Introduction
Studies have shown that the matrix
metalloproteinases (MMPs), including MMP-2 and MMP-9, are key
proteases in the invasion and metastasis of human oral squamous
carcinoma and breast cancer cells (1,2). The
levels of MMPs are regulated by tissue inhibitors of
metalloproteinases (TIMPs). TIMP-1 and TIMP-2 have the ability to
inhibit tumor invasion and metastasis (3). Therefore, numerous studies have
focused on MMPs and their regulatory pathways, to explore the
molecular mechanisms for preventing cancer metastasis.
Increased activity of the nuclear factor κB (NF-κB)
pathway has been observed in various signaling cascades, including
the apoptosis and metastasis cascades (4,5). It
has also been reported that follicle-stimulating hormone affects
the proliferation and invasion of ovarian cancer cells by
regulating the NF-κB signaling pathway (6). Inhibition of the NF-κB pathway may
potentially prevent proliferation and invasion in a broader range
of tumors. In addition, the NF-κB signaling pathways are
significant in regulating the expression of MMPs. The stilbenoid,
piceatannol, suppresses breast cancer cell invasion through the
inhibition of NF-κB pathways (7).
Thus, the NF-κB pathways are potential targets for the treatment of
tumors.
The epidermal growth factor receptor (EGFR) has been
observed to be upregulated in a number of tumors and is considered
a target for cancer therapy (8).
EGFR appears to be one of the most promising and effective targets
in the treatment of head and neck (9) and breast (10) cancer. Icotinib hydrochloride, a
novel and potent selective EGFR tyrosine kinase inhibitor (TKI),
has been used in the treatment of patients with non-small cell lung
cancer (NSCLC), and exhibits promising efficacy and safety
(11,12). In previous studies, icotinib has
shown an effective role in reducing proliferation and increasing
apoptosis in HCC827 cells (12),
and has exhibited antitumor activity in the A431 cell line
(13). A recent study also revealed
that the toxicity of icotinib was typically low in patients with
advanced NSCLC (14), where
icotinib was generally well tolerated and exhibited antitumor
activity (15).
Although the inhibitory effect of icotinib in the
growth of cancer cells is known, the role of icotinib in the
inhibition of tumor invasion and metastasis remains unclear. The
present study aimed to determine the inhibitory effect of icotinib
on metastasis in human tongue carcinoma Tca8113 cells, and its
possible underlying mechanisms of action.
Materials and methods
Reagents and antibodies
Icotinib was provided by Zhejiang Beta Pharma Co.,
Inc. (Yuhang, China) and dissolved in 5% dimethyl sulfoxide (DMSO).
Rabbit monoclonal anti-human MMP2, MMP9, TIMP1 and TIMP2 antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA), while rabbit polyclonal anti-human NF-κB p65 antibody was
purchased from Millipore (Billerica, MA, USA) and mouse monoclonal
anti-ox histone H1 was purchased from Millipore (Bedford, MA, USA).
Pyrrolidine dithiocarbamate (PDTC) was purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Cell culture
The human tongue carcinoma Tca8113 cell line was
obtained from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). The Tca8113 cells were cultured in
RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco-BRL) and 1% streptomycin
(Sigma-Aldrich), and incubated at 37°C in a 5% CO2
humidified atmosphere. The medium was replaced three times per
week.
Western blot analysis
Equal amounts of protein lysates were separated on
10% Bis-Tris gels (Invitrogen Life Technologies, Carlsbad, CA, USA)
and electrophoretically transferred to 0.22 μm polyvinylidene
difluoride membranes (Invitrogen Life Technologies). The membranes
were blocked with 5% non-fat milk for 2 h at room temperature and
then incubated with the primary rabbit monoclonal anti-human MMP2,
MMP9, TIMP1 and TIMP2 (Cell Signaling, Inc.), and rabbit polyclonal
anti-human NF-κB p65 (Millipore) antibodies. Following washing with
phosphate-buffered saline, the membranes were incubated with
horseradish peroxidase-conjugated IgG (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and then washed a further three times with
Tris-buffered saline with Tween 20. The immunoreactivity was
visualized by enhanced chemiluminescence (WP20005, Invitrogen Life
Technologies). β-actin or His H1 was used as an experimental
control, and the density of immunoblotting was quantified using
Quantity One software (Bio-Rad, Hercules, CA, USA).
