Introduction
Tumors of the head and neck form a heterogeneous
group of malignant neoplasms that typically arise from the upper
aerodigestive tract. The most common tumor entity (>90%) is head
and neck squamous cell carcinoma (HNSCC). HNSCC commonly affects
the oral cavity, the hypo-and oropharynx and the larynx. HNSCC
customarily originates from epithelial layers and often from
pre-cancerous lesions, including leukoplakia. Histologically,
HNSCCs are subclassified as verrucous, basaloid and adenosquamous
carcinomas. In 2008, the World Health Organization calculated
~631,800 new cases of HNSCC globally. This equates to a global
incidence of 13.7/100,000 (1).
HNSCC is the result of a multifactorial process caused by
carcinogenic substances (2–4). Chronic consumption of alcohol and
tobacco abuse are the main risk factors for HNSCC. Between 85 and
90% of all HNSCC cases are associated with nicotine or alcohol
abuse (2). In addition, the risk
for HNSCC rises with the amount and duration of abuse.
Consequently, a synergistic effect induced by alcohol and nicotine
has been hypothesized (5). HNSCCs
have a high invasive potency and even an early-stage tumor is at
risk for lymphogenic metastasis. In this context, the topographical
affection is linked to the cervical lymph nodes (6). Subsequent to incorporation into the
subcapsular sinus of the lymph nodes, the tumor cells start to
proliferate (7). Tumor size and
location, lymph node invasion, extracapsular spread and distant
metastases define the individual tumor prognosis without taking
account of the heterogeneous tumor biology of the tumor entity.
Vascular endothelial growth factor (VEGF) is a
highly potent angiogenic factor that is strongly expressed in a
multitude of neoplasias, including breast, lung and head and neck
cancer (8). It has been shown that
the serological VEGF levels of patients with head and neck cancer
correlate with the occurrence of lymph node metastasis and a poor
prognosis (9). Furthermore, high
levels of other angiogenic factors, for instance platelet-derived
growth factor (PDGF), and a high rate of p53 mutations have been
reported for cases of HNSCC with elevated VEGF levels (10,11).
Various isoforms of PDGF are involved in
inflammatory and angiogenic processes and in cellular migration of
HNSCC. The autocrine stimulation caused by PDGF leads to tumor
growth and facilitates the infiltration of tumor stromal cells
(12). In contrast to healthy
controls, patients with HNSCC show significantly higher PDGF
levels, but there is no significant correlation between clinical
stage and the PDGF serum level (13).
The epidermal growth factor receptor (EGFR) is a
tyrosine kinase that is expressed in normal tissue and in tumor
cells. When EGFR is activated by its physiological ligands,
transforming growth factor-α or EGF, various enabled G-protein
linked kinases affect the transcription and secretion of
growth-enhancing mediators. These mediators lead to autocrine and
paracrine stimulation of pathological growth and the angiogenic
affiliation of tumor cells in head and neck cancer (14).
Interleukin-4 (IL-4) is an anti-inflammatory
cytokine that is produced and secreted by type 2 T-helper cells and
mast cells, and that plays a crucial role in allergic reactions of
the skin and mucosa membrane. It has been shown that SCCs do not
produce IL-4 (15), but IL-4
expression can be found in the tumor stroma (16). In vitro studies have shown
that IL-4 triggers tumor growth of HNSCC cell cultures in a
dose-dependent manner (16,17). By contrast, a growth-inhibiting
effect was reported for melanomas and gastric and renal cancer
(18–20). Additionally, IL-4 has shown an
antiangiogenic effect in animal studies (15,21).
As a multifunctional cytokine, IL-6 has
proinflammatory properties and activates migration of immune cells.
Increased IL-6 levels have been determined in lung, ovarian and
head and neck cancer (22,23). Wang et al (24) showed that patients suffering from
malignant tumors of the head and neck exhibited distinctly elevated
IL-6 and IL-6-receptor levels compared with healthy controls. The
occurrence of metastases, relapses and reduced overall survival
rates were also significantly associated with elevated serological
IL-6 levels (24).
Osteopontin is an extracellular phosphoglycoprotein
that is physiologically involved in the formation of bone matrix.
With its ability to mediate cell adhesion, osteopontin can take
part in the process of tumor invasion, angiogenesis and metastasis
formation. Elevated osteopontin levels have been reported for 34
various tumor entities and their metastasis (25). Weber et al (26) revealed that low osteopontin levels
prior to therapy were associated with higher overall survival rates
and an improved therapy response in patients with head and neck
cancer. By contrast, Lim et al (27) failed to verify a correlation between
osteopontin and the overall survival rate or therapy response in
head and neck cancer. However, osteopontin was suitable for use as
a tumor marker, although it was not clear for which entity it was
most appropriate (26,28).
Granulocyte-colony stimulating factor (G-CSF) is
produced and released by macrophages, fibroblasts and epithelial
cells that are also part of the tumor stroma (29). In bone marrow, G-CSF works as a
mediator to stimulate cell differentiation of the progenitor cells
of neutrophil granulocytes. Increased G-CSF levels are detectable
in leukemia and also in solid tumors (30). In HNSCC, G-CSF stimulates the
proliferation and migration of tumor and inflammatory cells. In
contrast to G-CSF-negative tumors, G-CSF-positive tumors show
distinct invasiveness of bone and cartilage (31).
