1. Introduction
Recent studies have shown that tumor necrosis (TN)
influences metastasis-free survival in patients exhibiting
neoplasms (1,2). In particular, TN has been reported to
indicate poor prognosis in lung (3), breast (4,5),
thyroid (6), colorectal (7,8),
pancreatic (9) and renal (10–16)
malignancies. Therefore, it has been proposed that the
presence/absence of TN must be indicated in any histopathological
report (12,14), as this type of assessment has a high
rate of reproducibility among pathologists (9,12).
It is generally accepted that TN is a result of
chronic ischemic injury due to rapid tumor growth. Jain and
Carmeliet (17) suggested that
intratumoral mechanical stresses, resulting from tumor cell
proliferation, cause focal large-vessel obstruction, leading to
ischemic intratumoral infarcts. The compression, exerted by
surrounding neoplastic cells on the microvasculature, is considered
to be spatially and temporally heterogeneous (18) and this may explain the uneven
distribution of TN. However, whether insufficient tumor
vascularization and inadequate tumor cell oxygenation are the only
factors causing TN remains controversial. We hypothesize that
hypoxia may indirectly induce coagulative necrosis in tumor cells
harboring p53 mutations via mitotic catastrophe. Mitotic
catastrophe is a cell death mechanism, which occurs as a result of
dysregulated/failed mitosis that may be accompanied by
morphological alterations, including micronucleation,
multinucleation and abnormal mitoses.
In this review, the morphologic features of TN in
malignant epithelial tumors are investigated. In addition, the
associations between hypoxia, mitotic catastrophe and TN are
briefly reviewed within the framework of our hypothesis.
2. Definition of apoptosis and TN
Depending on the lethal stimulus, tumor cells may
die as a result of distinct cellular death mechanisms, including
apoptosis and necrosis. The term ‘apoptosis’ was coined by Kerr
et al (19) to distinguish
the phenomenon as a mechanism of cell death that is morphologically
separate from coagulative necrosis. Ultrastructural features of
apoptosis include margination and condensation of chromatin,
nuclear fragmentation in apoptotic bodies (corresponding to the
histological terms, pyknosis and karyorrhexis), and ruffling of the
plasma membrane, which maintains integrity until the final stages
of the process (19–21). Phagocytosis of apoptotic bodies is
carried out by professional phagocytes, including macrophages and
dendritic cells, and non-professional ‘neighboring’ phagocytes,
including epithelial cells, endothelial cells, smooth muscle cells
and fibroblasts. By contrast, necrosis is characterized by cellular
swelling, which is accompanied by chromatin flocculation,
dilatation of the mitochondria and endoplasmic reticulum, plasma
membrane rupture and eventual shedding of the cytoplasmic contents
into the extracellular space, with subsequent inflammation
(20–21).
3. Detection of TN
According to the recommendations of the Nomenclature
Committee on Cell Death (NCCD) (21–23),
electron microscopy remains the ‘gold standard’ for identification
of the specific features of cells undergoing death. However, the
detection of cell death must be based on at least two techniques,
one to reveal morphological changes and the second to demonstrate
biochemical changes (21). For
example, pathologists use combined immunohistochemical methods and
light microscopy to identify dying necrotic cells. Histologically,
coagulative necrosis appears acellular and stains homogeneously
with red eosin. However, careful examination shows retention of the
general architectural pattern of the tissue, despite the death of
its constituent elements. Coagulative necrosis is also
characterized by an abrupt transition from viable to necrotic cells
without an interposed zone of granulation tissue or hyalinized
tissue between the viable and necrotic cells (21). Generally, these histological
observations are supplemented with electron microscopy images to
identify the morphological characteristics of dying necrotic cells.
In addition, Tdt-mediated dUTP nick end labeling (TUNEL) and
anti-active caspase-3 staining are often used to identify apoptotic
cell death (21). Usually, cells
that stain positively for TUNEL but negatively for active caspase-3
are considered to be necrotic (24). On the other hand, there are no
specific positive discriminative biochemical markers for the
detection of necrosis in vitro or in vivo. However,
it has been demonstrated that certain candidate necrotic
biomarkers, including high-mobility group box 1 protein and
cyclophilin A, are released by cells dying from secondary necrosis
following apoptosis (24).