Boyden chamber invasion assay
The ability of Tca8113 cells to pass through the
Matrigel-coated polycarbonate filters (Becton-Dickinson,
Heidelberg, Germany) was determined by the Boyden chamber invasion
assay. Following treatment with various concentrations of icotinib
for 24 h, the cells (1×104 cells/well) in serum-free
medium were added to the upper chamber. The complete medium
containing 10% FBS was applied to the lower chamber as a
chemoattractive agent, and the chamber was subsequently incubated
for 24 h at 37°C. Following incubation, the cells of the upper
surface of the membrane were removed with a cotton swab. The cells
that had invaded across the Matrigel to the lower surface of the
membrane were stained with hematoxylin and eosin. These cells were
then scored by ImageJ quantification software (National Institutes
of Health, Bethesda, MD, USA) under a microscope (DM6000B, Leica
Microsystems, Wetzlar, Germany) (16).
Nuclear protein extraction
The cytosolic and nuclear extracts were prepared as
previously described with slight modifications (17). Briefly, the cells were centrifuged
in a homogenization buffer [10 mM Hepes, 10 mM KCl, 0.1 mM EGTA,
0.1 mM EDTA, 0.5 mM phenylmethanesulfonyl fluoride (PMSF) and
protease inhibitor cocktail (1:100); Active Motif, Carlsbad, CA,
USA] at 13,000 × g for 10 min at 4°C, and the supernatant was
collected. For the nuclear protein extracts, the pellets were
resuspended in nuclear extraction buffer (20 mM Hepes, 1.5 mM
MgCl2, 0.4 M NaCl,1 mM EGTA, 1 mM EDTA, 1 mM
dithiothreitol, 0.5 mM PMSF and protease inhibitor cocktail
(1:100)] for 30 min and centrifuged at 13,000 × g for 20 min at
4°C. The supernatants containing the nuclear protein were collected
and stored at −80°C.
Results
Icotinib inhibits the proliferation of
Tca8113 cells
Icotinib has been observed to inhibit tumor cell
proliferation (18). In the present
study, the antiproliferative effects of icotinib at various
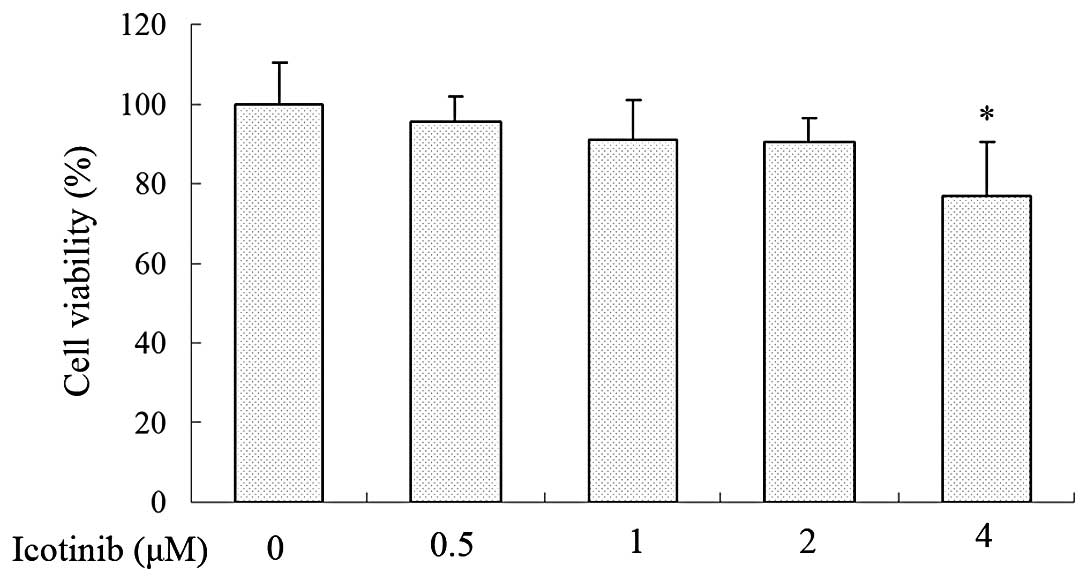
concentrations (0, 0.5, 1, 2 and 4 μM) on Tca8113 cells for 24 h
were determined by MTT assay, and the results are shown in Fig. 1. The viability of Tca8113 cells was
not significantly affected by ≤2 μM icotinib compared with the
DMSO-treated control group. As a result, a concentration ranging
between 0.5 and 2 μM was selected for the subsequent experiments
with icotinib.
