Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1). Recent
studies on personalized treatment, conducted by selecting patients
who are likely to respond to a particular therapeutic agent, may
allow improved treatment efficacy. Activated mutations of the EGFR
gene are normally located in exons 18 to 21, >90% of which
consist of deletions in exon 19 and L858R substitution in exon 21
(2). Patients with non-small cell
lung cancer (NSCLC) harboring mutations in the epidermal growth
factor receptor (EGFR) gene exhibit a significant response to
EGFR-tyrosine kinase inhibitors (TKIs) (3,4).
Clinical trials have demonstrated that gefitinib improves
progression-free and overall survival in the treatment of NSCLC
(5). Gefitinib is now approved for
these indications (6). Gefitinib
has also been proposed for the treatment of patients with locally
advanced or metastatic NSCLC with EGFR-activating mutations
(7), which targets the tyrosine
kinase (TK) domain of EGFR, inhibiting the downstream signaling
processes for growth and proliferation. Mutations in the EGFR gene
may also affect the behavior of the receptor and its response to
inhibitors.
The majority of NSCLC patients with EGFR mutations
initially benefit favorably from treatment with gefitinib,
suggesting that these mutations promote tumorigenesis. However,
among tumors that initially respond to EGFR-TKIs, the majority of
patients eventually acquire resistance, often due to the emergence
of a secondary mutation, such as T790M, in the kinase domain of
EGFR (8). Patients with both L858R
and T790M EGFR mutations are extremely rare (9). Written informed consent was obtained
from the patient’s family.
Case report
A 77-year-old male with a history of smoking was
admitted to the Department of Interventional Radiology, The First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) in
November 2011 due to an abnormal shadow in the right upper field
following a chest X-ray. Physical examination revealed no
significant abnormalities; however, computed tomography (CT) of the
chest revealed a tumor measuring 66×74×80 mm in size in the right
S1+2 with multiple lung and bone metastases (cT3N3M1; stage IV),
according to the TNM classification (10). A transbronchial lung biopsy (TBLB)
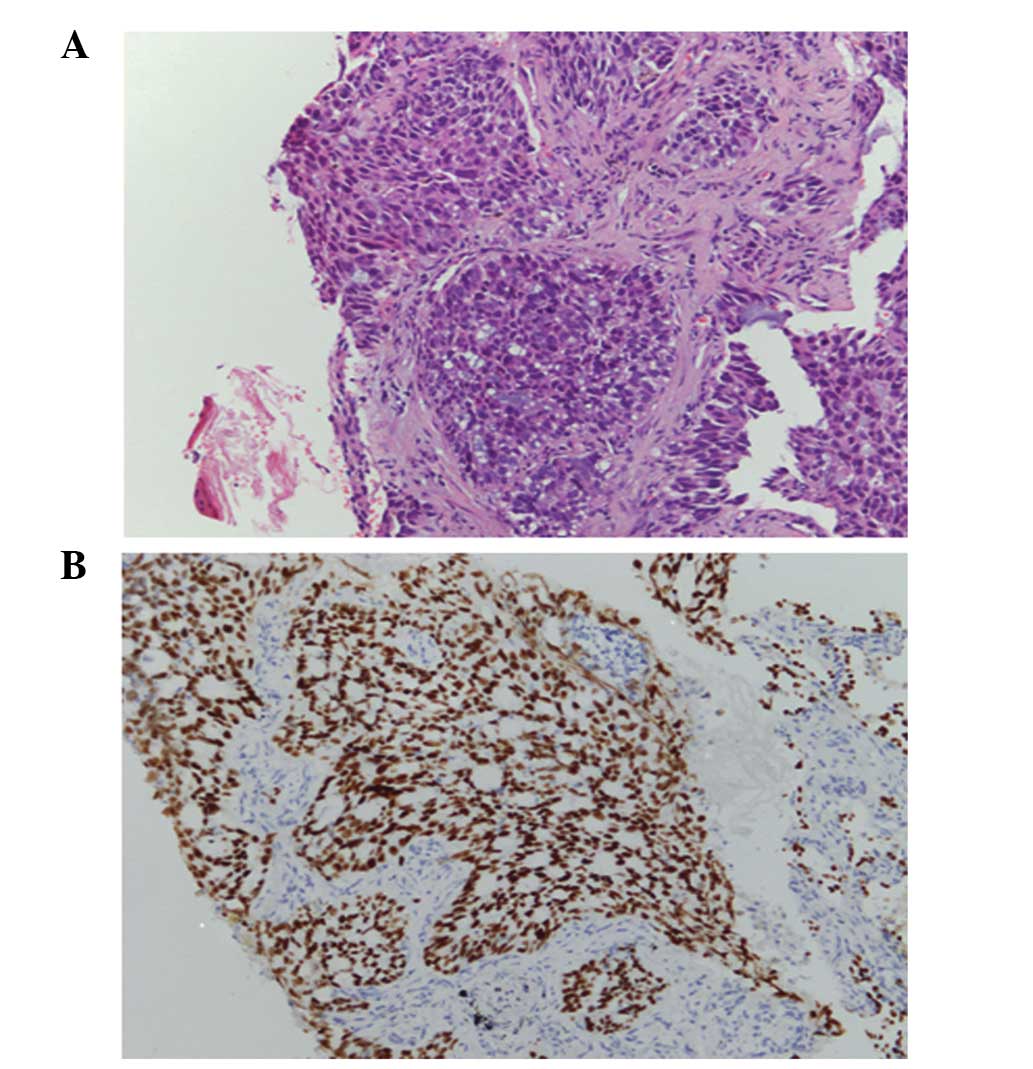
was conducted and the pathological diagnosis of the TBLB specimen
was acinar adenocarcinoma (Fig.
