Introduction
The well-organized architectures of the normal
colonic epithelia are inevitably associated with the apical and
basolateral polarity (1). The
basolateral polarity of the normal epithelium is maintained by
conservation of the basement membrane (BM) between the cells and
the extracellular matrix (ECM), as well as the expression of
adhesion molecules on the plasma membranes, including E-cadherin
and β-catenin, between the epithelial cells. In certain situations,
the cell polarity is disturbed and the remodeling of the epithelial
cell organization, including wound healing and cancer progression,
is required. In these processes, the epithelial cells obtain
increased motility showing front-rear polarity similar to that of
mesenchymal cells, instead of apical and basolateral polarity.
Epithelial cell polarity is regulated by highly
conserved polarity proteins and the atypical protein kinase C
(aPKC) is a protein family that is one of the most important
signaling components controlling cell polarity. In particular, aPKC
λ/ι is a pivotal regulator of cell polarity and has been reported
to be associated with the pathogenesis and progression of neoplasms
(1). Changes in aPKC λ/ι expression
have been reported in several types of tumors and the
overexpression of aPKC λ/ι is associated with the progression and
prognosis of various carcinomas (2–5).
Laterally spreading tumors (LSTs) are flat-type
colorectal tumors that are gross morphological concepts in contrast
to polypoid-type tumors. Kudo (6)
defined LSTs as colorectal tumors growing superficially along the
mucosal surface with a short vertical length despite horizontal
diameters of >10 mm. Their superficial replacing growth is
confirmed by light microscopy and, according to technical
improvements in endoscopy, an increased number of LSTs have been
diagnosed and resected. The majority of LSTs are histologically
adenoma, however, several cases have been identified as cancerous.
Notably, the majority of cancerous LSTs also show superficial
replacing growth and less invasive behavior. A flat depressed-type
tumor is an additional type of non-polypoid colorectal neoplasm
(7), which shows expanding growth
and massive submucosal invasion in early-stage cancer in comparison
with LST (8,9). Protruded-type tumors also show
expanding growth and, therefore, the cell polarity of LSTs
approaches that of the normal mucosal epithelia. However, flat
depressed- or protruded-type tumors also show invasive growth.
These observations suggested that there may be a
difference between LST and flat depressed- and polypoid-type
lesions in the cell polarity status. These results prompted an
investigation of the expression and localization of cell
polarity-related proteins in LST, as well as in flat depressed-and
polypoid-type lesions, using four immunomarkers against aPKC λ/ι,
β-catenin, E-cadherin and type IV collagen.
Materials and methods
Samples
In total, 37 flat-type and 20 polypoid-type
colorectal tumors were selected. All of the lesions were
endoscopically or surgically resected at the Yokohama City
University Hospital (Yokohama, Japan), between 1998 and 2011. The
resected tissue was immediately fixed in 20% formalin and embedded
in paraffin. Next, 4-μm thick paraffin sections were stained with
hematoxylin and eosin and subjected to pathological diagnosis. The
flat-type tumors included 15 adenomas (LST, adenoma; LST-A), nine
non-invasive adenocarcinoma in adenomas (LST, cancer in adenomas;
LST-CAs) and 13 flat depressed-type tumors. The polypoid-type
tumors included 11 adenomas (polypoid-type adenomas; P-As) and nine
non-invasive adenocarcinoma in adenomas (polypoid-type cancer in
adenomas; P-CAs) (Table IA and B).
The flat depressed-type cancers (FD-CAs) were all invasive cancers.
The study was approved by the institutional ethical committee,
Yokohama City University Ethical Review Board (Yokohama, Japan) and
written informed consent was obtained from all the enrolled
patients for the use of the samples.
 | Table IClinicopathological characteristics of
flat- and polypoid-type tumors. |
Table I
Clinicopathological characteristics of
flat- and polypoid-type tumors.
| A,
Clinicopathological characteristics of LSTs and FD-CAs |
|---|
|
|---|
| Characteristics | LST-A | LST-CA | FD-CA | P-value |
|---|
| n | 15 | 9 | 13 | |
| Age, years (mean ±
SE) | 63.2±4.2 | 62.1±6.2 | 63.9±6.8 | NS |
| Gender, n (%) |
| Male | 9 (60.0) | 6 (66.7) | 7 (53.8) | NS |
| Female | 6 (40.0) | 3 (33.3) | 6 (46.2) | |
| Diameter, mm (mean ±
SE) | 17.1±6.2 | 22.8±5.3 | 38.3±15.5 | <0.005 |
| LST subtype, n
(%) |
| G-type | 11 (73.3) | 4 (44.4) | - | NS |
| F-type | 4 (26.7) | 5 (63.6) | - | |
| Site (relative to
splenic flexure), n (%) |
| Proximal colon | 5 (33.3) | 5 (55.6) | 8 (61.5) | NS |
| Distal colon | 8 (53.3) | 3 (33.3) | 3 (23.1) | |
| Rectum | 2 (13.4) | 1 (11.1) | 2 (15.4) | |
|
| B,
Clinicopathological characteristics of polypoid-type tumors |
|
| Characteristics | P-A | P-CA | P-value |
|
| n | 11 | 9 | |
| Age, years (mean ±
SE) | 64.7±8.5 | 57.7±7.7 | NS |
| Gender, n (%) |
| Male | 9 (81.2) | 7 (77.8) | NS |
| Female | 2 (18.2) | 2 (22.2) | |
| Diameter, mm (mean ±
SE) | 12.1±2.3 | 15.3±4.3 | NS |
| Site (relative to
splenic flexure), n (%) |
| Proximal
colon | 4 (36.4) | 2 (22.2) | NS |
| Distal colon | 3 (27.3) | 3 (33.3) | |
| Rectum | 4 (36.4) | 4 (44.4) | |
Immunohistochemistry
The expression and localization of aPKC λ/ι,
β-catenin, E-cadherin and type IV collagen were
immunohistochemically examined as previously described (2,3).
