Introduction
Photodynamic therapy (PDT) is a treatment modality
for various tumors and non-malignant diseases, in which visible
light is used to activate a photosensitizer (1,2). The
precise mechanism of PDT on cells and tissues has not been fully
elucidated. However, singlet oxygen generated following exposing
the sensitizer to an appropriate light wavelength has been
identified as the possible cytotoxic agent responsible for direct
tumor cell damage or cell death (3).
Phthalocyanines belong to a second generation
photosensitizer and are reported as the most effective drugs for
PDT (4). Phthalocyanines constitute
a large class of compounds with high extinction coefficients in the
red spectral region (630 and 800 nm), which have been identified to
present excellent tumor-localizing properties and high
photosensitizing efficiency (5).
Cellular components are adhered to the extracellular
matrix (ECM) and among them are cell adhesion proteins, which allow
cell anchorage, survival, proliferation and migration. There are
four main cell adhesion protein superfamilies, including integrins,
selectins, immunoglobulins and cadherins (6). Integrins are ubiquitous glycoproteins
that modulate cell adhesion to the ECM components, including
collagen, fibronectin, laminin and vitronectin. These elements form
a link between the extracellular environment and the cytoskeleton,
through interactions with adaptor proteins that constitute focal
adhesion contacts. In particular, integrins participate in the
regulation of survival, proliferation, migration and
differentiation (6).
It has been established that PDT produces changes in
the ECM and to cell adhesion, which are largely dependent on the
type of photosensitizer and the treatment doses (7); however, the mechanisms underlying this
effect remain elusive. In one study, the cells subjected to PDT,
using an hematoporphyrin derivative as a photosensitizing agent,
required a longer time to adhere to a plastic substrate and a
confluent layer of untreated cells when compared with the control
group, suggesting that the damaging effects involve cytoskeletal
proteins (8). Furthermore,
cytoskeletal reorganization damage following photodynamic treatment
has been reported in several other studies (1,9,10), and
it has been observed that changes in the capacity of PDT-induced
cell adhesion is accompanied by remodeling of actin filaments
(11,12).
The present study aimed to investigate the adhesion
process of the cell line HEp-2 (human laryngeal carcinoma) that
have been subjected to PDT with the photosensitizing aluminum
phthalocyanine tetrasulfonated (AlPcS4) and zinc
phthalocyanine (ZnPc).
Materials and methods
Cell line
The HEp-2 human laryngeal cancer cells, (Adolfo Lutz
Institute, São Paulo, Brazil) were cultured as a monolayer of cells
in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10%
fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin
(100 mM/ml; Gibco-BRL, Carlsbad, CA, USA).
Chemicals
ZnPc, violet crystal, human collagen type IV,
phalloidin-TRITC, anti-focal adhesion kinase (FAK) and
anti-β1-integrin monoclonal antibodies were obtained from Sigma
Chemical Co. (St. Louis, MO, USA). AlPcS4 was obtained
from Frontier Scientific, Inc., (Logan, UT, USA). Mouse anti-rabbit
fluorescein isothiocyanate (FITC)-conjugated and calcein-AM IgG, as
well as primers for β1-integrin, FAK and β-actin, were obtained
from Invitrogen Life Technologies (Carlsbad, CA, USA).
Photodynamic therapy
The cells were exposed to the photosensitizers
AlPcS4 (10 μM ml−1) or ZnPc (10 μM ml−1) for
1 h and were irradiated with an As-Ga-Al diode laser (wavelength,
650 nm; energy density, 4,5 J/cm2; Bio Wave LLLT
Dual-Kondortech, São Carlos-SP, Brazil).