1A). Immunohistochemical staining was positive for
transcription factor-1 protein (Fig.
1B). Laboratory findings were within the normal range, with the
exception of the carcinoembryonic antigen (CEA) level of 12.65
ng/ml (normal range, 0–3.4 ng/ml) in the serum. A diagnosis of lung
adenocarcinoma was determined and the patient was treated with
first-line chemotherapy consisting of cisplatin (80
mg/m2) and docetaxel (60 mg/m2), every three
weeks for up to three cycles. However, no marked response was
observed.
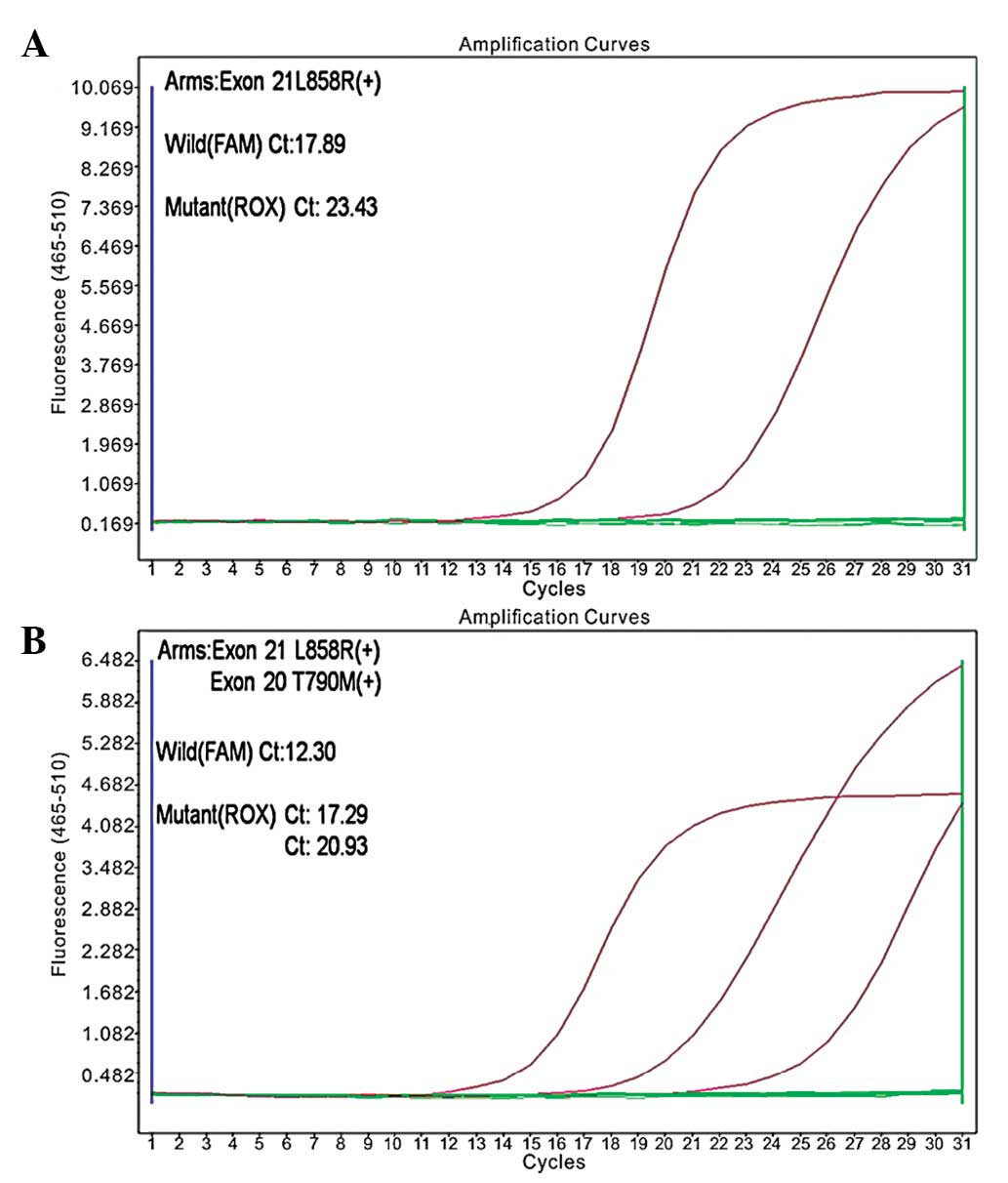