Icotinib inhibits the invasion of Tca8113
cells
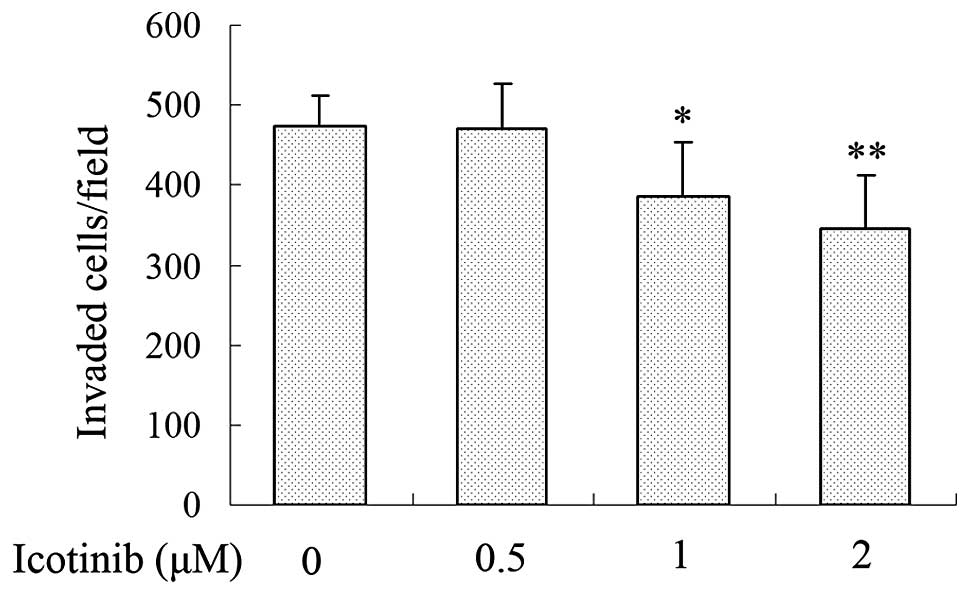
To investigate the effect of icotinib on the
invasive ability of Tca8113 cells, the motility of Tca8113 cells
was examined by Transwell invasion assay. Tca8113 cells treated
with icotinib at various concentrations (0, 0.5, 1 and 2 μM) were
plated in the upper chamber for 24 h. After 24 h, the number of
cells that had moved to the lower membrane was counted under a
light microscope (Leica Microsystems). The results demonstrated
that icotinib reduced the invasion of Tca8113 cells in a
concentration-dependent manner when treated with 0, 0.5, 1 and 2 μM
of icotinib for 24 h (Fig. 2).