4. Morphological variants of coagulative
TN
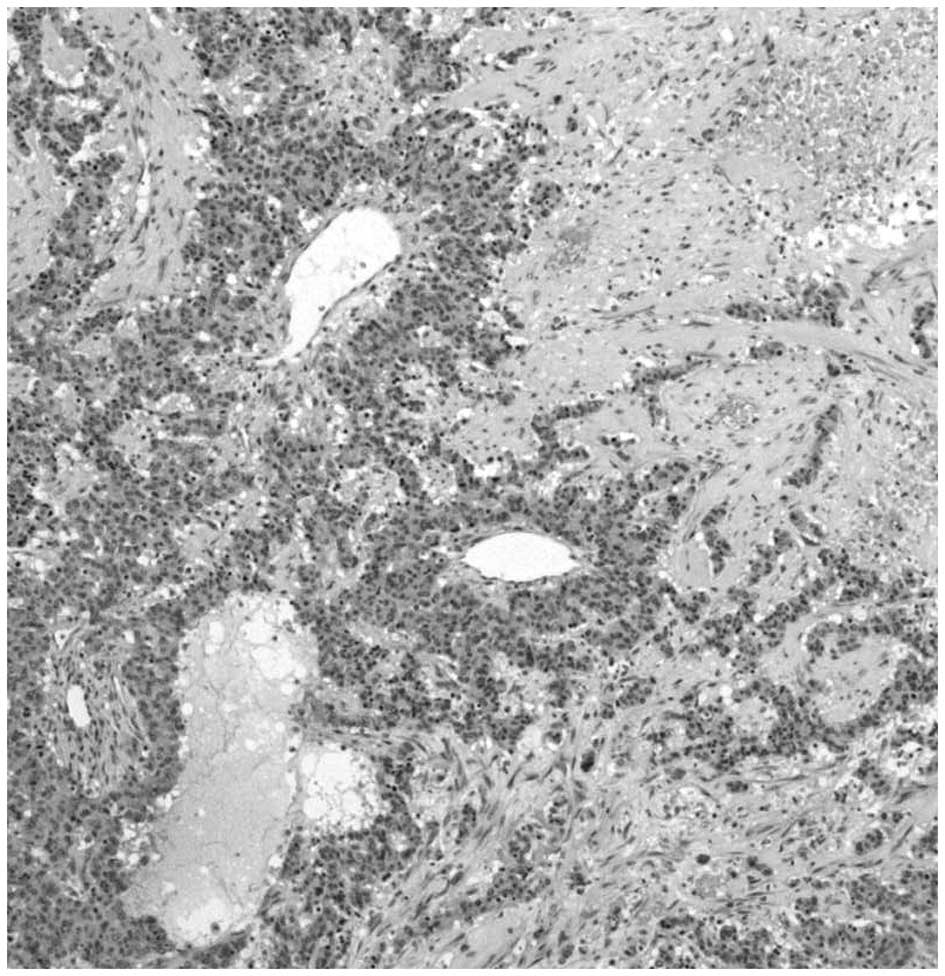
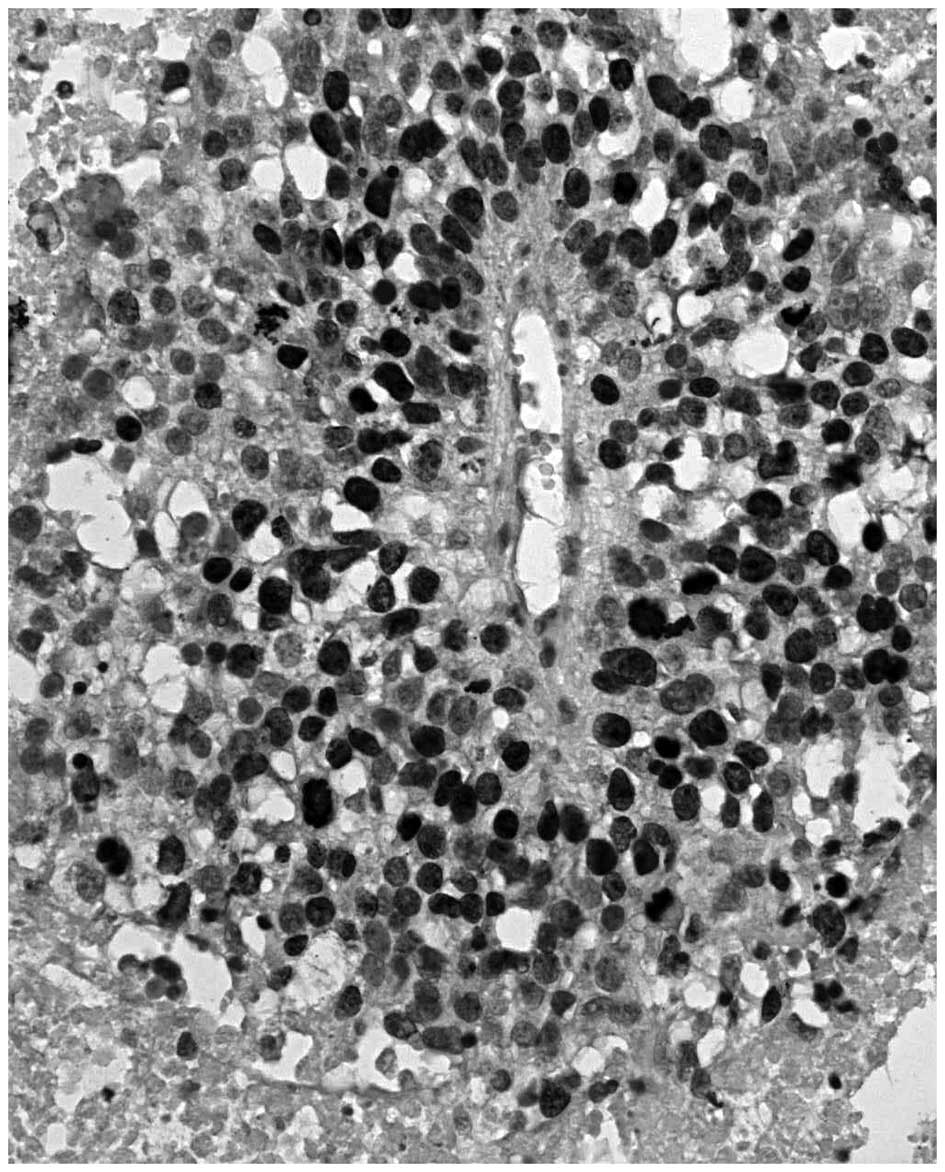
Peritheliomatous necrosis and comedo-type necrosis
may be considered as morphological variants of coagulative TN. The
term peritheliomatous necrosis refers to a microscopic pattern
which is characterized by large areas of coagulative necrosis with
sheets or cords of viable tumor cells surrounding a centrally
disposed blood vessel (Fig. 1)
(18). The term ‘comedo’ describes
the appearance of compressed ducts exuding necrotic material, often
observed in ductal carcinoma in situ (DCIS) of the breast,
which is a neoplastic expansion of ductal lining cells confined by
the basement membrane (25). As
blood vessels remain in the stromal compartment, DCIS occurs in an
avascular microenvironment and inevitably develops hypoxic regions
near the oxygen diffusion limit, due to persistent proliferation of
intraepithelial tumor cells. Pathologists have distinguished two
types of DCIS, comedo and non-comedo (25), based on the presence of necrosis,
which is often associated with microcalcifications in the center of
the breast ducts (25).
A pattern similar to comedo-type necrosis,
characteristically found in DCIS of the breast, has also been
identified in invasive carcinomas. It is characterized by the
presence of well-circumscribed epithelial nests containing central
necrotic material, including neuroendocrine carcinomas; carcinoma
arising in pleomorphic adenoma, duct carcinomas of the salivary
glands; cervical carcinoma in situ with features of
impending invasion; and basaloid squamous carcinoma of the lung,
salivary glands, esophagus, anal canal and sinonasal tract
(25). Therefore, coagulative
necrosis and its variants (peritheliomatous and comedo-type
necrosis) are usually observed in epithelial tumors, in situ
and invasive, characterized by a solid growth pattern.
5. TN and fibrotic focus
Following a certain period of time, coagulative
necrosis may be replaced by colliquative necrosis, in which the
cellular structures are broken down by proteolitic enzymes released