The present pilot study assessed the applicability
of these seven serological factors as biomarkers for malignant
tumors of the head and neck. The pre- and post-therapeutical serum
samples were determined from 20 patients receiving concomitant
radiochemotherapy with two cycles of cisplatin or carboplatin and
5-fluorouracil (5-FU) with curative intent, and the expression of
these markers was compared with that in healthy controls. The pilot
study sought to investigate whether the serum of patients showed
significant concentration differences in the analyzed factors at
the start of concomitant radiochemotherapy compared with the
controls, and whether these markers indicated a neoplastic process.
The study also examined whether concomitant radiochemotherapy with
cisplatin or carboplatin and 5-FU induced significant alterations
of concentration compared with pre-therapeutic levels.
Patients and methods
Patient characteristics and
treatment
The present study was approved by the Ethics
Committee II of the Medical Faculty of Mannheim at the University
of Heidelberg (file number 2011-279N-MA; Mannheim, Germany).
Written informed consent was obtained from all patients and members
of the control group. The study assessed 20 patients (17 male and 3
female; mean age, 62.4 years; and range, 41–77 years) and 40
healthy control subjects (25 male and 15 female; mean age, 50.3
years; and range, 19–81 years). All patients underwent concomitant
radiochemotherapy due to a malignant tumor of the head and neck
with two cycles of 5-FU (1,000 mg/m2; treatment days 1–4
and 22–25) and cisplatin (80 mg/m2; treatment days 1 and
22) or carboplatin [dose calculated using the Calvert formula
(32); treatment days 1 and 22].
All patients were treated with curative intent. No participant
received palliative therapy or ‘best supportive care’. A total of
80% of the tumor patients received adjuvant (postoperative)
concomitant radiochemotherapy with 60–66 Gy of the tumor
localization and 44–66 Gy of the un- or involved nodal levels
following surgical resection of the tumor and reconstruction, and
uni- or bilateral neck dissection. The remaining 20% of patients
with HNSCC underwent definitive radiochemotherapy with two cycles
of chemotherapy and a cumulative dose of radiation from 66–74 Gy
(primary tumor localization) and 44–64 Gy (un- and involved nodal
stations).
In total, 30% of the diagnosed tumors were localized
in the oropharynx, particularly in the tonsil area, with 20% in the
oral cavity, 20% in the larynx, 15% in the salivary gland, 15% in
the lower lip, 5% in the hypopharynx and 15% were cancer of unknown
primary syndrome. A total of 10% of the head and neck malignancies
were locoregional metastasis (lymph node metastasis) or local tumor
recurrences following initial tumor resection. At the initiation of
therapy, none of the patients presented with distant metastasis,
although 80% of the patients were affected by lymph node
metastasis. According to the Union for International Cancer Control
classification (33), 75% of the
patients had stage IVA cancer, 15% had stage III, 5% had stage I
and 5% had stage II. Along with cardiopulmonary comorbidities
(arterial hypertension, coronary heart disease and cardiac
arrhythmias), diabetes and ethyltoxic hepatic cirrhosis were
coexisting. Of the 20 patients, 13 stated regular nicotine use. All
patients with HNSCC completed the definitive or postoperative
radiochemotherapy. No patients dropped out of the study or had to
be excluded. All of the 40 controls were healthy patients from the
Sleep Laboratory of the Ear Nose and Throat Department without
clinical or laboratory signs of inflammation or a history of a
malignancy. During the course of regular pre- and
post-chemotherapeutic blood draws, one ethylenediaminetetraacetic
acid and one serum sample with S-Monovette® (Sarstedt,
Nuembrecht, Germany) was obtained from each patient one week prior
to and one week following chemotherapeutic treatment. The interval
between pre- and post-therapeutic blood draws was approximately six
weeks. The collected samples were centrifuged with 2,000 × g for 10
min and the supernatant plasma was pipetted into
Eppendorf® tubes, labeled and stored at −20°C.
Assays
The serological levels of each factor were measured
by enzyme linked immunosorbent assay (ELISA) (R&D Systems,
Abingdon, UK). All required reagents were warmed from a storage
temperature of 2°C to room temperature for analysis. To prepare the
wash buffer, 20 ml of wash buffer concentrate was diluted into 480
ml of distilled water. To produce a stock solution of 2,000 pg/ml,
the provided factor-standard was reconstituted with 1 ml of
calibrator diluent and incubated for 15 min under gentle agitation.
Following incubation, a standard dilution series was prepared with
seven stages (2,000, 1,000, 500, 250, 125, 62.5 and 31.2 pg/ml). At
the beginning of the test, 100 μl of assay diluent was added to
each well of the mouse anti-human monoclonal capture
antibody-coated microplate (IL-4, IL-6, EGFR, osteopontin, PDGF,
G-CSF or VEGF)(R&D Systems). The first seven wells were filled
with 100 μl of each standard dilution, and all other wells were
filled with 100 μl of defrosted patient or control plasma samples.
After a 2-h incubation, the wells were washed with wash buffer to
remove unbound material. Subsequently, each well was filled with
200 μl of horseradish peroxidase-linked polyclonal goat anti-human
detection antibody (IL-4, IL-6, EGFR, osteopontin, PDGF, G-CSF or
VEGF)solution (factor conjugate; R&D Systems). The detection
antibody bound another epitope of the antigen than the capture
antibody, and as a result a sandwich of antibody-antigen-antibody
emerged. After another 2-h incubation and washing, the wells were
filled with the color substrate solution (stabilized horseradish
peroxidase and tetramethylbenzidine; R&D Systems) and incubated
for 25 min. The resulting color change in each well indicated the
amount of antigen (factor) detected in the plasma. To terminate the
enzymatic reaction, 50 μl of stop solution (2-N-sulfuric acid;
R&D Systems) was added to each well. In the final step, the
exact quantity of antigen was detected by a microplate reader
(MRX-Reader; Dynatech Laboratories, Denkendorf, Germany) set at 450
nm.