Briefly, 4 μm-thick paraffin sections were deparaffinized and
rehydrated. Next, the antigen retrieval was performed by
autoclaving (for aPKC λ/ι), microwaving three times for 3 min each
time (for β-catenin and E-cadherin) or digestion with proteinase K
(0.4 mg/ml; DakoCytomation, Glostrup, Denmark) at room temperature
for 6 to 15 min (for type IV collagen). The endogenous peroxidase
activity was quenched by immersing the sections in 0.3% hydrogen
peroxide/phosphate-buffered saline for 30 min at room temperature,
and the sections were incubated with 10% goat serum (Pierce
Biotechnology, Inc., Rockford, IL, USA) at room temperature for 20
min to block non-specific protein binding. The primary antibodies
were applied to the sections and incubated for 1 h at room
temperature for type IV collagen, and overnight at 4°C for aPKC
λ/ι, β-catenin and E-cadherin staining. The monoclonal mouse
anti-human antibodies against aPKC λ/ι (clone 23/PKCi; cat. no.
610176; BD Transduction Laboratories, Lexington, KY, USA),
β-catenin (clone 14; 1:100; BD Transduction Laboratories),
E-cadherin (BV-6; 1:100; Chemicon, Temecula, CA, USA) and type IV
collagen (CIV 22; 1:50; DakoCytomation) were used as primary
antibodies. The labeled antigens were visualized by the HistoFine
kit (Nichirei, Tokyo, Japan) followed by 3,3′-diaminobenzidine
reaction. The sections were then counterstained with hematoxylin
and microscopically observed (Olympus BX41; Olympus Corporation,
Tokyo, Japan).
Evaluation of aPKC λ/ι expression
The intensities of the immunopositive signals for
aPKC λ/ι in the neoplasms were semi-quantitatively scored by one
pathologist not blinded to the study according to the following
previously employed criteria (2):
1+, weak to normal intensity staining in the cytoplasm in
comparison to the normal epithelium; 2+, moderate intensity
staining in the cytoplasm and/or nucleus; and 3+, strong intensity
staining in the cytoplasm and nucleus.
Evaluation of β-catenin and E-cadherin
expression
The expression of β-catenin and E-cadherin in the
cancer cells was compared with that of the normal epithelial cells
as a standard, as normal epithelial cells exhibit strong expression
of these proteins at the intercellular boundaries. The expression
of β-catenin in the colorectal tumors was classified into the
following three subclasses: i) Preserved type, staining localized
on the cell surface membrane; ii) cytoplasmic type, diffuse
cytoplasmic staining; and iii) nucleic type, nuclear staining
(10). E-cadherin expression was
also classified into three subclasses according to Oka et al
(11), as follows: i) Preserved
type, ≥90% of neoplastic cells positive for E-cadherin; ii)
heterogeneous type,>10 and <90%; and iii) lost type,
≤10%.
Evaluation of type IV collagen
expression
Type IV collagen expression in colorectal tumors was
classified into the following three subclasses: i) Continuous type,
continuous linear staining in the BM of glands; ii) discontinuous
type, discontinuous staining in the BM of glands; and iii) lost
type, no staining in the BM of glands (12).
Statistical analysis
Statistical analyses were performed using the SPSS
program version 17 for Windows (SPSS, Inc., Chicago, IL, USA). The
differences in the expression patterns of the antigens between all
of the tumor groups were compared using the χ2 test, and
χ2 tests with Fisher’s exact correction were applied
when the incidence was <5. To analyze the correlation between
the expression intensity of aPKC λ/ι staining and other staining,
Pearson’s correlation coefficient (r) was used.