from ruptured lysosomes and similar enzymes released by
infiltrating inflammatory cells (20). Finally, colliquative/coagulative
necrosis is replaced by a scar-like area, defined as the fibrotic
focus (26). It appears as a
radially expanding fibrosclerotic core and consists of loose, dense
or hyalinized collagen bundles and a variable number of fibroblasts
(26). In addition, elastic tissue
may be abundant. The arrangements of fibroblasts or collagen fibers
forming fibrotic foci differ from that of the surrounding stroma,
which is more ordered (26). The
presence of a fibrotic focus was found to positively correlate with
disease progression, increased tumor size, lymph node metastases
and a poor outcome in breast, colorectal and pancreatic cancer
(26–28).
6. TN in invasive adenocarcinomas
Colliquative necrosis, dirty necrosis and
intraglandular necrotic debris are usually identified in invasive
adenocarcinomas (29–35). In these tumors, necrosis may remain
confined to single neoplastic glands, whereas in other areas it may
involve neoplastic glands and intervening stroma. The term ‘dirty
necrosis’ is used to describe the presence of intraglandular
eosinophilic material frequently in combination with necrotic cell
debris and neutrophils (29). This
intraglandural material stains positively with periodic acid-Schiff
and expresses the transmembrane glycoprotein MUC1 (29). Furthermore, dirty necrosis has been
identified in colorectal adenocarcinomas and is often accompanied
by segmental necrosis of the glandular lining (29). Foci of dirty necrosis are also
common in pulmonary metastases of colonic carcinomas, but are
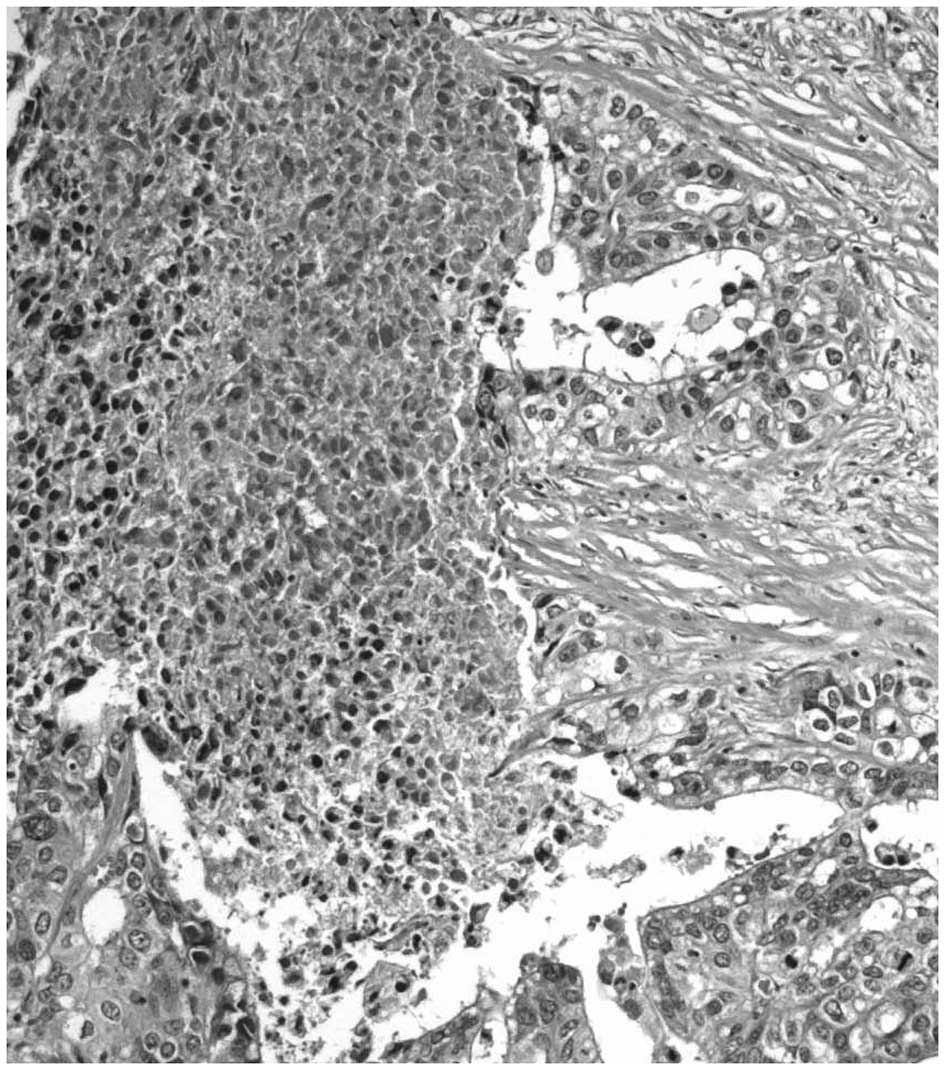
rarely observed in primary lung adenocarcinomas (36). Necrotic areas involving the stroma
and glands are frequently infiltrated by neutrophils in a pattern
similar to that observed in colliquative necrosis (Fig. 2) (7). Notably, mucinous adenocarcinomas are
characterized by MUC2 overexpression and the absence of dirty
necrosis (37). Colliquative and/or