Icotinib downregulates the expression of
MMP-2 and MMP-9 and promotes the expression of TIMP-1 and TIMP-2 in
Tca8113 cells
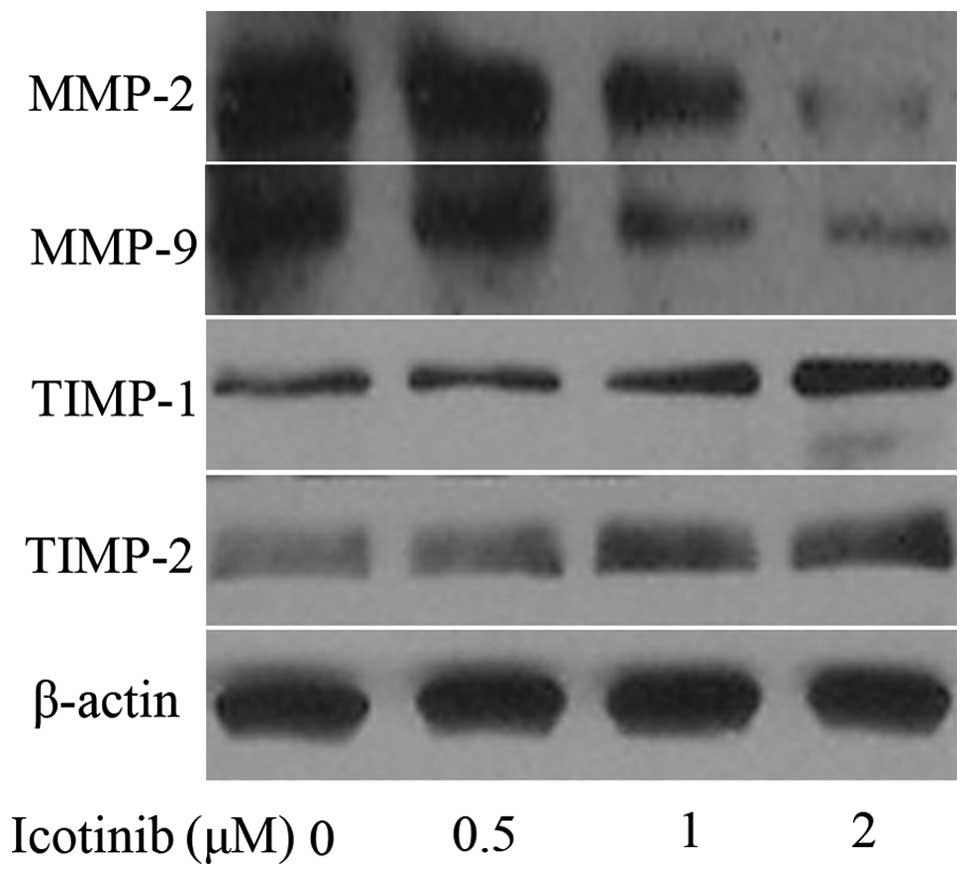
As MMP-2 and MMP-9 have a critical function in tumor
cell invasion, the inhibitory effect of icotinib on the expression
of MMP-2 and MMP-9 was investigated. Tca8113 cells were treated
with 0, 0.5, 1 and 2 μM icotinib for 24 h. As shown in Fig. 3, icotinib markedly reduced MMP-2 and
MMP-9 expression in a concentration-dependent manner. Additionally,
icotinib significantly increased the protein levels of TIMP-1 and
TIMP-2 in a concentration-dependent manner, as shown in Fig. 3 by western blot analysis.
NF-κB pathway is involved in the
antimetastatic mechanism of icotinib
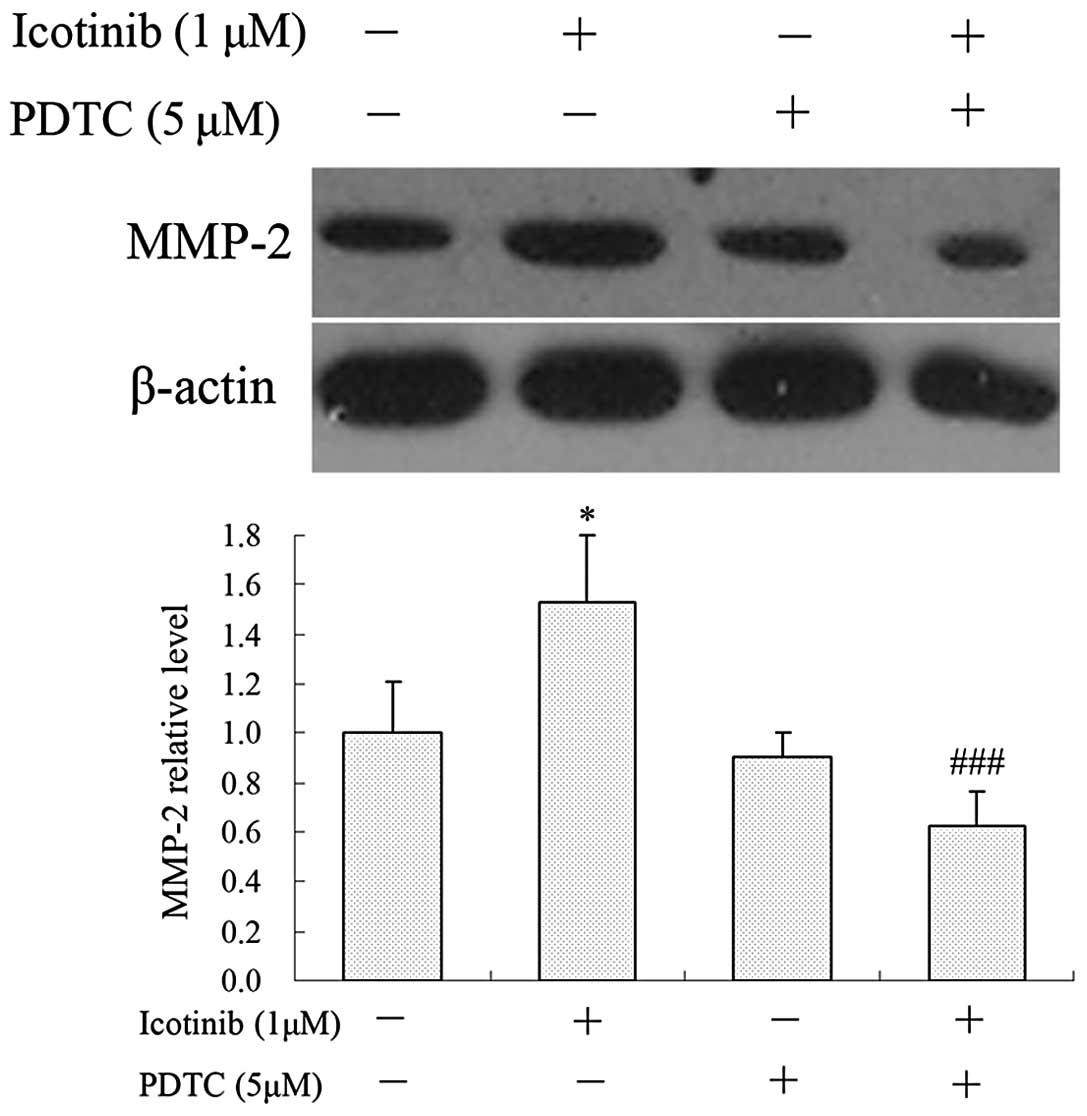
To elucidate whether the inhibitory effect of
icotinib on the lower expression of MMP-2 is regulated by the NF-κB
pathway, the effect of icotinib on the NF-κB pathway in Tca8113
cells was investigated. The results shown in Fig. 4 demonstrate that the nuclear levels
of NF-κB p65 were significantly decreased following treatment with
icotinib in comparison with the control. To further determine
whether the effect of icotinib on lowering the levels of MMP-2
occurred through the inhibition of NF-κB pathway, Tca8113 cells
were then pretreated with the NF-κB inhibitor, PDTC (5 μM), for 1 h