Immunostaining
Tissue culture plates were coated overnight with
human collagen type IV (5 μg/well) at room temperature under
sterile conditions. The wells were washed with phosphate-buffered
saline (PBS; Sigmal Chemical Co.) and non-specific binding sites
were blocked with 100 μl of 0.2% bovine serum albumin (BSA; Sigma
Chemical Co.) in DMEM (Gibco-BRL) for 90 min at 37°C. The wells
were seeded with 500 μl of the appropriate cell suspension,
105 cells/ml, and incubated at 37°C in a humidified 5%
CO2 incubator for 24 h. The attached cells following
incubation with AlPcS4 or ZnPc were irradiated and immediately the
groups were separated at the times of 0 and 12 h, and cells were
incubated in 4% paraformaldehyde (Sigma Chemical Co.) in PBS for 15
min at room temperature. Cells were permeabilized with 0.2% of
Triton X-100 (Sigma Chemical Co.) and 4% paraformaldeyde in PBS for
10 min, and then blocked with 1% BSA solution in PBS for 30 min.
Subsequently, cells were incubated with phalloidin-TRITC (1:100/1
h; Sigma Chemical Co.), mouse anti-human monoclonal antibody
against β1-integrin (1:500/1 h) or mouse anti-human monoclonal
antibody against FAK (1:500/1 h) (both Sigma Chemical Co.), and
then incubated with the rabbit anti-mouse polyclonal secondary
antibody conjugated with fluorescein (FITC; 1:1,000/1 h).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA was extracted by TRIzol
(Invitrogen Life Technologies). Reverse transcription of 1 μg RNA
was conducted using Taq Man® reverse transcription
reagents (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Equal amounts of cDNA (1/20 of the
reaction volume) were subjected to PCR amplification using the
following primers: β1-integrin: forward,
5′-GGACAGTGTGTTTGTAGGAAGAGG-3′ and reverse,
5′-GCACTGAACAGATTCTTTATGCTC-3′; FAK: forward,
5′-TGCAAGTAAGGAAATACAGTTTGG-3′ and reverse,
5′-CCACATACACACACCAAACATCATCCA-3′, and were then visualized by
ethidium bromide-stained agarose gel (Sigma Chemical Co.)
electrophoresis. RT-PCR was performed using standard
conditions.
Cell-cell adhesion assay
Following PDT, cells were incubated with calcein-AM
(2 μM/30 min) and seeded (105 cells/well) over a HEp-2
culture in a confluent monolayer in 96-well plates. Cells were
incubated at 37°C for 2 h. Following this time, the non-adherent
calcein-labeled cells were removed for washing with the culture
medium. The attached cells were incubated for 12 and 24 h. The
number of cells attached was determined at the end of each period
using a Leica DMLB fluorescence microscope (Leica Microsystems,
Milton Keynes, Buckinghamshire, UK).
Cell-matrix adhesion assay
Cells submitted to treatment following the
incubation periods, were seeded at a concentration of
105 cells/well in coverslips coated with human collagen
type IV and incubated at 37°C with DMEM with 2% FBS to allow
adhesion. Following this period, the non-adherent cells were
removed with PBS and fixed with 96% ethanol for 10 min at room
temperature. The cells were incubated with 0.1% crystal violet
(Sigma Chemical Co.) for 30 min. Excess dye was removed with
distilled water and then 300 μl dimethyl sulfoxide (Sigma Chemical
Co.) was added for extraction of the label. The optical density of
the plates was read at 570 nm on a microplate reader (Packard
SpectraCount; Packard BioScience Co., Meriden, CT, USA). Each
experiment was run in triplicate.
Statistical analyses
The data are presented as the mean ± standard
deviation. All results presented a Gaussian distribution allowing
the use of analysis of variance to compare means among the groups.
P<0.05 was considered to indicate a statistically significant
difference. To conduct the statistical analysis and graphics,
GraphPad InStat® and Microcal Origin® 6.0
software were used, respectively.