dirty necrosis are predominantly found in MUC1-positive
adenocarcinomas of the pancreas (38) and colorectum (29), whereas the absence of necrotic
phenomena is characteristically found in MUC2-positive mucinous
adenocarcinomas of the gastrointestinal tract (36,39).
7. p53
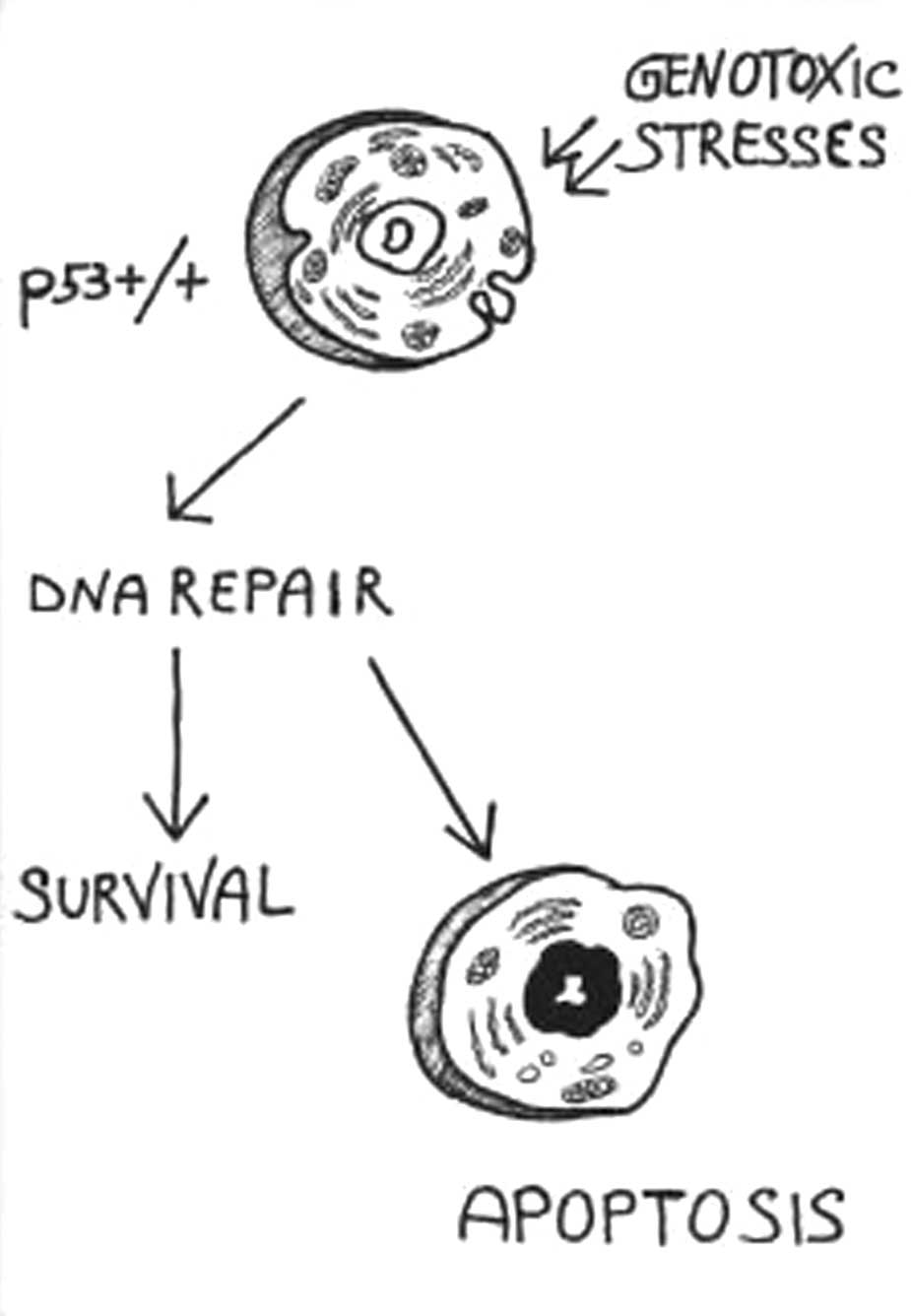
p53 acts as a guardian of the genome, protecting
cells against cancer (40). In
response to a variety of genotoxic stresses (DNA-damaging agents,
UV damage, antimicrotubule agents and hypoxia), the p53 protein
promotes cell-cycle arrest, which is necessary to repair any DNA
damage, or apoptosis, if repair cannot be achieved (Fig. 3) (40). Cell-cycle arrest may be used to
repair any damage, whereas apoptosis is a genetically controlled
response whereby cells commit suicide when repair cannot be
achieved. These cellular responses allow p53 to inhibit
tumorigenesis and genomic instability (40). Furthermore, when p53 is mutated, it
accumulates at the nuclear level and the cell-cycle checkpoint
becomes defective. Thus, a cell may enter mitosis prematurely,
prior to the completion of DNA replication or DNA damage repair.
This aberrant mitosis may lead to apoptosis or necrosis (41). Of note, mitotic catastrophe is not
considered a form of cell death, but rather an irreversible trigger
for cell death (22).
The p53 tumor suppressor gene is mutated in ~50% of
all human cancers. Following severe genotoxic damage, numerous
p53-mutated tumors undergo mitotic catastrophe (41–44).
According to NCCD, mitotic catastrophe refers to cell death that is
triggered by aberrant mitosis and executed during mitosis or in the
subsequent interphase (22).
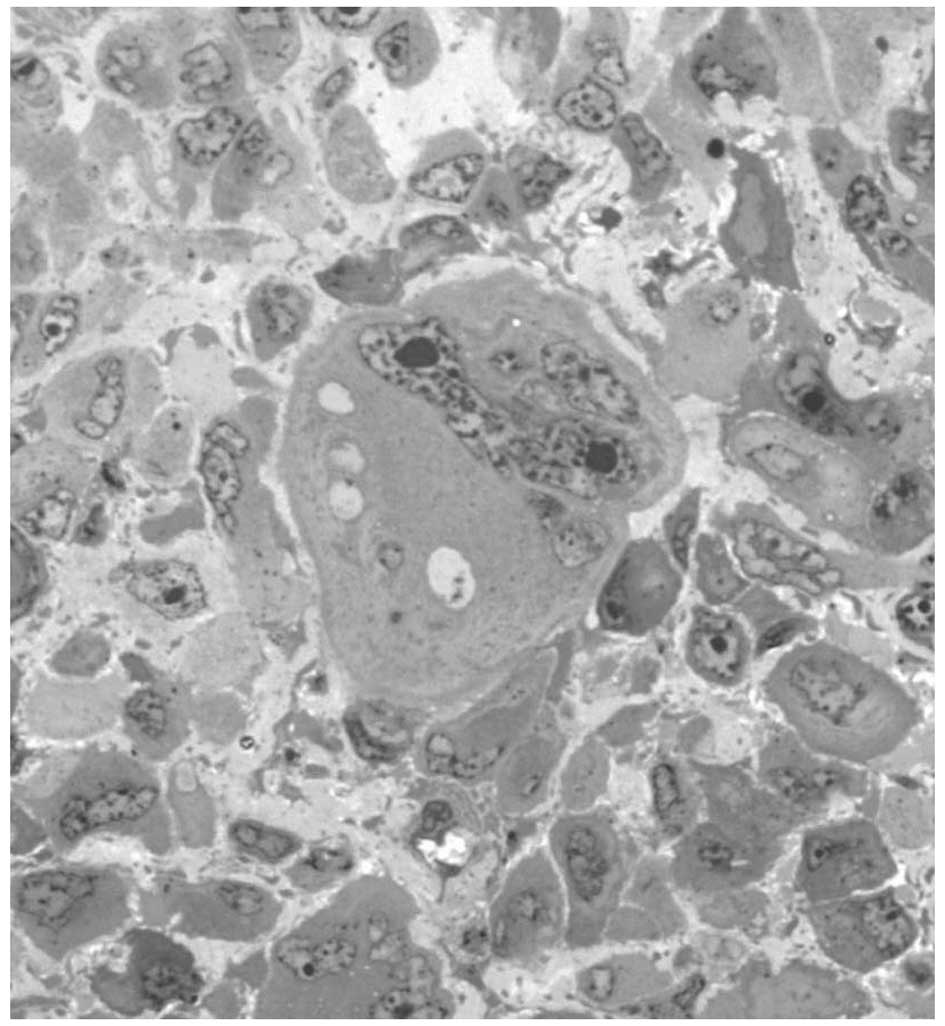
Mitotic catastrophe is morphologically characterized by
anisocytosis and anisokaryosis (heterogeneity in cytoplasmic and
nuclear size, respectively), presence of micronuclei (derived from
chromosomes and/or chromosome fragments that have been irregularly
distributed between daughter nuclei) and multinucleation (two or
more nuclei with similar or heterogeneous sizes in a single cell,