Statistical analysis
To calculate alterations in the pre- and
post-therapeutic serum levels, the Wilcoxon signed-rank test for
dependent samples was performed. For comparison of patients and
controls, the t-test was used for normally distributed markers
(Osteopontin, PDGF, EGFR, IL-4 and G-CSF). The levels of VEGF and
IL-6 were not normally distributed. Consequently, both markers were
analyzed with the non-parametric Mann-Whitney U-test. The
statistical evaluation was conducted in cooperation with Dr C Weiss
(Department of Medical Statistics, Biomathematics and Information
Processing, Mannheim University Hospital, Mannheim, Germany).
P<0.05 was considered to indicate a statistically significant
difference.
Results
IL-4
IL-4 exhibited the lowest values of all measured
factors for patients and controls. The mean pre-therapeutic value
of the patient group was 2.42±0.81 pg/ml, while the mean value of
the control group was 1.37±0.63 pg/ml. Significant differences were
shown between the control and patient groups (P=0.0001), with
considerably higher IL-4 levels in the patient group. Following
radiochemotherapy, the patient group showed a decline of 0.16±0.90
pg/ml in serum concentration (Table
I and Fig. 1). Considering
possible therapy-induced changes, the Wilcoxon signed-rank test
revealed P=0.8500. Thus, a significance for therapy-associated IL-4
alterations was not shown, but a trend towards decreased levels was
found.
 | Table ISerum levels of patients with HNSCC