Results
Histopathological characteristics of
colon tumors
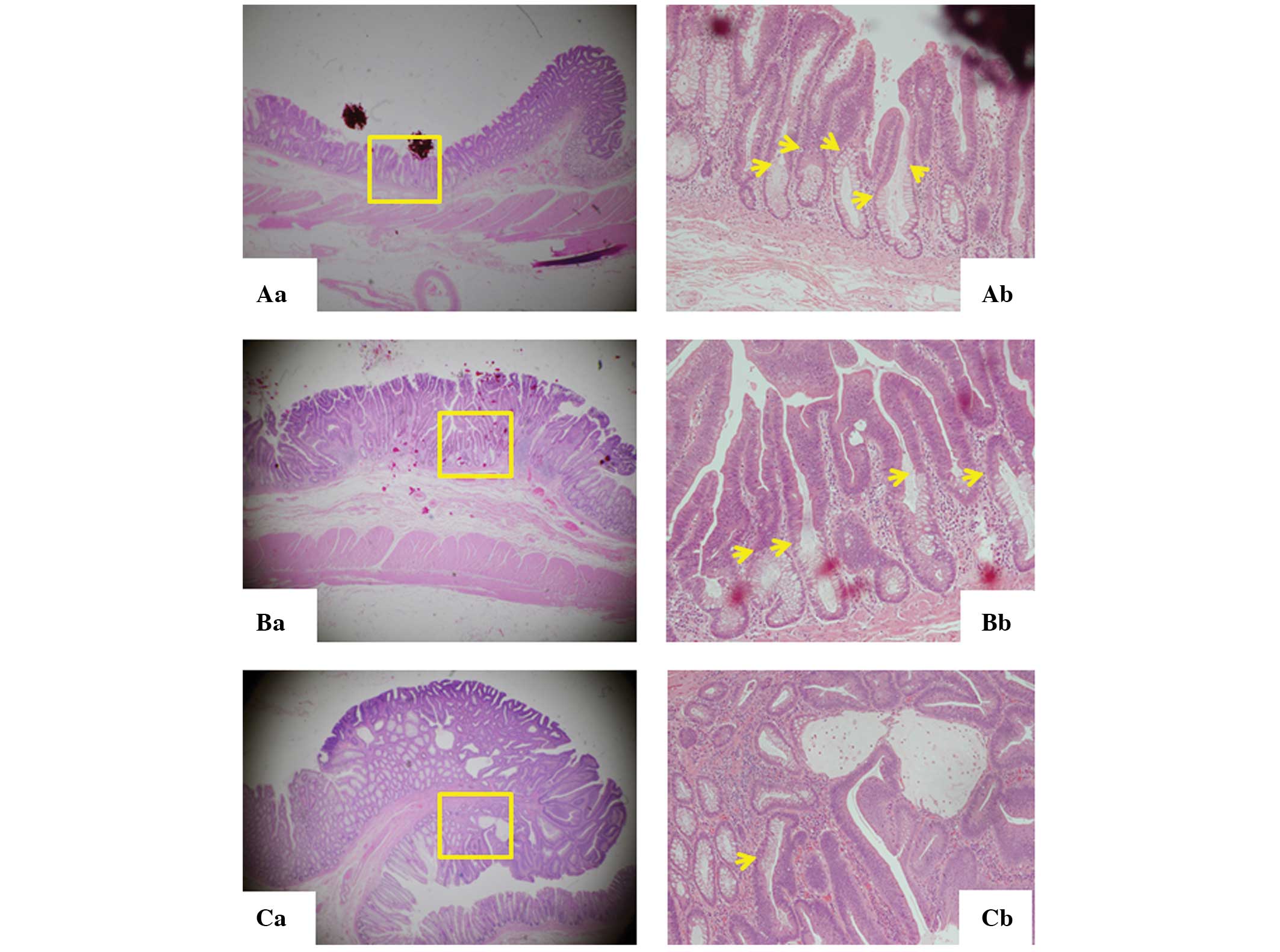
LST-As exhibited evident boundaries with the normal
epithelium, known as the lesional front, at multiple sites in the
lesion (Fig. 1Aa and Ab). The LST-A
occupies the surface mucosa with bottom-situated normal mucosa,
presenting a two-layered elevated lesion. The same architecture was
observed in LST-CAs showing the top layer of cancer in adenoma and
the bottom layer of normal tissue (Fig.
1Ba and Bb). However, the tumor front of polypoid-type tumors
and FD-CAs was detected only in the border of tumors, and they did
not show the two-layered structures (Fig. 1Ca and Cb).
Expression of aPKC λ/ι
Representative images of the various intensities of
immunostaining for aPKC λ/ι, 1+ to 3+, are shown in Fig. 2B–D, respectively. As shown in
Table IIA, the intensities of aPKC
λ/ι immunostaining were 1+ in 86.6% of LST-As and 2+ in 45.5% of
P-As. Additionally, ~70% of P-CAs and FD-CAs were 3+ . On the other
hand, ~55.6% of LST-CAs were 1+, and LST-As and LST-CAs showed
significantly lower expression of aPKC λ/ι than P-As or P-CAs
(P=0.038 and 0.029, respectively; Fig.