as a result of failed separation during cytokinesis) (Fig. 4) (22). Morphological features associated
with mitotic catastrophe may be observed in pleomorphic, giant cell
carcinoma, a tumor without any identifiable glandular, squamous or
any other type of differentiation (45). It consists of sheets of highly
undifferentiated pleomorphic cells, often with areas of coagulative
necrosis (22) with numerous
bizarre/multinucleated cells (46–48)
and many abnormal mitotic figures. Pleomorphic, giant cell
carcinomas are highly malignant tumors that are most commonly found
in the lungs, breast, pancreas and thyroid (22).
8. Mitotic catastrophe in anticancer
therapy
Mitotic catastrophe has been characterized as the
predominant form of cell death induced by ionizing radiation, and
occurs in response to several anticancer drugs (49,50).
Since preoperative chemotherapy is being used more frequently in
the management of advanced tumors, pathologists must be aware of
the resultant morphological effects, which may result in
difficulties in tumor typing and grading and in the identification
of residual neoplasia (51,52). The morphological features of lung,
breast and ovarian cancers treated with chemotherapy include
nuclear and cytoplasmic alterations and pronounced stromal changes
(52,53). Nuclei exhibit significant
enlargement with extremely irregular outlines, and occasionally
appear similar to multinucleated giant cells (52,53), a
feature associated with mitotic catastrophe. Nuclear size has been
shown to represent a useful prognostic indicator in ovarian and
breast cancer, and therefore an increased nuclear size
post-chemotherapy may influence the results if this measurement is
used as a predictor of outcome (53). Post-chemotherapy tumor cells are
observed singularly or in small clusters, often without tubular
differentiation, and mitotic activity is rare (53). Therefore, preoperative chemotherapy
causes difficulty in tumor grading, which is based on cytological
and architectural features, as well as mitotic activity.
9. Pathogenesis of TN
It is hypothesized that TN is caused by chronic