and the control group prior and subsequent to therapy. |
Table I
Serum levels of patients with HNSCC
and the control group prior and subsequent to therapy.
| Marker | Patientsa | Controlsa | Difference pre-post
treatment patientsa | P-value
patients-controls | P-value patients
pre-post treatment |
|---|
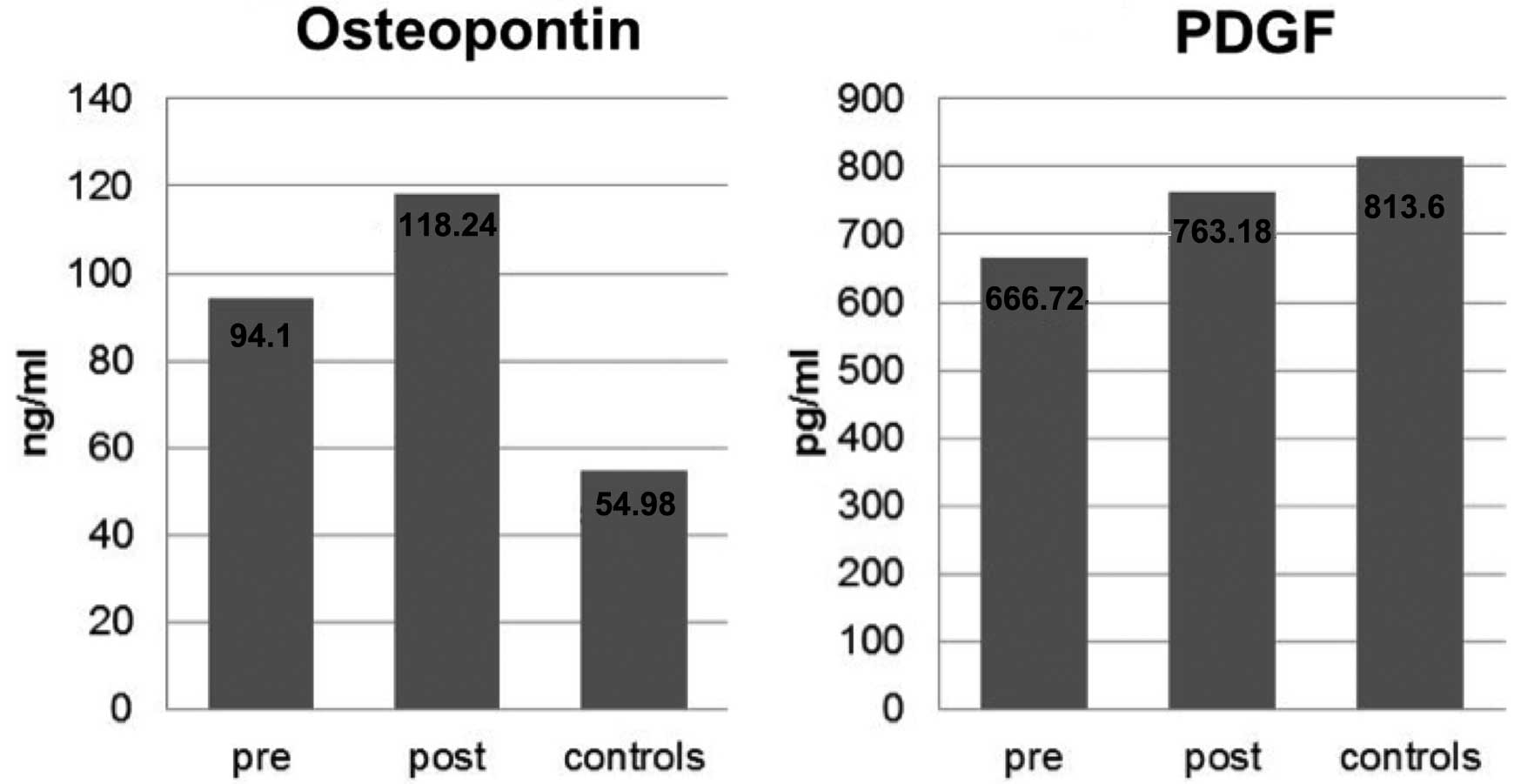
| Osteopontin
(ng/ml) | 94.10±38.96 | 54.98±20.97 | 24.13±46.54 | 0.0003b | 0.06 |
| PDGF (pg/ml) | 666.72±789.74 | 813.60±819.68 | 96.46±480.68 | 0.4300 | 0.26 |
| VEGF (pg/ml) | 349.05±403.61 | 215.08±208.39 | −139.26±405.56 | 0.9100 | 0.31 |
| EGFR (ng/ml) | 49.06±15.99 | 63.01±12.78 | 1.90±18.86 | 0.0005b | 0.43 |
| IL-4 (pg/ml) | 2.42±0.81 | 1.37±0.63 | −0.16±0.90 | 0.0001b | 0.85 |
| IL-6 (pg/ml) | 17.01±25.62 | 5.35±17.89 | 15.66±46.50 | 0.0001b | 0.03b |
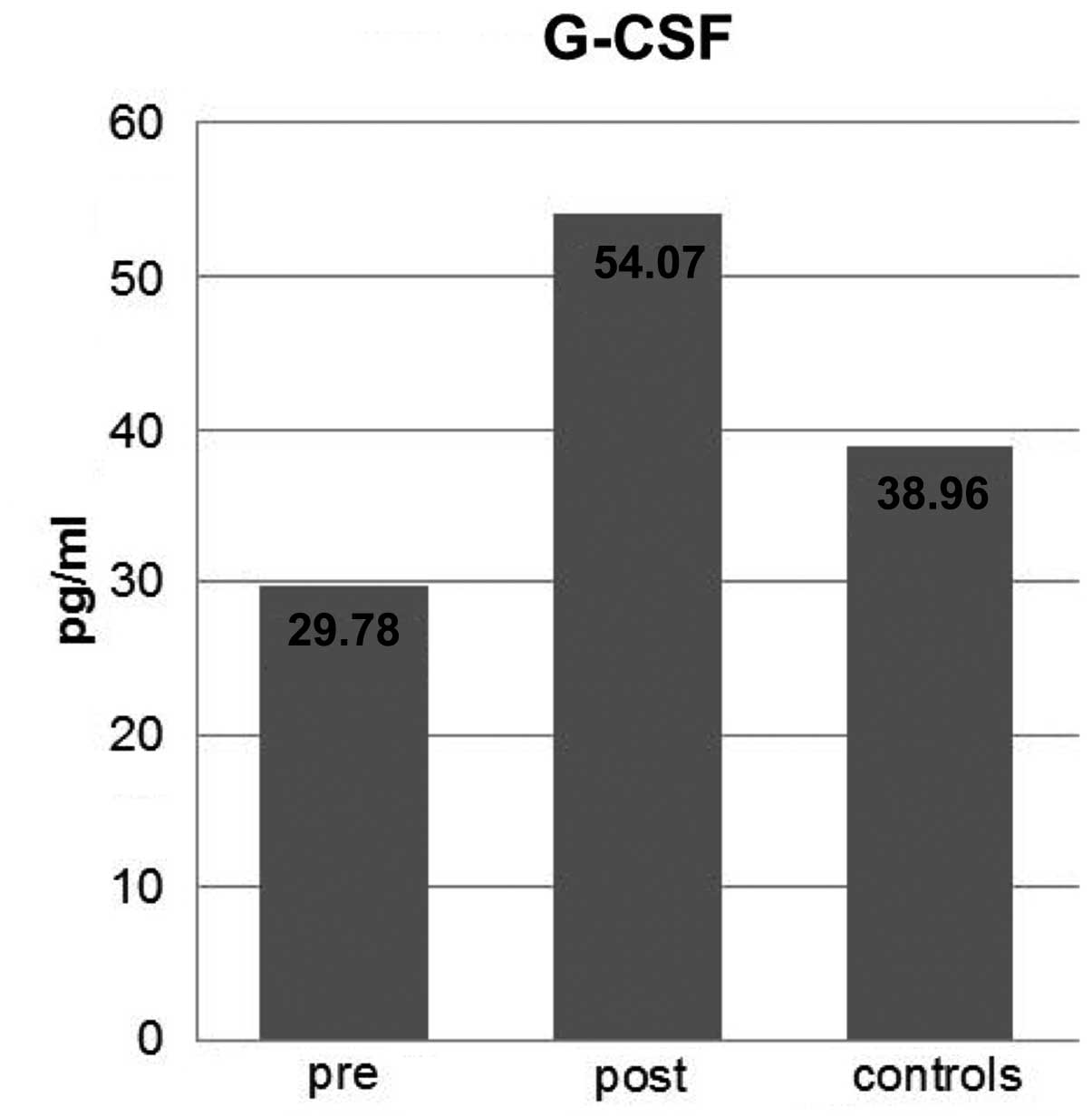
| G-CSF (pg/ml) | 29.79±10.83 | 38.96±51.27 | 24.29±81.40 | 0.9100 | 0.06 |
IL-6
Consistent with the results for IL-4, a significant
increase of IL-6 levels in patients, P=0.0001, was found when
comparing the IL-6 serum levels of patients and controls. The mean
serum levels in the patient group (17.01±26.62 pg/ml) were three
times those of the control group (5.35±17.89 pg/ml). A significant
therapy-associated alteration of IL-6 (P=0.0300) was also shown in
the patient group. The patients showed significantly elevated IL-6
serum levels (Table I and Fig. 1) following radiochemotherapy, with
an average increase of 15.66±46.50 pg/ml.