3A, Table IIA).
 | Figure 3(A) Expression of aPKC was compared
among five types of tumors. The χ2 test or χ2
test with Fisher’s exact correction was applied to the ratio of 1+
vs. 2+ or 3+ for each tumor type. LST-As showed an extremely high
ratio of aPKC 1+ compared with P-As and FD-CAs. This distinction
was also observed in LST-CAs. P-CAs and FD-CAs did not show aPKC
1+; however, 55.6% of LST-CAs showed aPKC 1+. (B) Expression of
β-catenin was compared among five types of tumor. The ratio of the
preserved type was ~90% in the LST-As and P-As. The ratio was lower
in P-CAs and the FD-CAs, at 22.2 and 7.7%, respectively, and
LST-CAs showed a statistically higher ratio (77.8%) than the other
two groups. (C) The expression of E-cadherin was compared among
five types of tumors. E-cadherin was not preserved in all five
groups and the ratio of the lost type was high in the P-CAs and
FD-CAs. (D) The expression of type IV collagen was compared among
five types of tumor. The ratio of the continuous type was
significantly higher in LST-As and -CAs than in the P-As, P-CAs and
FD-CAs. aPKC, atypical protein kinase C; LST-A, laterally spreading
tumor, adenoma; LST-CA, LST, cancer in adenoma; FD-CA, flat
depressed-type cancer; P-A, polypoid-type adenoma; P-CA,
polypoid-type cancer in adenoma. |
 | Table IIExpression of aPKC λ/ι, β-catenin,
E-cadherin and type IV collagen in LSTs and polypoid-type tumors of
each histological type. |
Table II
Expression of aPKC λ/ι, β-catenin,
E-cadherin and type IV collagen in LSTs and polypoid-type tumors of
each histological type.
| A, aPKC λ/ι |
|---|
|
|---|
| Type | LST-A, n (%) | LST-CA, n (%) | FD-CA, n (%) | P-A, n (%) | P-CA, n (%) |
|---|
| 1+ | 13 (86.6) | 5 (55.6) | 0 (0.0) | 5 (45.5) | 0 (0.0) |
| 2+ | 1 (6.7) | 3 (33.3) | 3 (23.1) | 5 (45.5) | 3 (33.3) |
| 3+ | 1 (6.7) | 1 (11.1) | 10 (76.9) | 1 (9.0) | 6 (66.7) |
| Total | 15 (100.0) | 9 (100.0) | 13 (100.0) | 11 (100.0) | 9 (100.0) |
|
| B, β-catenin |
|
| Type | LST-A, n (%) | LST-CA, n (%) | FD-CA, n (%) | P-A, n (%) | P-CA, n (%) |
|
| P | 13 (86.6) | 7 (77.8) | 1 (7.7) | 10 (99) | 2 (22.2) |
| C | 2 (13.3) | 1 (11.1) | 4 (30.8) | 1 (9) | 2 (22.2) |
| N | 0 (0) | 1 (11.1) | 8 (61.5) | 0 (0) | 5 (55.6) |