ischemia (i.e. hypoxia, low pH, low glucose and high lactate)
within tumors, due to vascular collapse, high interstitial pressure
and/or rapid tumor growth exceeding its blood supply. Anemia, the
most common cancer-associated morbidity, further reduces the blood
capacity for O2 transportation (54), and it is an adverse prognostic
factor for survival, which is independent of tumor type (55). The contiguous or sheet-like nature
of the necrosis indicates that the cause of death is due to
ischemic injury, affecting a field or group of tumor cells,
supplied or drained by a single vessel (18). The subsequent necrosis suggests that
the large feeding artery or exit vein becomes obstructed, leading
to an arterial or a venous infarct (18). By contrast to this hypothesis, it
has been revealed that TN frequently occurs within regions that
display relatively increased microvessel density (56). However, there are tumors in which
coagulative necrosis is rare, although the tumor stage is advanced.
For example, the lowest frequency of TN is observed in mucinous
adenocarcinomas of the gastrointestinal tract (36). Furthermore, Tollefson et al
(57) revealed that renal
carcinomas exhibiting coagulative necrosis also exhibited
relatively high proportions of proliferative Ki-67-positive tumor
cells. Similar findings have been demonstrated in gastric
carcinomas (45). Fig. 5 shows the morphological association
between peritheliomatous necrosis, atypical mitoses and high
proportion of cycling Ki-67 immunoreactive tumor cells.
We hypothesize that hypoxia, a known genotoxic
factor, may indirectly induce TN via mitotic catastrophe in tumor
cells harboring p53 mutations. A similar pathway has been suggested
for TN occurring in vivo following treatment with anticancer
drugs or radiation (42,44,58).
Our hypothesis for the association between hypoxia, mitotic
catastrophe and TN is shown in Fig.
6. Further studies regarding the mechanisms associated with TN
may yield useful insights into epithelial malignant tumor biology
and improve patient management.