Following the initial treatment, a mutation in the
EGFR gene was identified (exon 21; L858R, in which the leucine at
amino acid position 858 is replaced by arginine; Fig. 2A). The second-line chemotherapy was
gefitinib (250 mg) once a day, administered between March and July
2012. The gefitinib therapy was effective, and no adverse events
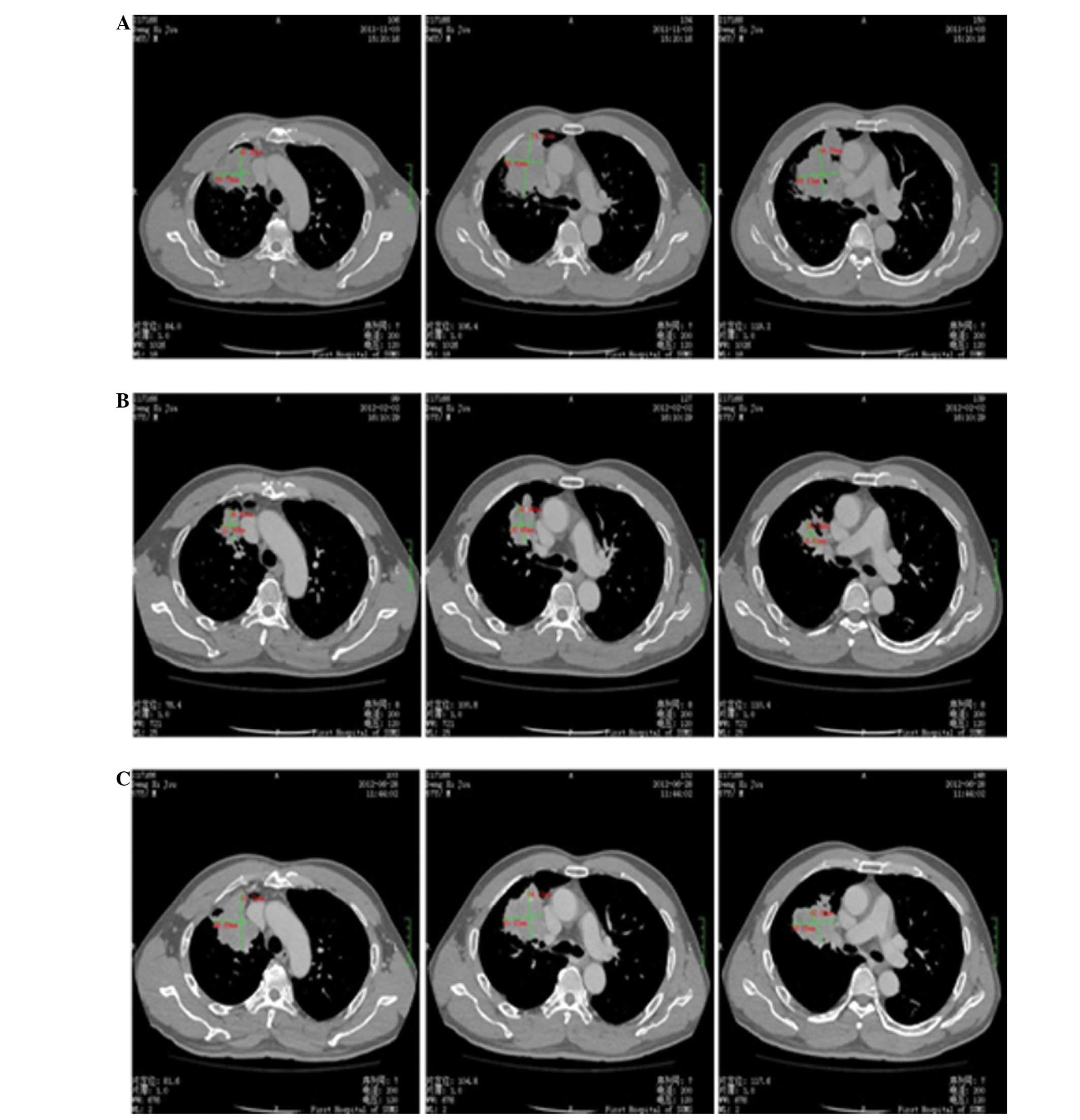
were reported. A CT scan of the thorax was performed in May 2012,
which revealed residual disease in the right lung (51×72×51 cm in
diameter) and few metastatic bone lesions (Fig. 3).