and incubated in the presence or absence of icotinib (1 μM) for 24
h. The results indicated that PDTC significantly reverses the
effect of icotinib on the expression of MMP-2 (Fig. 5).
Discussion
The antitumor effect of icotinib has been confirmed
in a number of cancer cell lines (13,18).
However, the anti-invasion effect and its associated mechanisms in
Tca8113 cells were unclear. The current study revealed that
icotinib significantly suppresses the invasion and metastasis of
Tca8113 cells by regulating the expression of MMPs and TIMPs via
the inhibition of the NF-κB pathway. To the best of our knowledge,
the antimetastatic effects of icotinib in vitro have rarely
been reported.
The present study demonstrated that icotinib does
not inhibit the viability of Tca8113 cells treated with icotinib at
non-toxic doses; however, the cell invasion was inhibited. These
results suggested that the invasion-inhibiting effect of icotinib
on Tca8113 cells was not due to its cytotoxicity. To clarify the
mechanism of icotinib in the inhibition of invasion, the potential
correlation between the inhibitory effect of icotinib on cell
invasion and the downregulation of MMP levels were investigated
Cancer cell invasion and metastasis are the
predominant causes of mortality in cancer patients. MMPs are
significant in degrading the matrix barriers around the tumor, and
contribute to cell invasion and metastasis (7,19).