Osteopontin
The osteopontin levels in the patient and control
samples showed distinct differences. The mean pre-therapeutic value
of the patient group was 94.10±38.96 ng/ml, while the mean value of
the control group was 54.98±20.97 ng/ml, and a statistical
comparison of the groups revealed significantly higher osteopontin
levels in patients (P=0.0003). Following radiochemotherapy, the
patients showed a discrete but not significant increase of
osteopontin levels (24.13±46.54 ng/ml; P=0.0600) (Table I and Fig. 2).
PDGF
The levels of PDGF for the patient group were
heterogeneous and unevenly distributed, with a mean value of
666.72±789.74 pg/ml. The mean value of the control group was
813.60±819.68 pg/ml. Statistical comparison of the groups showed
P=0.4300. Following therapy, a mean increase of 96.46±480.68 pg/ml
was measured in the patient group (Table I and Fig. 2). The P-value for the comparison of
pre- and post-therapeutic levels was not significant
(P=0.2600).
VEGF
The results were inhomogeneous, with certain
patients showing marked increases ≤444 pg/ml and others showing
declines of 915 pg/ml following treatment. No reproducible tendency
could be detected. As shown in Table
I and Fig. 3, the mean
concentration of VEGF decreased from 349.05±393.39 to 209.79±261.79
pg/ml following treatment. A statistically significant result was
not exhibited for the statistical comparison of VEGF concentrations
in patient serum prior and subsequent to treatment (P=0.3100).
Furthermore, the comparison of controls and tumor patients was not
statistically significant (P=0.9100). Consequently, a statistically
significant result could not be stated for either the comparison of
VEGF levels prior and subsequent to multimodal treatment or for the
comparison of the levels in healthy controls and patients with
HNSCC.
EGFR
A homogenous distribution was found for the EGFR
concentration in the tumor patients and control groups. The mean
value of the control group (63.01±12.62 ng/ml) was significantly
higher than the mean pre-therapeutic value of the patient group
(49.06±15.58 ng/ml). Using the t-test for comparison of patients
and controls, P=0.0005. In terms of the therapeutic process, the
patient group presented with a mean difference of 1.90±18.86 ng/ml
(data shown in Table I and Fig. 3). Statistical analysis of pre- and
post-therapeutic results showed that P=0.4300 for therapy-induced
concentration changes. Thus, no significant changes in EGFR were
observed during therapy.
G-CSF
The mean G-CSF concentration values for the patient
(pre-therapy) and control groups were 29.79±10.83 and 38.96±51.27
pg/ml, respectively. However, no significant difference was
identified between these two groups in terms of G-CSF concentration
(P=0.9100). Following therapy, an increase of 24.29±81.40 pg/ml was
measured in the patient group. The P-value for therapy-associated
changes was 0.0600. Neither the differences between the pre- and
post-therapy levels nor the comparison with the control group were
significant (data shown in Table I
and Fig. 4).
Logistic regression
For multivariate analysis, logistic regression was
performed with osteopontin (P=0.0003) and IL-4 (P=0.0001) to
compare patients and controls (Table
I). Using these results, a formula was generated in which the
osteopontin and IL-4 levels of a random patient could be calculated
(Fig. 5). With this formula, an
individual risk figure (from 0=low risk to 1=high risk) for the
emergence of HNSCC can be created, including a corresponding Youden
index (sensitivity + specificity − 1), which is applied as a marker
for the quality of each test. The Youden index may be a value
between −1 and +1, it is reasonable to apply a diagnostic test when
the value is between 0 and +1. The closer the Youden index is to
+1, the higher the diagnostic quality of a test. Thus, the higher
the Youden index of a patient, the higher the likelihood for
developing HNSCC depending on the individual serum levels of the
two combined markers (osteopontin and IL-4). The higher the Youden
index, the more reliable the generated risk figure (34).
The results of the formula in Fig. 5 were used to create a receiver
operating characteristic (ROC) curve in which sensitivity and
1-specificity were opposed (see Fig.
6). For each risk figure and corresponding Youden index, the
curve shows the association between sensitivity and specificity and
could aid in the diagnosis for each patient.
Discussion
The present pilot study was performed to assess the
validity of seven serological factors as biomarkers for malignant
tumors of the head and neck. Furthermore, the study sought to
investigate whether there are significant serum concentration
differences of the analyzed factors between patients with HNSCC
pre-therapeutic and healthy controls, and whether these markers are
valid to indicate a neoplastic process of the head and neck as a
screening instrument in primary diagnostic algorithms. Until now,
tumor size and location, lymph node invasion, extracapsular spread
and metastatic disease define the individual tumor prognosis
without taking into account the heterogeneous tumor biology of the
tumor entity (12). As another aim
of the study, whether concomitant radiochemotherapy with cisplatin
or carboplatin and 5-FU induces significant alterations of the
serological levels of the seven surrogate markers compared with
pre-therapeutic expression levels was examined.
Although there was no significant association with
clinical and pathological parameters, two independent studies
showed that patients with HNSCC present with higher levels of IL-4
compared with healthy controls (15,17).
Klein (35) contradicted the study
by Mojtahedi et al (17) and
stated that IL-4 is not suitable for use as an HNSCC-screening
marker. Therefore, the results concerning IL-4 are inconclusive.
The results of the present study showed a significant
tumor-associated increase of IL-4 when comparing patients with
HNSCC and controls (P=0.0001), but unlike Mojtahedi et al
(17), a significant decrease of
IL-4 post-therapeutic (P=0.8500) was not found. Therefore, IL-4
appeared to be able to indicate a neoplastic process but was
insufficient for monitoring the therapy response.