In November 2012, a further CT scan revealed a
number of new lesions (one in the right lung, and several in the
bone and brain), indicating disease progression. The patient was
subjected to a rebiopsy to detect EGFR mutations, with analysis by
the amplification refractory mutation system. L858R and T790M point
mutations were detected in the tumor cells (Fig. 2B). Subsequently, the patient
underwent three cycles of third-line chemotherapy (150 mg erlotinib
per day for three months); however, further metastases emerged in
the brain and, therefore, palliative care was administered in May
2013. The gefitinib therapy was discontinued to introduce the
third-line chemotherapy, which induced an infusion reaction, and no
remarkable response was observed. Following discontinuation of the
third-line chemotherapy, the tumor growth induced empyema and the
patient’s general condition gradually deteriorated and the patient
succumbed to the disease in August, 2013.
Discussion
It has been demonstrated that the majority of
patients with lung cancer that are responsive to EGFR-TKIs harbor
activating mutations in the TK domain of EGFR (11–13).
This further supports the hypothesis that the identification of
genetic signatures associated with oncogenic alterations may serve
as predictive biomarkers for corresponding molecular target
inhibitors. In comparison with smokers, EGFR mutations have
consistently been found to be more common in non-smokers (14). In the present study, the patient did
not have a history of smoking. Histopathologically, mutation rates
among adenocarcinoma are predominantly higher than those in
squamous cell lung carcinomas (15). The diagnosis of squamous cell
carcinoma or adenocarcinoma is based on histomorphological grounds
in cases where the appearances are characteristic; additionally,
immunohistochemical staining is performed using antibodies against
TTF-1, p63, M-CEA and CK. Immunohistochemical nuclear expression of
TTF-1 also confirms a primary pulmonary origin. Furthermore,
M-CEA-positive and p63-negative tumor cells indicate a glandular
epithelium origin (16).
According to the Food and Drug Administration
regulations, EGFR inhibitors have been approved as the first-line
treatment for advanced NSCLC patients positive for EGFR-activating
mutations (17). However, EGFR
inhibitors are not used to treat patients with wild-type EGFR and,
by contrast, a poor outcome has been observed in response to the
treatment (18,19). Activating mutations of the EGFR gene
are predominantly located in exons 18–21 and >90% are deletions
in exon 19 or the L858R substitution in exon 21. These activating
mutations are eligible for treatment with modern TKIs, for example
gefitinib (20–22). Therefore, the accurate detection of
EGFR mutations is critical for determining the efficacy in the
adoption of gefitinib for advanced NSCLC in any given population.
In the current study, the L858R point mutation of exon 21 was
detected in the tumor cells, and an effective and curative outcome
was observed following treatment with gefitinib. However, after
several months, a CT scan revealed new lesions in the brain,
indicating disease progression. The L858R point mutation of exon 21
and a compound T790M EGFR substitution mutation were detected in
the tumor cells, which was consistent with the study by Pao et
al (23), indicating that the
efficacy of EGFR-TKIs in lung cancer is severely compromised by the
rapid emergence of targeted therapy-resistant clones within one
year.
In conclusion, the current study reports a rare case
of lung cancer harboring an L858R point mutation of exon 21 and a
compound T790M EGFR substitution mutation following treatment with
gefitinib. However, following the detection of the T790M EGFR
substitution mutation in the tumor cells, the patient exhibited
poor curative effect when treatment with gefitinib was continued.
Therefore, to improve the selection of optimal treatment regimens
in individual patients, further investigation into determining the
genetic causes of drug resistance at various points during the
clinical course is required.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (grant nos. 30900650/H1615,
81372501/H1615, 81172232/H1615 and 81172564/H1625), Guangdong
Natural Science Foundation (grant nos. S2012010008378 and
S2013010015327), the Research Fund for the Doctoral Program of
Higher Education of China (grant no. 20120171120086) and the
Science and Technology Planning Project of Guangdong Province
(grant no. 2012B061700078).