References
|
1
|
Richards CH, Mohammed Z, Qayyum T, Horgan
PG and McMillan DC: The prognostic value of histological tumour
necrosis in solid organ malignant disease: a systematic review.
Future Oncol. 7:1223–1235. 2011.
|
|
2
|
Caruso R, Parisi A, Bonanno A, et al:
Histologic coagulative tumour necrosis as a prognostic indicator of
aggressiveness in renal, lung, thyroid and colorectal carcinomas: A
brief review. Oncol Lett. 3:16–18. 2012.
|
|
3
|
Swinson DE, Jones JL, Richardson D, Cox G,
Edwards JG and O’Byrne KJ: Tumour necrosis is an independent
prognostic marker in non-small cell lung cancer: correlation with
biological variables. Lung Cancer. 37:235–240. 2002.
|
|
4
|
Jimenez RE, Wallis T and Visscher DW:
Centrally necrotizing carcinomas of the breast: a distinct
histologic subtype with aggressive clinical behavior. Am J Surg
Pathol. 25:331–337. 2001.
|
|
5
|
Livasy CA, Karaca G, Nanda R, et al:
Phenotypic evaluation of the basal-like subtype of invasive breast
carcinoma. Mod Pathol. 19:264–271. 2006.
|
|
6
|
Hiltzik D, Carlson DL, Tuttle RM, Chuai S,
et al: Poorly differentiated thyroid carcinomas defined on the
basis of mitosis and necrosis: a clinicopathologic study of 58
patients. Cancer. 106:1286–1295. 2006.
|
|
7
|
Pollheimer MJ, Kornprat P, Lindtner RA, et
al: Tumour necrosis is a new promising prognostic factor in
colorectal cancer. Hum Pathol. 41:1749–1757. 2010.
|
|
8
|
Richards CH, Roxburgh CS, Anderson JH, et
al: Prognostic value of tumour necrosis and host inflammatory
responses in colorectal cancer. Br J Surg. 99:287–294. 2012.
|
|
9
|
Hiraoka N, Ino Y, Sekine S, et al: Tumour
necrosis is a postoperative prognostic marker for pancreatic cancer
patients with a high interobserver reproducibility in histological
evaluation. Br J Cancer. 103:1057–1065. 2010.
|
|
10
|
Lam JS, Shvarts O, Said JW, Pantuck AJ, et
al: Clinicopathologic and molecular correlations of necrosis in the
primary tumour of patients with renal cell carcinoma. Cancer.
103:2517–2525. 2005.
|
|
11
|
Leibovich BC, Blute ML, Cheville JC, Lohse
CM, Frank I, Kwon ED, et al: Prediction of progression after
radical nephrectomy for patients with clear cell renal cell
carcinoma: a stratification tool for prospective clinical trials.
Cancer. 97:1663–1671. 2003.
|
|
12
|
Sengupta S, Lohse CM, Leibovich BC, et al:
Histologic coagulative tumour necrosis as a prognostic indicator of
renal cell carcinoma aggressiveness. Cancer. 104:511–520. 2005.
|
|
13
|
Katz MD, Serrano MF, Grubb RL 3rd, et al:
Percent microscopic tumour necrosis and survival after curative
surgery for renal cell carcinoma. J Urol. 183:909–914. 2010.
|
|
14
|
Delahunt B, McKenney JK, Lohse CM, et al:
A novel grading system for clear cell renal cell carcinoma
incorporating tumor necrosis. Am J Surg Pathol. 37:311–322.
2013.
|
|
15
|
Pichler M, Hutterer GC, Chromecki TF, et
al: Histologic tumour necrosis is an independent prognostic
indicator for clear cell and papillary renal cell carcinoma. Am J
Clin Pathol. 137:283–289. 2012.
|
|
16
|
Pichler M, Hutterer GC, Chromecki TF,
Pummer K, Mannweiler S and Zigeuner R: Presence and extent of
histological tumour necrosis is an adverse prognostic factor in
papillary type 1 but not in papillary type 2 renal cell carcinoma.
Histopathology. 62:219–228. 2013.
|
|
17
|
Jain RK and Carmeliet PF: Vessels of death
or life. Sci Am. 285:38–45. 2001.
|
|
18
|
Weidner N: Tumour vascularity and
proliferation: clear evidence of a close relationship. J Pathol.
189:297–299. 1999.
|
|
19
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wideranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
|
|
20
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.
|
|
21
|
Kroemer G, El-Deiry WS, Golstein P, et al;
Nomenclature Committee on Cell Death. Classification of cell death:
recommendations of the Nomenclature Committee on Cell Death. Cell
Death Differ. 12(Suppl 2): 1463–1467. 2005.
|
|
22
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, et al: Molecular definitions of
cell death subroutines: recommendations of the Nomenclature
Committee on Cell Death 2012. Cell Death Differ. 19:107–120.