TIMPs are inhibitors of MMPs, which control MMP activities and
minimize matrix degradation (20,21).
To determine whether the inhibitory effect of icotinib on cell
invasion and metastasis is associated with MMPs in Tca8113 cells, a
Boyden chamber invasion assay was performed with icotinib-treated
cells. Icotinib markedly inhibited the expression of MMPs, while
the levels of TIMP-1 and TIMP-2 were increased following icotinib
treatment in Tca8113. The results presented in this study suggested
that icotinib suppresses tumor cell invasion by downregulating MMPs
and upregulating TIMP-1/2.
Several studies have revealed that NF-κB activation
is involved in stimulating the secretion of MMPs in tumor cells
(22,23). A previous study has also
demonstrated that the steroid saponin, diosgenin, inhibits tumor
necrosis factor-induced NF-κB activation and blocks the
proliferation of tumor cells (24).
Furthermore, when activated, NF-κB has been shown to stimulate
invasion and metastasis in a number of cancer cell lines, which
mediates the resistance to chemo- and radiotherapies (2,25). The
current study demonstrated that icotinib suppresses the protein
expression of NF-κB p65, and that treatment with the NF-κB
inhibitor, PDTC, significantly reduces the cell invasion and
decreases the levels of MMP. These results demonstrated that the
reduction in MMP expression levels is caused by icotinib and
attributed to the blocking of NF-κB activation.
In conclusion, the results of this study
demonstrated that icotinib has the ability to inhibit the migration
and invasion of the squamous cells of tongue carcinoma in
vitro. This effect may be via the inactivation of
NF-κB-mediated MMP-9 expression. Therefore, icotinib may present as
a promising antimetastatic drug for the prevention of malignant
cancer.
References
|
1
|
Chen HJ, Lin CM, Lee CY, et al: Phenethyl
isothiocyanate suppresses EGF-stimulated SAS human oral squamous
carcinoma cell invasion by targeting EGF receptor signaling. Int J
Oncol. 43:629–637. 2013.
|
|
2
|
Li F, Li C, Zhang H, et al: VI-14, a novel
flavonoid derivative, inhibits migration and invasion of human
breast cancer cells. Toxicol Appl Pharmacol. 261:217–226. 2012.
|
|
3
|
Zhou H, Kimura K, Orita T, Nishida T and
Sonoda KH: Inhibition by medroxyprogesterone acetate of
interleukin-1β-induced collagen degradation by corneal fibroblasts.
Invest Ophthalmol Vis Sci. 53:4213–4219. 2012.
|
|
4
|
Kuo PL, Shen KH, Hung SH and Hsu YL:
CXCL1/GROα increases cell migration and invasion of prostate cancer
by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic
regulation. Carcinogenesis. 33:2477–2487. 2012.
|
|
5
|
Wang Z, Sengupta R, Banerjee S, et al:
Epidermal growth factor receptor-related protein inhibits cell
growth and invasion in pancreatic cancer. Cancer Res. 66:7653–7660.
2006.
|
|
6
|
Xu CL, Lu XL, Yan XN, Wang HL and Chen SQ:
Effects of PI3K/Akt/NF-κB signal pathway on FSH facilitation on
cell proliferation and invasion by human epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 47:134–138. 2012.(In Chinese).
|
|
7
|
Ko HS, Lee HJ, Kim SH and Lee EO:
Piceatannol suppresses breast cancer cell invasion through the
inhibition of MMP-9: involvement of PI3K/AKT and NF-κB pathways. J
Agric Food Chem. 60:4083–4089. 2012.
|
|
8
|
Gan HK, Burgess AW, Clayton AH and Scott
AM: Targeting of a conformationally exposed, tumor-specific epitope
of EGFR as a strategy for cancer therapy. Cancer Res. 72:2924–2930.
2012.
|
|
9
|
Sobhakumari A, Schickling BM, Love-Homan
L, et al: NOX4 mediates cytoprotective autophagy induced by the
EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol
Appl Pharmacol. 272:736–745. 2013.
|
|
10
|
Li P, Zhang Q, Torossian A, et al:
Simultaneous inhibition of EGFR and PI3K enhances radiosensitivity
in human breast cancer. Int J Radiat Oncol Biol Phys. 83:e391–e397.