Results
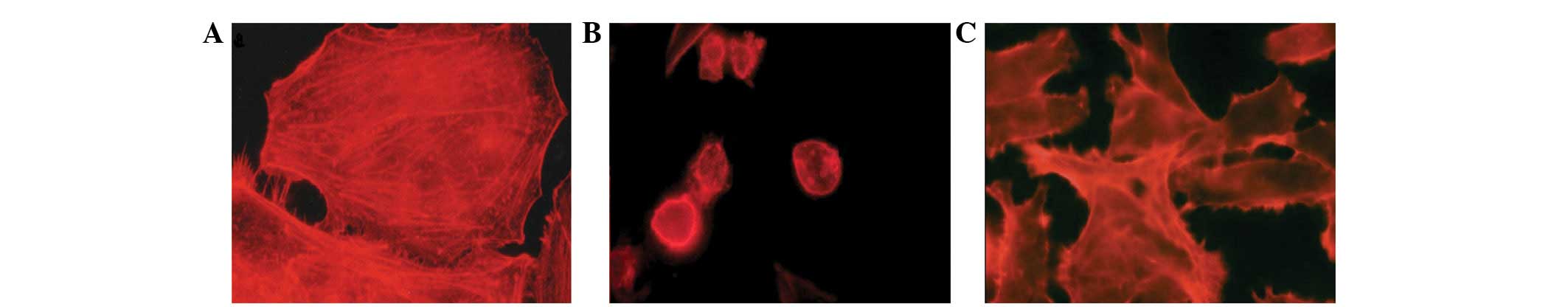
Effect of PDT on cell morphology
Immunostaining analysis revealed that PDT acted on
the actin filaments of the cytoskeleton and the adhesion proteins
β1-integrin and FAK. A total of 12 h following PDT, immunostaining
analysis observations of the control group revealed a homogeneous
distribution of actin filaments with stress fiber characteristics
(Fig. 1A). Following PDT, intense
retraction in the actin filaments was observed in the
AlPcS4 and ZnPc groups when compared with control group,
revealing severe damage that was compromising the cellular
morphology and the loss of integrity of the filaments with the
disappearance of stress fibers (Fig. 1B
and C). This consequently led to damage to the internal
organization, mechanical and structural stability of the cells.
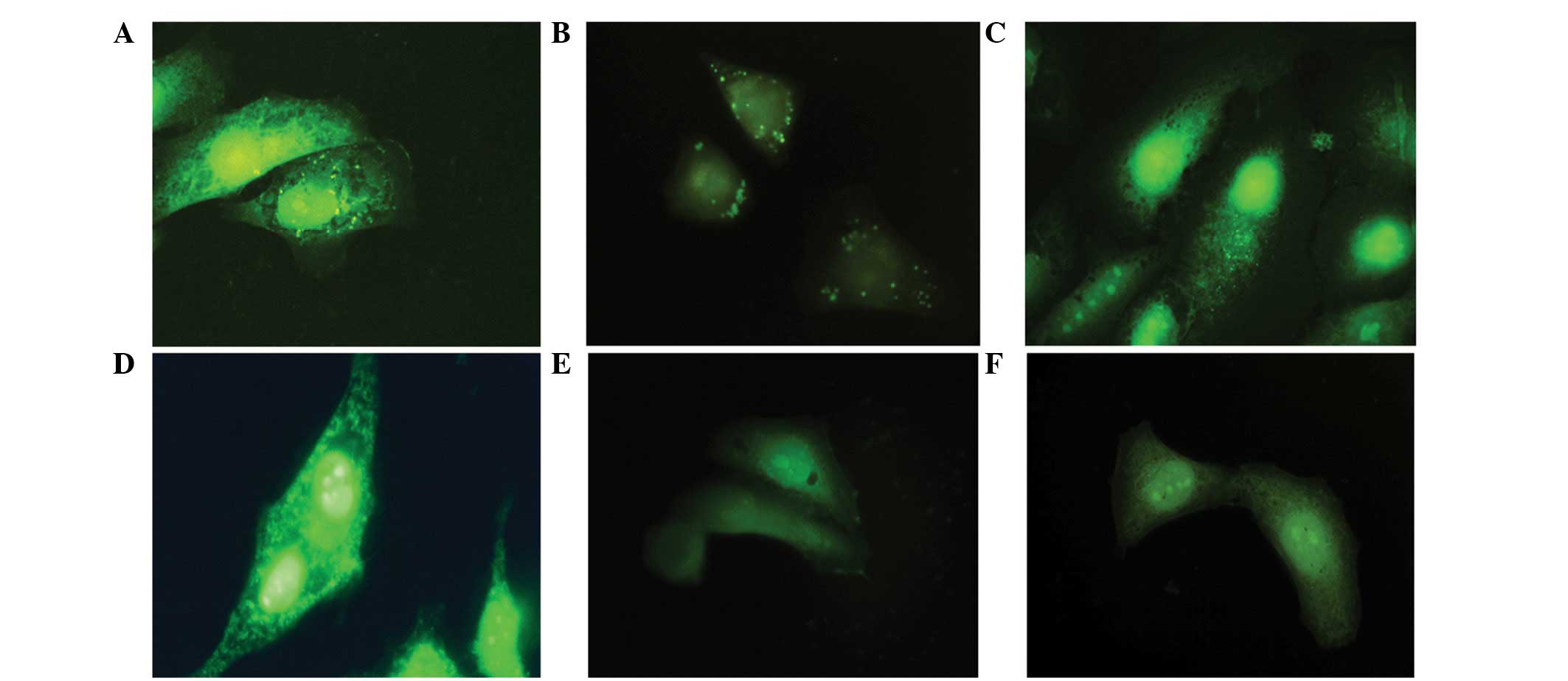
Cell adhesion features and changes in commitment, adhesive proteins
β1-integrin and FAK are illustrated in Fig. 2. Immediately following treatment,
the cells were labeled for these adhesive proteins, but after 12 h