Wang et al (24) showed that patients with HNSCC
present with increased IL-6 and IL-6 receptor levels compared with
a healthy control group. Similarly, the present study documented an
association between IL-6 levels, tumor size and histological
grading (24). To identify IL-6 as
a potential biomarker for HNSCC, Sato et al (36) proposed post-therapeutic saliva
analysis for early detection of relapses. The results of the
present study are consistent with the findings of Wang et al
(24). IL-6 was significantly
elevated in the patient serum (P=0.0001). However, a significant
increase of IL-6 levels was also detected following therapy
(P=0.0300). Therefore, IL-6 appears to be a suitable serological
biomarker for malignant tumors of the head and neck. Clearly,
therapy response cannot be indicated by IL-6 as the expression
levels do not decrease following therapy.
Both Snitcovsky et al (37) and Weber et al (26) reported a significant correlation
between the serological osteopontin concentration and tumor stage
(26,37). Contrary to this, Lim et al
(27) could not verify a
correlation between elevated osteopontin levels in patients with
HNSCC and a decreased overall survival rate or reduced therapy
response (27). To a certain
extent, the results of the present study confirmed the findings of
Snitcovsky et al (37) who
postulated greater levels of osteopontin in patients with advanced
tumor stage. A significantly higher expression level was shown in
the patient group compared with the chemotherapy-naive control
group. During therapy, the patients in the study by Snitcovsky
et al (37) presented with a
mean decline of 14.5 ng/ml. By contrast, the patients in the
present study showed an increase of 24.14±45.36 ng/ml following
therapy (P=0.0600). These results found osteopontin to be
potentially applicable for clinical use as a marker for tumor
screening. However, osteopontin appeared to be unsuitable for use
as a therapy response marker, as the results showed no significant
changes in serum levels following radiochemotherapy with curative
intent.
According to a study by Thariat et al
(38), an overexpression of EGFR is
detectable in 90% of all HNSCC cases and is associated with a poor
overall survival rate. Regarding therapy-induced EGFR changes in
patients, Bergler and Bier (39)
recorded a 30% decline in therapy response among patients receiving
platinum-based chemotherapy. On the contrary, the control group in
the present study showed higher expression levels of EGFR compared
with the patient group. However, a pathological overexpression of
EGFR in oncologic patients could not be confirmed and, in fact, the
opposite was true. Furthermore, a decrease of EGFR or any other
significant therapy-associated changes was not found following
treatment (P=0.4300). Therefore, EGFR cannot be recommended for use
as either a biomarker or a screening parameter. This is in contrast
to the findings of Riedel et al (40) who reported a downregulation of VEGF
and endothelial cell migration following EGFR-targeted therapy.
In a multitude of neoplasmas, including breast, lung
and head and neck cancer, an overexpression of VEGF has been
detected previously (8). Various
studies have been published on the correlation between
tumor-node-metastasis (TNM) staging and VEGF levels. A study by
Boonkitticharoen et al (9)
showed a significant correlation between TNM staging and VEGF
levels, however, a study by Riedel (11) did not. The results of the present
study did not show a significant difference in the VEGF serum
levels of patients prior or subsequent to treatment (P=0.3100). Nor
was a difference between patients and controls detected (P=0.9100).
Based on these results, VEGF serum levels cannot be recommended as
a prognostic parameter.
Palmer et al (13) showed that patients with HNSCC have
significantly higher PDGF levels compared with a control group,
however, similar results were not stated in the present study. In
the patient and control groups, the PDGF levels were heterogeneous
and unevenly distributed. Therefore, significant results were not
shown for either the comparison of patients and controls (P=0.4300)
or for therapy-associated changes of the patient group (P=0.2600).
Considerable discrepancies were found between the mean values of
patients and controls in the present study and the study by Palmer
et al (13). The results of
the present study revealed mean values of 813.60±809.36 pg/ml in
controls and 666.72±769.74 pg/ml in patients, while Palmer et
al revealed mean values of 1,708.52 pg/ml in controls and
5,945.28 pg/ml in patients. Both studies used ELISA for the
detection of PDGF. However, the results of the study by Palmer
et al exceed the present study by nine-fold, which is a
remarkable difference. Based on the present study results, it can
be concluded that PDGF is not suitable as a biomarker for HNSCC or
for the analysis of therapy response.
In HNSCC, G-CSF stimulates proliferation and
migration of tumor and inflammatory cells. In contrast to
G-CSF-negative tumors, G-CSF-positive tumors are distinctively
invasive of the bone and cartilage tissues (31). Besides HNSCC, lung, uterus and
hepatocellular carcinomas present with elevated G-CSF levels and
are associated with a poor outcome (41–43).
The present study revealed approximately the same mean values in
the patient and control groups. There was no significant difference
in the comparison of the groups (P=0.9100) and elevated serum
levels following therapy (P=0.0600) were not significant. Based on
these results, G-CSF is not suitable as a screening marker or as a
marker for therapy-induced alterations of the serological marker
signature.
In conclusion, the present pilot study revealed a
significant correlation between three serological markers
(osteopontin, IL-4 and IL-6) and a histopathologically confirmed
neoplasm of the head and neck. The comparison between serum samples
of tumor patients and the control group showed significantly
elevated serum levels of osteopontin, IL-4 and IL-6. Therefore,
these markers could be a suitable tool in the primary diagnostic
algorithm of a head and neck tumor (screening instrument). Only
IL-6 showed a significant difference (an increase) in the
expression levels post-therapeutically. Thus, none of the markers
may be used as an indicator of treatment response, since a
reduction of the elevated expression levels would be expected
following sufficient therapy. Taking into account the clinically
observed post-therapeutic local and regional tumor control of the
tumor patient collective, the present study failed to identify a
serological multi-marker strategy as sufficient to monitor
treatment success and predict the individual prognosis of tumor
disease.