References
|
1
|
Langevin SM, Kratzke RA and Kelsey KT:
Epigenetics of lung cancer. Transl Res. March 12–2014.(Epub ahead
of print).
|
|
2
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006.
|
|
3
|
Xu L, Kikuchi E, Xu C, et al: Combined
EGFR/MET or EGFR/HSP90 inhibition is effective in the treatment of
lung cancers codriven by mutant EGFR containing T790M and MET.
Cancer Res. 72:3302–3311. 2012.
|
|
4
|
Witta SE, Jotte RM, Konduri K, et al:
Randomized phase II trial of erlotinib with and without entinostat
in patients with advanced non-small-cell lung cancer who progressed
on prior chemotherapy. J Clin Oncol. 30:2248–2255. 2012.
|
|
5
|
D’Incecco A and Cappuzzo F: Gefitinib for
non-small-cell lung cancer treatment. Expert Opin Drug Saf.
10:987–996. 2011.
|
|
6
|
Costanzo R, Piccirillo MC, Sandomenico C,
et al: Gefitinib in non small cell lung cancer. J Biomed
Biotechnol. 2011:8152692011.
|
|
7
|
Kobayashi T, Takeda M, Marumo S, Koshimo
Y, Teranishi T, Higami Y and Kato M: Long-term gefitinib treatment
of occult lung carcinoma with multiple brain metastases. Lung
Cancer. 80:109–111. 2013.
|
|
8
|
Pao W and Chmielecki J: Rational,
biologically based treatment of EGFR-mutant non-small-cell lung
cancer. Nat Rev Cancer. 10:760–774. 2010.
|
|
9
|
Tokumo M, Toyooka S, Ichihara S, et al:
Double mutation and gene copy number of EGFR in gefitinib
refractory non-small-cell lung cancer. Lung Cancer. 53:117–121.
2006.
|
|
10
|
Wrona A and Jassem J: The new TNM
classification in lung cancer. Pneumonol Alergol Pol. 78:407–417.
2010.(In Polish).
|
|
11
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004.
|
|
12
|
Sequist LV, Martins RG, Spigel D, et al:
First-line gefitinib in patients with advanced non-small-cell lung
cancer harboring somatic EGFR mutations. J Clin Oncol.
26:2442–2449. 2008.
|
|
13
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004.
|
|
14
|
Cortes-Funes H, Gomez C, Rosell R, et al:
Epidermal growth factor receptor activating mutations in Spanish
gefitinib-treated non-small-cell lung cancer patients. Ann Oncol.
16:1081–1086. 2005.
|
|
15
|
Weiss GJ, Liman AK, Allen J, et al:
Squamous cell carcinoma of the lung with metastasis to the GI tract
associated with EGFR exon 19 deletion. Case Rep Med.
2013:8748362013.
|
|
16
|
Fang W, Zhang J, Liang W, et al: Efficacy
of epidermal growth facor receptor-tyrosine kinase inhibitors for
Chinese patients with squamous carcinoma of lung harboring EGFR
mutation. J Thorac Dis. 5:585–592. 2013.
|
|
17
|
Khozin S, Blumenthal GM, Jiang X, et al:
U.S. Food and Drug Administration Approval Summary: Erlotinib for
the first-line treatment of metastatic non-small cell lung cancer
with epidermal growth factor receptor exon 19 deletions or exon 21
(L858R) substitution mutations. Oncologist. May 27–2014.(Epub ahead
of print).
|
|
18
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009.
|
|
19
|
Rosell R, Moran T, Queralt C, et al:
Spanish Lung Cancer Group: Screening for epidermal growth factor
receptor mutations in lung cancer. N Engl J Med. 361:958–967.
2009.
|
|
20
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: role in clinical
response to EGFR tyrosine kinase inhibitors. Oncogene. 28(Suppl 1):
S24–S31. 2009.
|
|
21
|
Shigematsu H and Gazdar AF: Mutations of
EGFR in lung cancers and their implications for targeted therapy.
Discov Med. 4:444–447. 2004.
|
|
22
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006.
|
|
23
|
Bean J, Riely GJ, Balak M, Marks JL,
Ladanyi M, Miller VA and Pao W: Acquired resistance to epidermal
growth factor receptor kinase inhibitors associated with a novel
T854A mutation in a patient with EGFR-mutant lung adenocarcinoma.
Clin Cancer Res. 14:7519–7525. 2008.
|