occur a reduction of the same, when compared with the control
group.
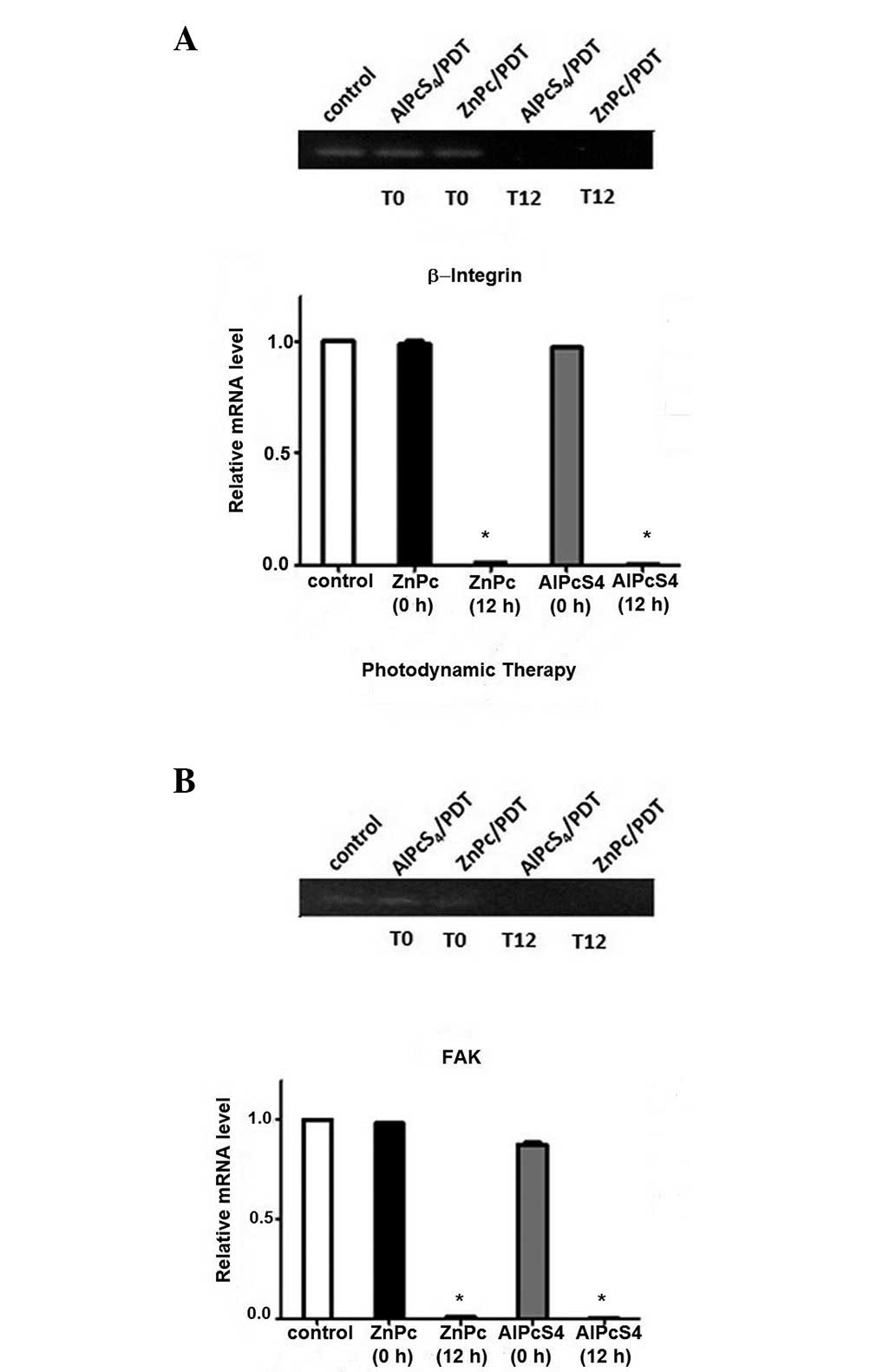
Effect of PDT on adhesion protein
expression
The results observed in the immunostaining were
confirmed by the analysis of the protein expression of FAK and
β1-integrin following PDT. The expression of the adhesion proteins
FAK and β1-integrin following PDT was assessed by RT-PCR. The
analysis of the β1-integrin mRNA expression in the
AlPcS4 and ZnPc groups compared with the control group
revealed no significant differences at the baseline time (0 h;
Fig. 3). However, 12 h following
PDT, a significant reduction in the expression of β1-integrin in
the ZnPc and AlPcS4 groups, as compared with the
controls, was observed. The expression of FAK mRNA in the two
groups did not demonstrate a significant reduction when compared
with the control group at 0 h. By contrast, 12 h following PDT
there was a significant reduction in the FAK mRNA expression in the
ZnPc and AlPcS4 groups, when compared with the control
group. Therefore, the two photosensitizers used demonstrated
efficacy in reducing the expression of β1-integrin and FAK,
influencing the accession process following PDT.
Effect of PDT on cell-cell adhesion
To study cell-cell interactions, calcein-AM was used
as an indicator of cell viability. The control and treatment groups
were incubated with calcein-AM and cocultured in HEp-2 monolayer
cells. The cells were incubated for 6, 12, 24 and 48 h to evaluate
the adhesion ability following treatment with PDT. The behavior of
the laser and control groups at all times demonstrated an increase
in cell adhesion throughout the period analyzed. Cultures submitted
to photodynamic treatment after 6, 12, 24 and 48 h (Fig. 4) exhibited a marked reduction in the
number of cells adhered to monolayer compared with the cells in the
control group. In the ZnPc PDT group, the adherence rates were low
at 6 h, but demonstrated no significant change until 24 h, with a
significant reduction (P<0.001) observed at 48 h. In the
PDT-AlPcS4 group, <100 cells were attached in the
same period.