| Total | 15 (100.0) | 9 (100.0) | 13 (100.0) | 11 (100.0) | 9 (100.0) |
|
| C, E-cadherin |
|
| Type | LST-A, n (%) | LST-CA, n (%) | FD-CA, n (%) | P-A, n (%) | P-CA, n (%) |
|
| P | 4 (26.7) | 1 (11.1) | 0 (0.0) | 2 (18.2) | 1 (11.1) |
| H | 11 (73.3) | 7 (77.8) | 4 (30.8) | 9 (81.8) | 3 (33.3) |
| L | 0 (0.0) | 1 (11.1) | 9 (69.2) | 0 (0.0) | 5 (55.6) |
| Total | 15 (100.0) | 9 (100.0) | 13 (100.0) | 11 (100.0) | 9 (100.0) |
|
| D, Type IV
collagen |
|
| Type | LST-A, n (%) | LST-CA, n (%) | FD-CA, n (%) | P-A, n (%) | P-CA, n (%) |
|
| Cont | 14 (93.3) | 6 (66.7) | 0 (0.0) | 3 (27.3) | 0 (0.0) |
| D | 1 (6.7) | 2 (22.2) | 1 (7.6) | 8 (72.7) | 2 (22.2) |
| L | 0 (0.0) | 1 (11.1) | 12 (92.4) | 0 (0.0) | 7 (77.8) |
| Total | 15 (100.0) | 9 (100.0) | 13 (100.0) | 11 (100.0) | 9 (100.0) |
Expression of β-catenin, E-cadherin and
type IV collagen
Fig. 3B–D and
Table IIB-D summarize the
expression of β-catenin, E-cadherin and type IV collagen,
respectively, in LSTs and polypoid-type tumors of each histological
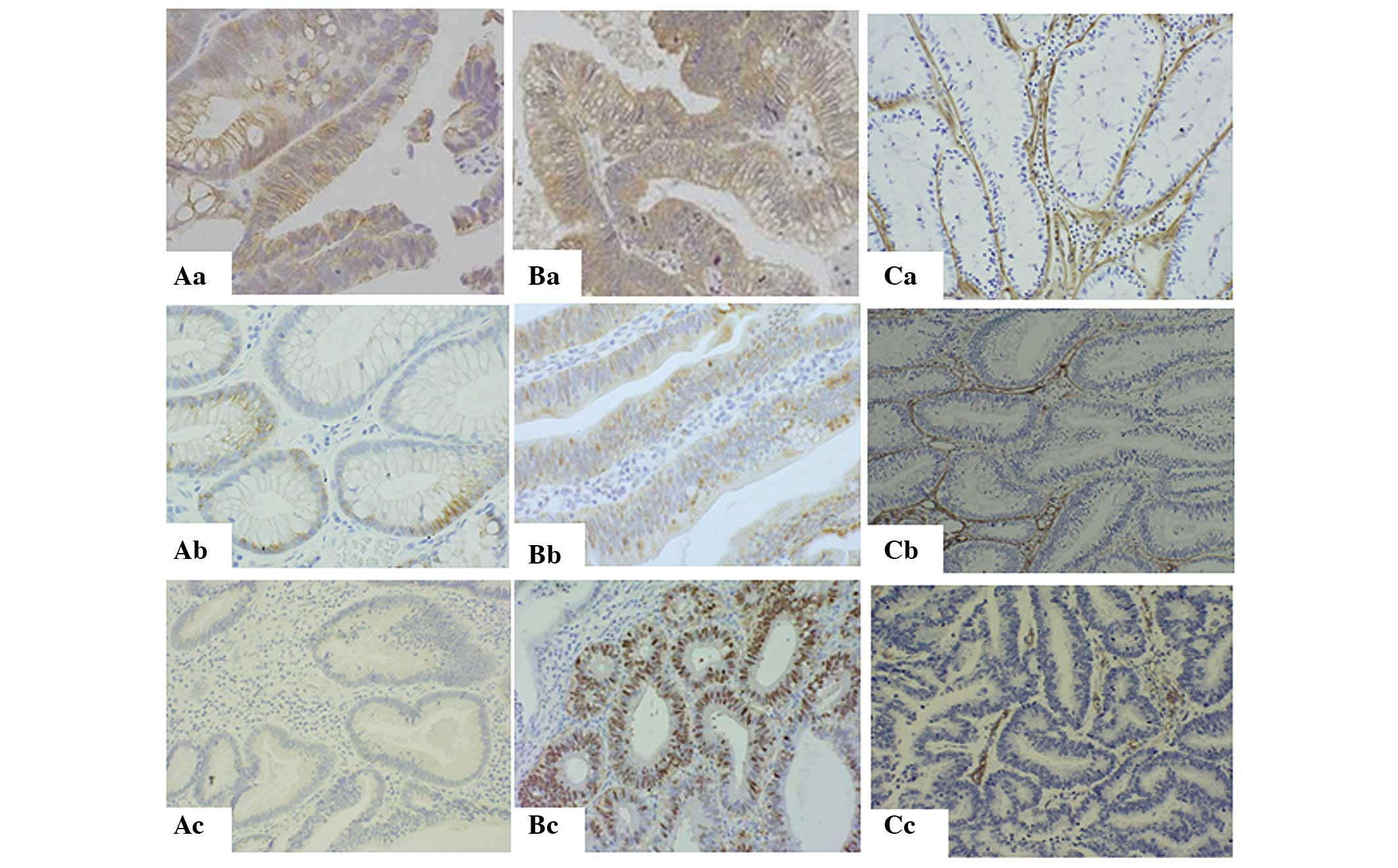
type. The results showed that 86.6% of the LST-As and 99% of the
P-As (Fig. 3B, Table IIB) showed the preserved type of
expression of β-catenin (Fig. 4Aa).
Furthermore, 77.8% of LST-CAs also showed the preserved type of
expression. On the other hand, 55.6% of P-CAs and 61.5% of FD-CAs
showed the nucleic type (Fig. 4Ac).
The expression of E-cadherin in the preserved type (Fig. 4Ba) was only observed in 26.7% of
LST-As, 18.2% of P-As and 11.1% of LST-CAs and P-As (Fig. 3C, Table
IIC). The expression of type IV collagen was observed in 93.3%
of LST-As (Fig. 3D, Table IID); however, only 27.3% of P-As
were of the continuous type (Fig.
4Ca). In addition, 92.4% of FD-CAs and 77.8% of P-CAs were of
the lost type (Fig. 4Cc). Notably,
66.7% of LST-CAs showed the continuous type (Fig. 4Cb).
Pearson’s correlation coefficient using the
expression results of all the samples showed a significant positive
correlation between the expression of aPKC λ/ι and β-catenin
(r=0.842; P<0.001) and type IV collagen (r=0.823; P<0.001)
(Fig. 5). A significant correlation
was identified between PKC and E-cadherin, but a marginally weaker
positive correlation/r value was identified (r=0.674; P<0.001;
Fig. 5).