Logistic regression facilitates the calculation of
the individual risk for HNSCC using osteopontin and IL-4. By using
the results of the present study with the formula (Fig. 5), a ROC curve was created in which
sensitivity and 1-specificity were opposed (see Fig. 6). For each risk figure and
corresponding Youden index, the curve shows the association between
sensitivity and specificity. This can be observed as a
quantification of test quality for the screening of HNSCC. The
suitability of this procedure for clinical use requires
investigation in clinical trials. Based on these results, a
serological multi-marker strategy for screening diagnosis and
follow-up requires further evaluation. IL-4, IL-6 and osteopontin
appeared to be suitable as screening parameters in the diagnosis of
HNSCC. However, none of these parameters were sufficient for
indicating the therapy response as the possible markers for
screening and diagnosis that showed elevated levels in tumor
patients did not reveal a consistent decrease following sufficient
therapy.
Acknowledgements
The authors would like to thank Mrs. Petra Prohaska
for her outstanding technical assistance and Dr C Weiss for the
distinguished advice in the statistical analysis.
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
EGF receptor
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
G-CSF
|
granulocyte-colony stimulating
factor
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
IL
|
interleukin
|
|
PDGF
|
platelet-derived growth factor
|
|
ROC
|
receiver operating characteristic
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics 2008. CA Cancer J Clin. 58:71–96. 2008.
|
|
2
|
Blot WJ, McLaughlin JK, Winn DM, et al:
Smoking and drinking in relation to oral and pharyngeal cancer.
Cancer Res. 48:3282–3287. 1988.
|
|
3
|
Riedel F and Hörmann K: Alcohol related
diseases of the head and neck. HNO. 52:590–598. 2004.(In
German).
|
|
4
|
Petti S: Lifestyle risk factors for oral
cancer. Oral Oncol. 45:340–350. 2009.
|
|
5
|
Brugere J, Guenel P, Leclerc A and
Rodriguez J: Differential effects of tobacco and alcohol in cancer
of the larynx, pharynx, and mouth. Cancer. 57:391–395. 1986.
|
|
6
|
Dietz A and Wichmann G: Translational
research in head and neck cancer. Biological characteristics and
general aspects. HNO. 59:874–884. 2011.(In German).
|
|
7
|
Sugiura T, Inoue Y, Matsuki R, et al:
VEGF-C and VEGF-D expression is correlated with lymphatic vessel
density and lymph node metastasis in oral squamous cell carcinoma:
Implications for use as a prognostic marker. Int J Oncol.
34:673–680. 2009.
|
|
8
|
Ninck S, Reisser C, Dyckhoff G, Helmke B,
Bauer H and Herold-Mende C: Expression profiles of angiogenic
growth factors in squamous cell carcinomas of the head and neck.
Int J Cancer. 106:34–44. 2003.
|
|
9
|
Boonkitticharoen V, Kulapaditharom B,
Leopairut J, et al: Vascular endothelial growth factor a and
proliferation marker in prediction of lymph node metastasis in oral
and pharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck
Surg. 134:1305–1311. 2008.
|
|
10
|
Brieger J, Schroeder P and Mann WJ:
Vascular endothelial growth factor and basic fibroblast growth
factor are secreted by squamous cell carcinoma cell lines after
radiotherapy and induce resistance to radiation in vitro. GMS Curr
Posters Otorhinolaryngol Head Neck Surg. 1:932005.(In German).
|
|
11
|
Riedel F: Expression of VEGF and
inhibition of tumor angiogenesis by abrogation of VEGF in head and
neack cancer. Laryngorhinootologie. 82:436–437. 2003.(In
German).
|
|
12
|
Montag M, Dyckhoff G, Lohr J, et al:
Angiogenic growth factors in tissue homogenates of HNSCC:
expression pattern, prognostic relevance, and interrelationships.
Cancer Sci. 100:1210–1218. 2009.
|
|
13
|
Palmer B, Bran GM, Hörmann K and Riedel F:
Analysis of the serum concentration of PDGF (-AB) in patients with
HNSCC. Presented at 78. German Society for Otorhinolaryngology,
Head and Neck Surgery Congress; 2007; http://www.egms.de/stat-ic/de/meetings/hnod2007/07hnod458.shtml.
(In German).
|
|
14
|
Hofmann TK: Immunotherapy of head and neck
cancer. Identification of a novel mechanism for anti-EGFR mAb
anti-tumor effects. HNO. 59:224–229. 2011.(In German).
|
|
15
|
de Oliveira MV, Fraga CA, Gomez RS and
Paula AM: Immunohistochemical expression of interleukin-4, -6, -8
and -12 in inflammatory cells in surrounding invasive front of oral
squamous cell carcinoma. Head Neck. 31:1439–1446. 2009.
|
|
16
|
Myers JN, Yasumura S, Suminami Y, et al:
Growth stimulation of human head and neck squamous cell carcinoma
cell lines by interleukin 4. Clin Cancer Res. 2:127–135. 1996.
|
|
17
|
Mojtahedi Z, Khademi B, Yehya A, et al:
Serum levels of interleukins 4 and 10 in head and neck squamous
cell carcinoma. J Laryngol Otol. 126:175–179. 2012.
|
|
18
|
Obiri NI, Hillman GG, Haas GP, et al:
Expression of high affinity interleukin-4 receptors on human renal
cell carcinoma cells and inhibition of tumor cell growth in vitro
by interleukin-4. J Clin Invest. 91:88–93. 1993.
|
|
19
|
Obiri NI, Siegel JP, Varricchio F and Puri
RK: Expression of high-affinity IL-4 receptors on human melanoma,
ovarian and breast carcinoma cells. Clin Exp Immunol. 95:148–155.