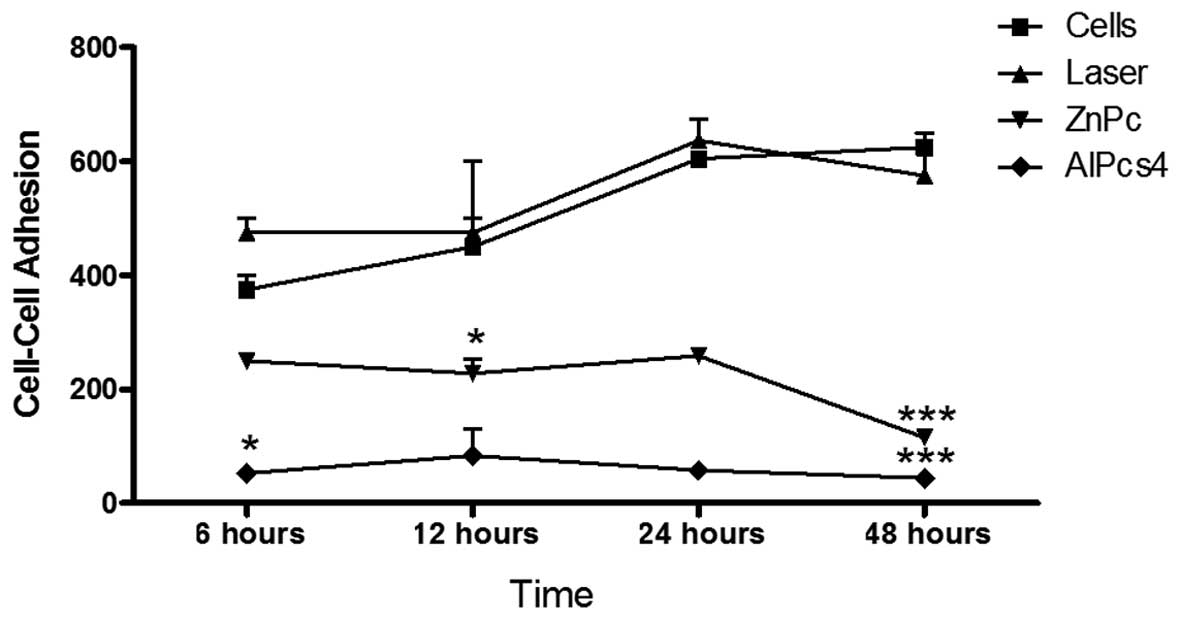 | Figure 4Cell-cell adhesion assay. Following
PDT, the cells were incubated for periods of 6, 12, 24 and 48 h. At
the end of these periods the cultures were analyzed by fluorescence
microscopy, counting the number of cells adhered to the monolayer.
After 24 h, the difference in the adhesion ability in the treatment
groups, compared with the control, was significant
(***P<0.001). When comparing the photosensitizers
ZnPc and AlPcS4, there was no significant difference.
PDT, photodynamic therapy; AlPcS4, aluminum
phthalocyanine tetrasulfonated; ZnPc, zinc phthalocyanine. |
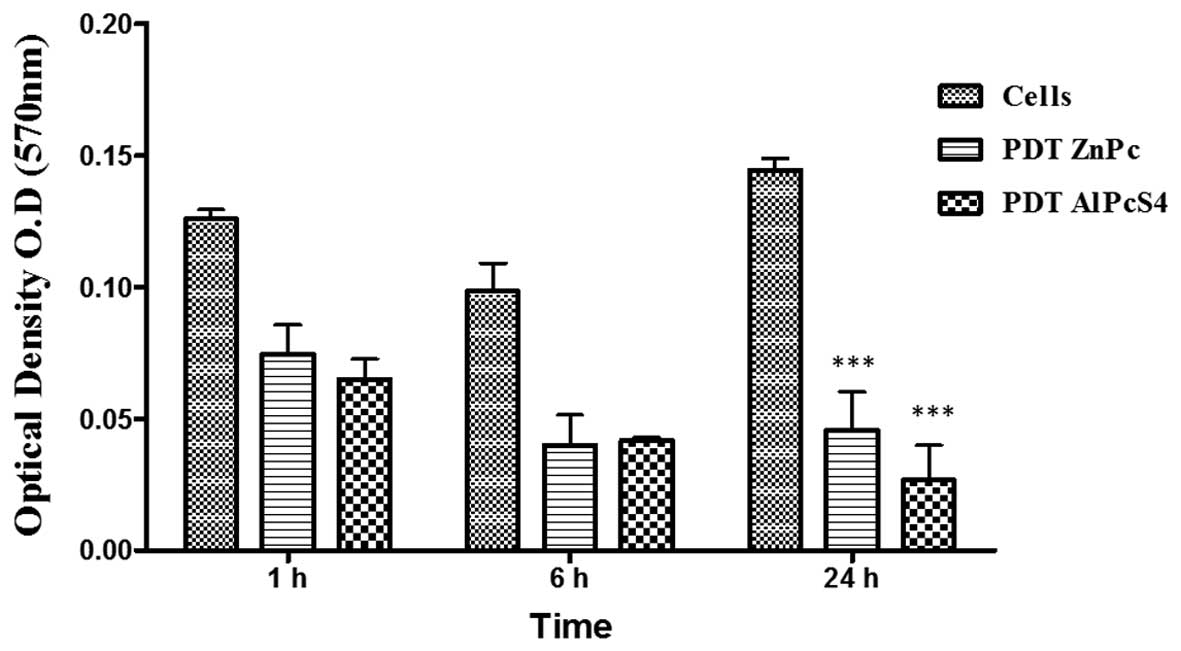
Effect of PDT on cell adhesion to the
matrix
The capacity of cells to adhere to matrix, was
evaluated using a colorimetric assay. Comparing the ZnPc/PDT and
AlPcS4/PDT groups with the controls, there was a
significant reduction in the number of cells adhered (P<0.01) at
the 1 and 6 h time points following incubation of the cells with
the matrix. After 24 h, this difference was significant
(P<0.001). There was no significant difference in cell adhesion
when comparing the two photosensitizers ZnPc and AlPcS4
(Fig. 5).
Discussion
The present study describes the effect of
photosensitizers ALPcS4 and ZnPc on cell adhesion.
AlPcS4 was found to modify the structure of actin filaments, with
more severe changes identified during the periods of 12, 24 and 48
h, as demonstrated by labeling with phalloidin-TRITC. In cultures
treated with ZnPc/PDT, it was possible to observe the presence of
small cytoplasmic projections, with concentrated actin filaments in
the cell edge, demonstrating that cellular organization was
affected by PDT treatment following 12 h, with a marginal recovery
at 48 h. The cellular structure of actin is recognized as crucial
to the maintenance of cell adhesion, and is therefore one of the
targets of PDT (13,14); however, changes induced by PDT in
the cytoskeletal proteins may be present in cells resistant to
treatment, leading to changes in the adhesion and organization of
the cytoskeletal components favoring the migration of these cells
