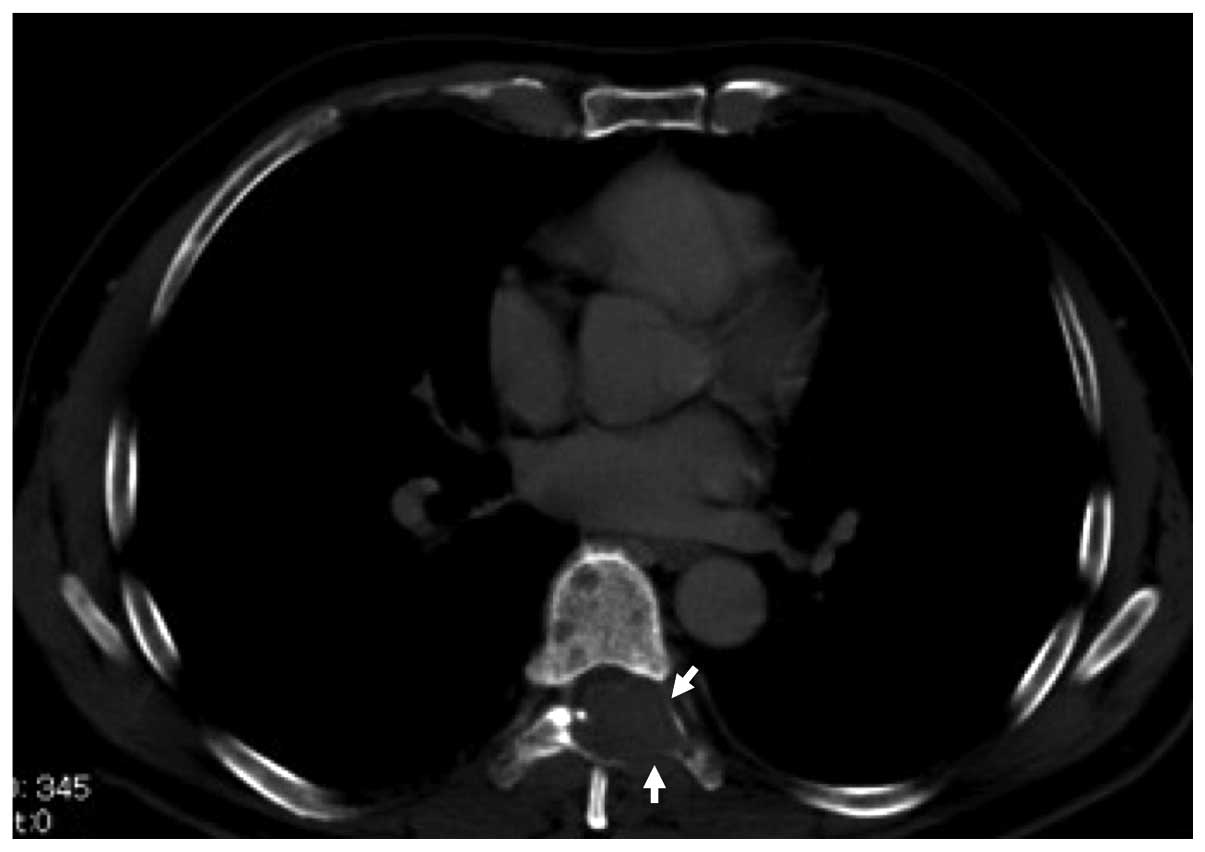
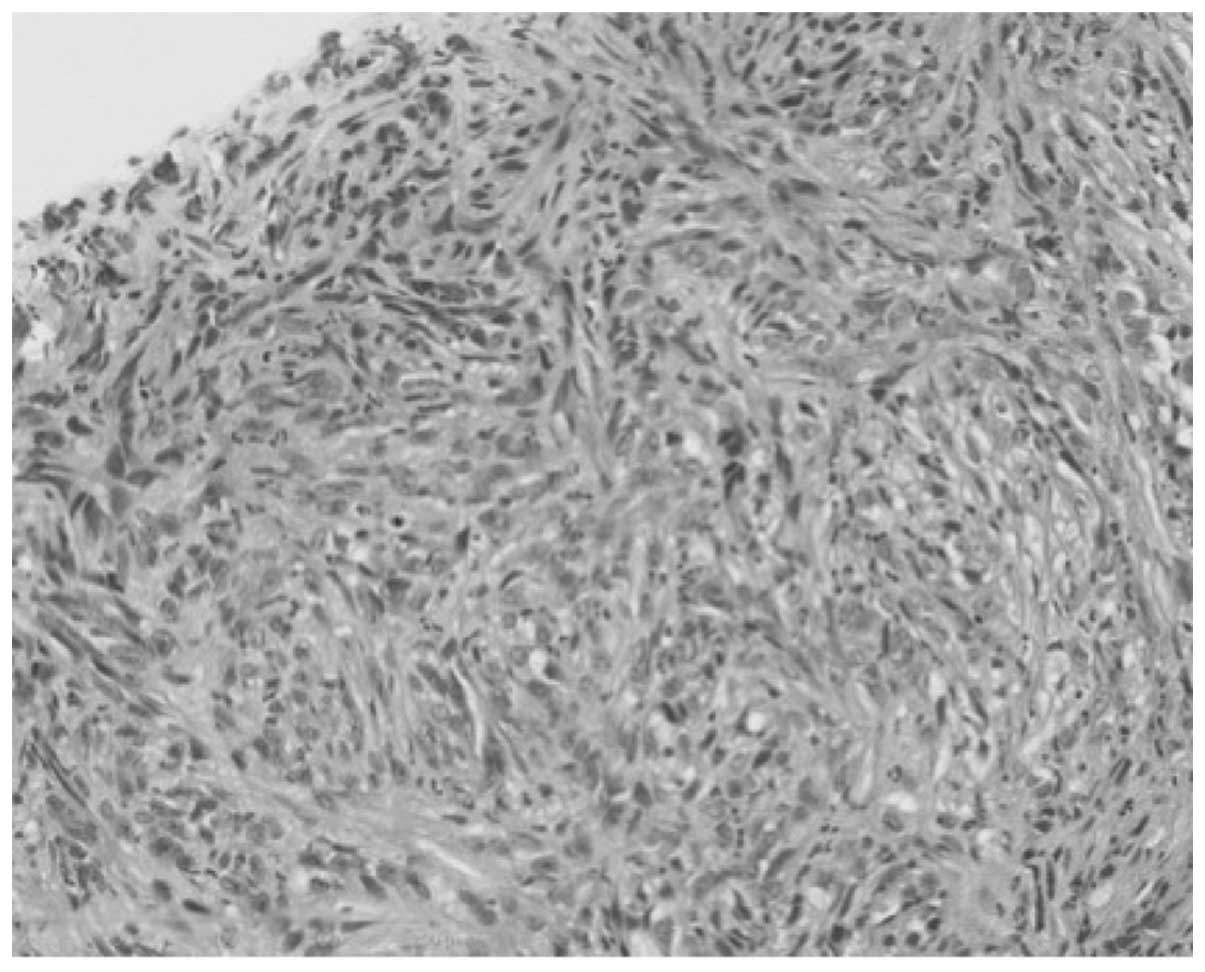
Spandidos Publications style
Numakura K, Tsuchiya N, Akihama S, Inoue T, Narita S, Huang M, Satoh S and Habuchi T: Successful mammalian target of rapamycin inhibitor maintenance therapy following induction chemotherapy with gemcitabine and doxorubicin for metastatic sarcomatoid renal cell carcinoma. Oncol Lett 8: 464-466, 2014.
APA
Numakura, K., Tsuchiya, N., Akihama, S., Inoue, T., Narita, S., Huang, M. ... Habuchi, T. (2014). Successful mammalian target of rapamycin inhibitor maintenance therapy following induction chemotherapy with gemcitabine and doxorubicin for metastatic sarcomatoid renal cell carcinoma. Oncology Letters, 8, 464-466. https://doi.org/10.3892/ol.2014.2118
MLA
Numakura, K., Tsuchiya, N., Akihama, S., Inoue, T., Narita, S., Huang, M., Satoh, S., Habuchi, T."Successful mammalian target of rapamycin inhibitor maintenance therapy following induction chemotherapy with gemcitabine and doxorubicin for metastatic sarcomatoid renal cell carcinoma". Oncology Letters 8.1 (2014): 464-466.
Chicago
Numakura, K., Tsuchiya, N., Akihama, S., Inoue, T., Narita, S., Huang, M., Satoh, S., Habuchi, T."Successful mammalian target of rapamycin inhibitor maintenance therapy following induction chemotherapy with gemcitabine and doxorubicin for metastatic sarcomatoid renal cell carcinoma". Oncology Letters 8, no. 1 (2014): 464-466. https://doi.org/10.3892/ol.2014.2118