1994.
|
|
20
|
Morisaki T, Yuzuki DH, Lin RT, et al:
Interleukin 4 receptor expression and growth inhibition of gastric
carcinoma cells by interleukin 4. Cancer Res. 52:6059–6065.
1992.
|
|
21
|
Volpert OV, Fong T, Koch AE, et al:
Inhibition of angiogenesis by interleukin 4. J Exp Med.
188:1039–1046. 1998.
|
|
22
|
Yamaji H, Iizasa T, Koh E, et al:
Correlation between interleukin 6 production and tumor
proliferation in non-small cell lung cancer. Cancer Immunol
Immunother. 53:786–792. 2004.
|
|
23
|
Riedel F, Zaiss I, Herzog D, et al: Serum
levels of interleukin-6 in patients with primary head and neck
squamous cell carcinoma. Anticancer Res. 25:2761–2765. 2005.
|
|
24
|
Wang YF, Chang SY, Tai SK, et al: Clinical
significance of interleukin-6 and interleukin-6 receptor
expressions in oral squamous cell carcinoma. Head Neck. 24:850–858.
2002.
|
|
25
|
Lu JG, Li Y and Kan X: Overexpression of
osteopontin and integrin αv in laryngeal and hypopharyngeal
carcinomas associated with differentiation and metastasis. J Cancer
Res Clin Oncol. 137:1613–1618. 2011.
|
|
26
|
Weber GF, Lett GS and Haubein NC:
Osteopontin is a marker for cancer aggressiveness and patient
survival. Br J Cancer. 103:861–869. 2010.
|
|
27
|
Lim AM, Rischin D, Fisher R, et al:
Prognostic significance of osteopontin in patients with
locoregionally advanced head and neck squamous cell carcinoma
treated on TROG 02.02 phase III trial. Clin Cancer Res. 18:301–307.
2012.
|
|
28
|
Wang HH, Wang XW and Tang CE: Osteopontin
expression in nasopharyngeal carcinoma: its relevance to the
clinical stage of the disease. J Cancer Res Ther. 7:138–142.
2011.
|
|
29
|
Tlsty TD: Stromal cells can contribute
oncogenic signals. Semin Cancer Biol. 11:97–104. 2001.
|
|
30
|
Mroczko B and Szmitkowski M: Hematopoietic
cytokines as tumor markers. Clin Chem Lab Med. 42:1347–1354.
2004.
|
|
31
|
Gutschalk CM, Herold-Mende CC, Fusenig NE
and Mueller MM: Granulocyte colony-stimulating factor and
granulocyte-macrophage colony-stimulating factor promote malignant
growth of cells from head and neck squamous cell carcinomas in
vivo. Cancer Res. 66:8026–8036. 2006.
|
|
32
|
van Warmerdam LJ, Rodenhuis S, ten Bokkel
Huinink WW, et al: The use of the Calvert formula to determine the
optimal carboplatin dosage. J Cancer Res Clin Oncol. 121:478–486.
1995.
|
|
33
|
Wittekind C: 2010 TNM system: on the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German).
|
|
34
|
Zhou XH, Obuchowski NA and McClish DK:
Measures of diagnostic accuracy. Statistical Methods in Diagnostic
Medicine. Wiley J; Hoboken, NJ: pp. 23–26. 2011
|
|
35
|
Klein F: Interleukins give poor evidence.
J Laryngol Otol. 126:175–179. 2012.(In German).
|
|
36
|
Sato J, Ohuchi M, Abe K, et al:
Correlation between salivary interleukin-6 levels and early
locoregional recurrence in patients with oral squamous cell
carcinoma: preliminary study. Head Neck. 35:889–894. 2013.
|
|
37
|
Snitcovsky I, Leitão GM, Pasini FS, et al:
Plasma osteopontin levels in patients with head and neck cancer
undergoing chemoradiotherapy. Arch Otolaryngol Head Neck Surg.
135:807–811. 2009.
|
|
38
|
Thariat J, Yildirim G, Mason KA, et al:
Combination of radiotherapy with EGFR antagonists for head and neck
carcinoma. Int J Clin Oncol. 12:99–110. 2007.
|
|
39
|
Bergler W and Bier H: Cisplatin reduces
epidermal growth factor receptors in squamous-cell carcinoma in
vitro. Preliminary results. ORL J Otorhinolaryngol Relat Spec.
52:297–302. 1990.
|
|
40
|
Riedel F, Götte K, Li M, et al: EGFR
antisense treatment of human HNSCC cell lines down-regulates VEGF
expression and endothelial cell migration. Int J Oncol. 21:11–16.
2002.
|
|
41
|
Pei XH, Nakanishi Y, Takayama K, et al:
Granulocyte, granulocyte-macrophage, and macrophage
colony-stimulating factors can stimulate the invasive capacity of
human lung cancer cells. Br J Cancer. 79:40–46. 1999.
|
|
42
|
Nasu K, Inoue C, Takai N, et al: Squamous
cell carcinoma of the cervix producing granulocyte
colony-stimulating factor. Obstet Gynecol. 104:1086–1088. 2004.
|
|
43
|
Snyder RA, Liu E and Merchant NB:
Granulocyte colony stimulating factor secreting hepatocellular
carcinoma. Am Surg. 78:821–822. 2012.
|