to other tissues. This alteration may be explained by the
involvement of adhesion proteins, mainly β1-integrin and FAK, as
these proteins are dependent on the disposition of the actin
filaments. In the present study, the immunostaining of β1-integrin
and FAK demonstrated a reduction in their protein expression 12 h
following PDT. The RT-PCR results confirmed the reduction of the
mRNA expression β1-integrin and FAK, 12 h following PDT for the two
photosensitizers. These results were corroborated in the study by
Milla Sanabria et al (6),
which suggested that the adhesion of the cell to the substratum is
mediated by integrins, and would therefore be interrupted following
photodynamic action by damage to the ECM and by damage directly to
the integrin proteins.
The stability of integrin (α and β) and FAK is
dependent on the interaction with other proteins, such as vinculin,
paxillin and actin filaments. In the present study, it was verified
that the mRNA expression for adhesion proteins was reduced
following PDT. This result indicates that PDT is not only acting to
destabilize the interaction between the proteins involved in
cell-substrate adhesion, as observed in the results of the
cell-matrix interaction, but that it is also acting on signaling
pathways in cells. The data of the violet crystal assay
demonstrated a significant reduction in cell-matrix adhesion 24 h
following PDT for the two phthalocyanines. This effect was also
observed in cell-cell interactions in which a reduction of adhesion
was observed following PDT, which was enhanced as the time
increased. These results confirm earlier evidence from Runnels
et al 1999 (15), who
performed a similar study assessing the adhesion to collagen IV,
laminin, fibronectin and vitronectin (components of ECM) with
PDT-AlPcS4. The reduction of cell adhesion OVCAR 3 (human ovarian
carcinoma) subjected to treatment with BPD-MA was attributed to the
high rate of cell death observed in culture; however, even with
decreasing rates of β1-integrin in the focal adhesion plaques,
there were no differences in the expression of this protein.