2012.
|
|
11
|
Li D, Ji H, Zaghlul S, et al: Therapeutic
anti-EGFR antibody 806 generates responses in murine de novo EGFR
mutant-dependent lung carcinomas. J Clin Invest. 117:346–352.
2007.
|
|
12
|
Yang G, Yao Y, Zhou J and Zhao Q: Effects
of icotinib, a novel epidermal growth factor receptor tyrosine
kinase inhibitor, in EGFR-mutated non-small cell lung cancer. Oncol
Rep. 27:2066–2072. 2012.
|
|
13
|
Gao Z, Chen W, Zhang X, et al: Icotinib, a
potent and specific EGFR tyrosine kinase inhibitor, inhibits growth
of squamous cell carcinoma cell line A431 through negatively
regulating AKT signaling. Biomed Pharmacother. 67:351–356.
2013.
|
|
14
|
Gu A, Shi C, Xiong L, Chu T, Pei J and Han
B: Efficacy and safety evaluation of icotinib in patients with
advanced non-small cell lung cancer. Chin J Cancer Res. 25:90–94.
2013.
|
|
15
|
Zhao Q, Shentu J, Xu N, et al: Phase I
study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine
kinase inhibitor, in patients with advanced NSCLC and other solid
tumors. Lung Cancer. 73:195–202. 2011.
|
|
16
|
Li Y, Xu Q, Zhang Z, Liu S, Shi C and Tan
Y: The impact of TGF-β1 on the mRNA expression of TβR I, TβR II,
Smad4 and the invasiveness of the JEG-3 placental choriocarcinoma
cell line. Oncol Lett. 4:1344–1348. 2012.
|
|
17
|
Bethea JR, Castro M, Keane RW, Lee TT,
Dietrich WD and Yezierski RP: Traumatic spinal cord injury induces
nuclear factor-kappaB activation. J Neurosci. 18:3251–3260.
1998.
|
|
18
|
Mu X, Zhang Y, Qu X, et al: Ubiquitin
ligase Cbl-b is involved in icotinib (BPI-2009H)-induced apoptosis
and G1 phase arrest of EGFR mutation-positive non-small-cell lung
cancer. Biomed Res Int. 2013:7263752013.
|
|
19
|
Stark AM, Anuszkiewicz B, Mentlein R,
Yoneda T, Mehdorn HM and Held-Feindt J: Differential expression of
matrix metalloproteinases in brain- and bone-seeking clones of
metastatic MDA-MB-231 breast cancer cells. J Neurooncol. 81:39–48.
2007.
|
|
20
|
Takino T, Miyamori H, Watanabe Y, Yoshioka
K, Seiki M and Sato H: Membrane type 1 matrix metalloproteinase
regulates collagen-dependent mitogen-activated
protein/extracellular signal-related kinase activation and cell
migration. Cancer Res. 64:1044–1049. 2004.
|
|
21
|
Sounni NE, Rozanov DV, Remacle AG,
Golubkov VS, Noel A and Strongin AY: Timp-2 binding with cellular
MT1-MMP stimulates invasion-promoting MEK/ERK signaling in cancer
cells. Int J Cancer. 126:1067–1078. 2010.
|
|
22
|
Tan BK, Adya R, Chen J, Lehnert H, Sant
Cassia LJ and Randeva HS: Metformin treatment exerts antiinvasive
and antimetastatic effects in human endometrial carcinoma cells. J
Clin Endocrinol Metab. 96:808–816. 2011.
|
|
23
|
Yan KH, Lee LM, Yan SH, et al: Tomatidine
inhibits invasion of human lung adenocarcinoma cell A549 by
reducing matrix metalloproteinases expression. Chem Biol Interact.
203:580–587. 2013.
|
|
24
|
Shishodia S and Aggarwal BB: Diosgenin
inhibits osteoclastogenesis, invasion, and proliferation through
the downregulation of Akt, I kappa B kinase activation and NF-kappa
B-regulated gene expression. Oncogene. 25:1463–1473. 2006.
|
|
25
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007.
|