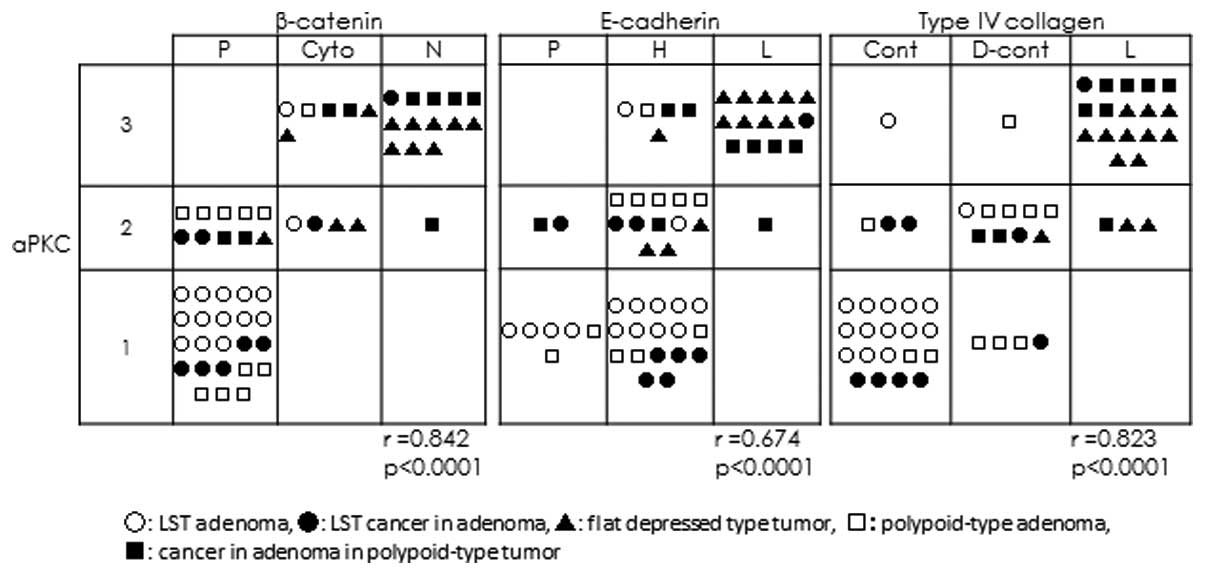 | Figure 5Correlation between aPKC λ/ι and
β-catenin, E-cadherin and type IV collagen. The expression of aPKC
λ/ι showed a strong positive correlation with that of β-catenin
(r=0.842) and type IV collagen (r=0.823), however, a weaker
positive correlation was identified between aPKC λ/ι and E-cadherin
expression (r=0.674) (P<0.0001 for all). aPKC, atypical protein
kinase C; r, Pearson’s correlation coefficient; LST, laterally
spreading tumor; P, preserved type; Cyto, cytoplasmic type; N,
nucleic type; H, heterogeneous type; L, lost type; Cont, continuous
type; D-cont, discontinuous type. |
Discussion
The present study showed the unique morphological
and functional characteristics of cell polarity proteins in LSTs,
including adenoma and cancer in adenoma.
LSTs of the colon and rectum are morphologically
defined as lesions of >10 mm in diameter with a low vertical
axis that extend laterally along the luminal wall. There are two
macroscopic subtypes of LST: G type, with a granule aggregating
surface (13); and NG type, with a
flat, smooth and non-granule aggregating surface. The majority of
LSTs remain as adenomas or early invasive cancers, thus LSTs are
considered to have potentially carcinogenic, but less invasive
characteristics (8,14). These characteristics can be
identified in the LST’s growth morphology as neoplastic cells that
tend to spread along the surface of the lumen. The microscopic
image in Fig. 1Ab and Bb, showing
the of the LST’s two layers, revealed a unique growth morphology,
which is maintained in cancerous LST lesions (4). By contrast, polypoid-type tumors,
another type of colorectal tumor, show expanding growth. The two
types of tumor show not only morphological differences, but also
some genetic or epigenetic differences (15). An additional flat type of colorectal
tumor besides LSTs is the flat depressed-type tumor (types IIc,
IIc+IIa and or IIa+IIc), shows more invasive characteristics than
LSTs. Ohno et al showed that flat depressed-type tumors also
show a low vertical axis that extends laterally along the luminal
wall similar to LSTs (8); however,
they show an expanding growth and do not exhibit two layers.
Furthermore, the authors also showed that ~70% of cancerous lesions
in LSTs are cancer in situ and ~64% of cancerous
lesions of flat depressed-type tumors show massive submucosal
invasion (8). Certain types of LSTs
become flat depressed-type tumors in the course of cancer
progression and the surface spreading growth pattern reveals that
LSTs show the expansive and invasive growth pattern of flat
depressed-type tumors during that progression.
Notably, the current study results showed that
LST-CAs maintain β-catenin and E-cadherin expression in the cell
membrane and that the BM was maintained around the tumor. However,
P-CAs and FD-CAs lost the expression of the proteins and the BM.
The BM structure was already lost in P-As.
The epithelial structure of colorectal mucosa has
apical and basolateral polarity (16), and the basal pole corresponds to the
contact between the cell membrane and extracellular BM molecules
(17). The BM is an important
structure that determines whether epithelial cells are aligned on
the ECM or migrate into it. No BM abnormalities have been noted in
hyperplastic polyps; however, discrete disruption in the BM may be
found in adenoma, depending on the degree of epithelial atypia
(18). Colorectal adenocarcinoma
shows various BM patterns and the majority of cancer cell nests do
not exhibit any BM. However, the current study found that
non-invasive cancer cells in LST structures have almost normal BM
structures.