In conclusion, the phthalocyanines AlPcS4
and ZnPc, following irradiation, induce damage that compromises the
cell adhesion ability and inhibits the metastatic potential of
HEp-2 cells. However, further studies are required to determine the
signaling pathways involved in the resistance of the surviving
cells.
Acknowledgements
A grant support for this study was provided by
Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP no.
2006/06736-5.
References
|
1
|
Juarranz A, Espada J, Stockert JC, et al:
Photodamage induced by Zinc(II)- phthalocyanine to microtubules,
actin, alpha-actinin and keratin of HeLa cells. Photochem
Photobiol. 73:283–289. 2001.
|
|
2
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387.
2003.
|
|
3
|
Allison RR and Moghissi K: Photodynamic
Therapy (PDT): PDT Mechanisms. Clin Endosc. 46:24–29. 2013.
|
|
4
|
Dougherty TJ, Gomer CJ, Henderson BW, et
al: Photodynamic therapy. J Natl Cancer Inst. 90:889–905. 1998.
|
|
5
|
Castano AP, Demidova TN and Hamblin MR:
Mechanisms in photodynamic therapy: Part three-Photosensitizer
pharmacokinetics, biodistribution, tumor localization and modes of
tumor destruction. Photodiagn Photodyn Ther. 2:91–106. 2005.
|
|
6
|
Milla Sanabria L, Rodríguez ME, Cogno IS,
et al: Direct and indirect photodynamic therapy effects on the
cellular and molecular components of the tumor microenvironment.
Biochim Biophys Acta. 1835:36–45. 2013.
|
|
7
|
Pazos MD and Nader HB: Effect of
photodynamic therapy on the extracellular matrix and associated
components. Braz J Med Biol Res. 40:1025–1035. 2007.
|
|
8
|
Uzdensky A, Kolpakova E, Juzeniene A,
Juzenas P and Moan J: The effect of sublethal ALA-PDT on the
cytoskeleton and adhesion of cultured human cancer cells. Biochim
Biophys Acta. 1722:43–50. 2005.
|
|
9
|
Ferreira SDRM, Tedesco AC, Sousa G, et al:
Analysis of mitochondria, endoplasmic reticulum and actin filaments
after PDT with AlPcS(4). Lasers Med Sci. 18:207–212. 2004.
|
|
10
|
Tsai T, Ji HT, Chiang PC, Chou RH, et al:
ALA-PDT results in phenotypic changes and decreased cellular
invasion in surviving cancer cells. Lasers Surg Med. 41:305–315.
2009.
|
|
11
|
Machado AH, Moraes KC, Pacheco-Soares C,
et al: Cellular changes after photodynamic therapy on HEp-2 cells
using the new ZnPcBr(8) phthalocyanine. Photomed Laser Surg.
28(Suppl 1): S143–S149. 2010.
|
|
12
|
Milla LN, Cogno IS, Rodríguez ME,
Sanz-Rodríguez F, Zammarrón A, Gilaberte Y, Carrasco E, Rivarola V
and Juarranz A: Isolation and characterization of squamous
carcinoma cells resistant to photodynamic therapy. J Cell Biochem.
112:2266–2278. 2011.
|
|
13
|
Liu T, Wu LY and Berkman CE:
Prostate-specific membrane antigen-targeted photodynamic therapy
induces rapid cytoskeletal disruption. Cancer Lett. 296:106–112.
2010.
|
|
14
|
Casas A, Sanz-Rodriguez F, Di Venosa G, et
al: Disorganisation of cytoskeleton in cells resistant to
photodynamic treatment with decreased metastatic phenotype. Cancer
Lett. 270:56–65. 2008.
|
|
15
|
Runnels JM, Chen N, Ortel B, et al:
BPD-MA-mediated photosensitization in vitro and in vivo: cellular
adhesion and beta1 integrin expression in ovarian cancer cells. Br
J Cancer. 80:946–953. 1999.
|