E-cadherin and β-catenin are necessary for the
cell-cell adhesion of normal colonic epithelial cells (19) and are important factors that
determine the lateral pole of colonic epithelial cells. The loss of
the staining pattern and lower level or absence of E-cadherin
expression in colorectal tumors is associated with increasing
histological grading and worse prognosis (20). β-catenin forms a complex with
glycogen synthase kinase 3β, adenomatous polyposis coli (APC) and
axis inhibition protein, which binds with a T-cell factor in the
nucleus to promote gene transcription and contribute to colorectal
carcinogenesis (21). The
distribution of nuclear β-catenin expression is utilized as a
prognostic marker in colorectal cancer (22). Hashimoto et al (23) showed that β-catenin in LSTs is
expressed more intensely in flat structure segments or invasive
lesions than in granulation structures or intramucosal lesions.
Wang et al (24) also
reported that β-catenin is expressed more prominently in LSTs than
in protruded-type adenoma. The authors evaluated the β-catenin
expression by counting stained cells and did not report the
distribution of β-catenin in the nucleus, cytoplasm or cell
membrane. The results of the current study showed that E-cadherin
and the expression of β-catenin were maintained in the cell
membrane in LST-As and LST-CAs, and that the expression was lost or
exhibited abnormal distribution in polypoid-type tumors.
Cell polarity is regulated by complex systems and
Par-6, Par-3 and aPKC are major regulators of basolateral polarity
(25). These regulators are
involved in tight junction-associated cell-cell adhesion opening
and assembly, and activate the mitogen-activated protein kinase
pathway (26). aPKC is associated
with the tumorigenesis and progression of cancer. Murray et
al (27) reported the
progression of an aPKC λ/ι-derived colon adenoma to carcinoma, and
that aPKC λ/ι is also necessary for APC/β-catenin mediated colon
tumorigenesis. aPKC λ/ι expression in breast cancer is weak in
ductal carcinoma in situ, and exhibits stronger staining in
invasive ductal carcinoma (7).
Furthermore, aPKC λ/ι overexpression in gastric cancer is a strong
prognostic factor for cancer recurrence (3). The current study found that aPKC λ/ι
expression becomes gradually stronger with progression from adenoma
to invasive cancer. Although, the cancer cells that showed normal
polarity, for example LST-CAs, showed weak expression for aPKC λ/ι,
as observed in adenomas. However, the aPKC λ/ι expression became
stronger in adenoma and early-stage cancer as a result of β-catenin
accumulation in the nucleus and loss of BM. Furthermore, aPKC λ/ι
expression was detected not only in the cytoplasm, but also in the
nucleus. Perander et al also reported that while wild-type
aPKC l is predominantly localized in the cytoplasm, two different
point mutations in the catalytic domain lead to nuclear
accumulation of full-length aPKC l (28).
These results suggested that LSTs of adenoma and
cancer in situ exhibit almost normal polarity, expression
and distribution of the BM, as well as β-catenin and E-cadherin. A
change in the polarity of colorectal tumors in expansive growth may
be associated with the expression of aPKC λ/ι. Therefore, further
investigation of the expression of aPKC λ/ι in colorectal cancer is
required.
Acknowledgments
The authors would like to thank Ms. Sakurada for
technical assistance. The current study was supported by
Grants-in-Aid from the Japanese Ministry of Education, Culture,
Sports, Science and Technology for Fundamental Research (C2) (grant
nos. 20590368, 20570138 and 205914076).
References
|
1
|
Fields AP, Frederick LA and Regala RP:
Targeting the oncogenic protein kinase Ciota signalling pathway for
the treatment of cancer. Biochem Soc Trans. 35:996–1000. 2007.
|
|
2
|
Kojima Y, Akimoto K, Nagashima Y, et al:
The overexpression and altered localization of the atypical protein
kinase C lambda/iota in breast cancer correlates with the
pathologic type of these tumors. Hum Pathol. 39:824–831. 2008.
|
|
3
|
Takagawa R, Akimoto K, Ichikawa Y, et al:
High expression of atypical protein kinase C lambda/iota in gastric
cancer as a prognostic factor for recurrence. Ann Surg Oncol.
17:81–88. 2010.
|
|
4
|
Wang JM, Li Q, Du GS, Lu JX and Zou SQ:
Significance and expression of atypical protein kinase C-iota in
human hepatocellular carcinoma. J Surg Res. 154:143–149. 2009.
|
|
5
|
Regala RP, Weems C, Jamieson L, et al:
Atypical protein kinase C iota is an oncogene in human non-small
cell lung cancer. Cancer Res. 65:8905–8911. 2005.
|
|
6
|
Kudo S: Endoscopic mucosal resection of
flat and depressed types of early colorectal cancer. Endoscopy.
25:455–461. 1993.
|
|
7
|
Kudo S, Kashida H, Tamura T, et al:
Colonoscopic diagnosis and management of nonpolypoid early
colorectal cancer. World J Surg. 24:1081–1090. 2000.
|
|
8
|
Ohno Y, Terai T, Ogihara T, Hirai S and
Miwa H: Laterally spreading tumor: clinicopathological study in
comparison with the depressed type of colorectal tumor. J
Gastroenterol Hepatol. 16:770–776. 2001.
|
|
9
|
Hiraoka S, Kato J, Tatsukawa M, et al:
Laterally spreading type of colorectal adenoma exhibits a unique
methylation phenotype and K-ras mutations. Gastroenterology.
131:379–389. 2006.
|
|
10
|
Hassan A, Yerian LM, Kuan SF, Xiao SY,
Hart J and Wang HL: Immunohistochemical evaluation of adenomatous
polyposis coli, beta-catenin, c-Myc, cyclin D1, p53, and
retinoblastoma protein expression in syndromic and sporadic fundic
gland polyps. Hum Pathol. 35:328–334. 2004.
|
|
11
|
Oka H, Shiozaki H, Kobayashi K, et al:
Expression of E-cadherin cell adhesion molecules in human breast
cancer tissues and its relationship to metastasis. Cancer Res.
53:1696–1701. 1993.
|
|
12
|
Oka Y, Naito I, Manabe K, et al:
Distribution of collagen type IV alpha1–6 chains in human normal
colorectum and colorectal cancer demonstrated by immunofluorescence
staining using chain-specific epitope-defined monoclonal
antibodies. J Gastroenterol Hepatol. 17:980–986. 2002.
|
|
13
|
Oka S, Tanaka S, Kanao H, Oba S and
Chayama K: Therapeutic strategy for colorectal laterally spreading
tumor. Dig Endosc. 21(Suppl 1): S43–S46. 2009.
|
|
14
|
Kaku E, Oda Y, Murakami Y, et al:
Proportion of flat- and depressed-type and laterally spreading
tumor among advanced colorectal neoplasia. Clin Gastroenterol
Hepatol. 9:503–508. 2011.
|
|
15
|
Takahashi T, Nosho K, Yamamoto H, et al:
Flat-type colorectal advanced adenomas (laterally spreading tumors)
have different genetic and epigenetic alterations from
protruded-type advanced adenomas. Mod Pathol. 20:139–147. 2007.
|
|
16
|
Tanos B and Rodriguez-Boulan E: The
epithelial polarity program: machineries involved and their
hijacking by cancer. Oncogene. 27:6939–6957. 2008.
|
|
17
|
Lelievre SA: Tissue polarity-dependent
control of mammary epithelial homeostasis and cancer development:
an epigenetic perspective. J Mammary Gland Biol Neoplasia.
15:49–63. 2010.
|
|
18
|
Bosman FT, de Bruine A, Flohil C, et al:
Epithelial-stromal interactions in colon cancer. Int J Dev Biol.
37:203–211. 1993.
|
|
19
|
Debruyne D, Oliveira MJ, Bracke M, Mareel
M and Leroy A: Colon cancer cells: pro-invasive signalling. Int J
Biochem Cell Biol. 38:1231–1236. 2006.
|
|
20
|
Van Aken J, Cuvelier CA, De Wever N, et
al: Immunohistochemical analysis of E-cadherin expression in human
colorectal tumours. Pathol Res Pract. 189:975–978. 1993.
|
|
21
|
Wong NA and Pignatelli M: Beta-catenin - a
linchpin in colorectal carcinogenesis? Am J Pathol. 160:389–401.
2002.
|
|
22
|
Horst D, Reu S, Kriegl L, Engel J,
Kirchner T and Jung A: The intratumoral distribution of nuclear
beta-catenin is a prognostic marker in colon cancer. Cancer.
115:2063–2070. 2009.
|
|
23
|
Hashimoto S, Higaki S, Amano A, et al:
Relationship between molecular markers and endoscopic findings in
laterally spreading tumors. J Gastroenterol Hepatol. 22:30–36.
2007.
|
|
24
|
Wang J, Wang X, Gong W, Mi B, Liu S and
Jiang B: Increased expression of beta-catenin, phosphorylated
glycogen synthase kinase 3beta, cyclin D1, and c-myc in laterally
spreading colorectal tumors. J Histochem Cytochem. 57:363–371.
2009.
|
|
25
|
Wodarz A and Nathke I: Cell polarity in
development and cancer. Nat Cell Biol. 9:1016–1024. 2007.
|
|
26
|
Gonzalez-Mariscal L, Tapia R and Chamorro
D: Crosstalk of tight junction components with signaling pathways.
Biochim Biophys Acta. 1778:729–756. 2008.
|
|
27
|
Murray NR, Weems J, Braun U, Leitges M and
Fields AP: Protein kinase C betaII and PKCiota/lambda:
collaborating partners in colon cancer promotion and progression.
Cancer Res. 69:656–662. 2009.
|
|
28
|
Perander M, Bjorkoy G and Johansen T:
Nuclear import and export signals enable rapid nucleocytoplasmic
shuttling of the atypical protein kinase C lambda. J Biol Chem.
276:13015–13024. 2001.
|