2012.
|
|
23
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, et al; Nomenclature Committee
on Cell Death 2009. Classification of cell death: recommendations
of the Nomenclature Committee on Cell Death 2009. Cell Death
Differ. 16:3–11. 2009.
|
|
24
|
Vanlangenakker N, Vanden Berghe T and
Vandenabeele P: Many stimuli pull the necrotic trigger, an
overview. Cell Death Differ. 19:75–86. 2012.
|
|
25
|
Al-Nafussi AI and Hughes DE: Histological
patterns of tumours and tumour-like conditions. Histological
Diagnosis of Tumours by Pattern Analysis. Arnold; London: pp.
11–18. 1997
|
|
26
|
Hasebe T, Sasaki S, Imoto S, Mukai K,
Yokose T and Ochiai A: Prognostic significance of fibrotic focus in
invasive ductal carcinoma of the breast: a prospective
observational study. Mod Pathol. 15:502–516. 2002.
|
|
27
|
Nishimura R, Hasebe T, Tsubono Y, et al:
The fibrotic focus in advanced colorectal carcinoma: a hitherto
unrecognized histological predictor for liver metastasis. Virchows
Arch. 433:517–522. 1998.
|
|
28
|
Watanabe I, Hasebe T, Sasaki S, et al:
Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin
expression correlate with outcome. Pancreas. 26:326–333. 2003.
|
|
29
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007.
|
|
30
|
Caruso RA, Napoli P, Nania A, Parisi A,
Fedele F and Zuccalà V: Mitochondrion-rich differentiated
adenocarcinomas of the stomach: clinicopathological,
immunohistochemical and electron microscopy study of nine cases.
Virchows Arch. 456:499–505. 2010.
|
|
31
|
Caruso RA, Fedele F, Finocchiaro G, et al:
Microvascular changes in human gastric carcinomas with coagulative
necrosis: an ultrastructural study. Ultrastruct Pathol. 32:184–188.
2008.
|
|
32
|
Caruso RA, Fedele F, Rigoli L, et al:
Apoptotic-like tumour cells and apoptotic neutrophils in
mitochondrion-rich gastric adenocarcinomas: a comparative study
with light and electronmicroscopy between these two forms of cell
death. Rare Tumours. 5:68–71. 2013.
|
|
33
|
Dutta S, Going JJ, Crumley AB, et al: The
relationship between tumour necrosis, tumour proliferation, local
and systemic inflammation, microvessel density and survival in
patients undergoing potentially curative resection of oesophageal
adenocarcinoma. Br J Cancer. 106:702–710. 2012.
|
|
34
|
Watanabe Y, Shimizu M, Itoh T and
Nagashima K: Intraglandular necrotic debris in gastric biopsy and
surgical specimens. Ann Diagn Pathol. 5:141–147. 2001.
|
|
35
|
Caruso RA, Rigoli L, Parisi A, et al:
Neutrophil-rich gastric carcinomas: light and electron microscopic
study of 9 cases with particular reference to neutrophil apoptosis.
Ultrastruct Pathol. 37:164–170. 2013.
|
|
36
|
Flint A and Lloyd RV: Pulmonary metastases
of colonic carcinoma. Distinction from pulmonary adenocarcinoma.
Arch Pathol Lab Med. 116:39–42. 1992.
|
|
37
|
Greenson JK, Bonner JD, Ben-Yzhak O, et
al: Phenotype of microsatellite unstable colorectal carcinomas:
Well-differentiated and focally mucinous tumours and the absence of
dirty necrosis correlate with microsatellite instability. Am J Surg
Pathol. 27:563–570. 2003.
|
|
38
|
Reid MD, Basturk O, Thirabanjasak D, et
al: Tumour-infiltrating neutrophils in pancreatic neoplasia. Mod
Pathol. 24:1612–1619. 2011.
|
|
39
|
Leteurtre E, Zerimech F, Piessen G, et al:
Relationships between mucinous gastric carcinoma, MUC2 expression
and survival. World J Gastroenterol. 12:3324–3331. 2006.
|
|
40
|
Baehrecke EH: Growth control: p53, the
guardian angel of compensatory proliferation. Curr Biol.
16:R840–R842. 2006.
|
|
41
|
Castedo M, Perfettini JL, Roumier T,
Andreau K, Medema R and Kroemer G: Cell death by mitotic
catastrophe: a molecular definition. Oncogene. 23:2825–2837.
2004.
|
|
42
|
Ianzini F, Bertoldo A, Kosmacek EA,
Phillips SL and Mackey MA: Lack of p53 function promotes
radiation-induced mitotic catastrophe in mouse embryonic fibroblast
cells. Cancer Cell Int. 6:112006.
|
|
43
|
Mackey MA and Ianzini F: Enhancement of
radiation-induced mitotic catastrophe by moderate hyperthermia. Int
J Radiat Biol. 76:273–280. 2000.
|
|
44
|
Roninson IB, Broude EV and Chang BD: If
not apoptosis, then what? Treatment-induced senescence and mitotic
catastrophe in tumour cells. Drug Resist Updat. 4:303–313.
2001.
|
|
45
|
Caruso R, Fedele F, Lucianò R, et al:
Mitotic catastrophe in malignant epithelial tumours: the
pathologist’s viewpoint. Ultrastruct Pathol. 35:66–71. 2011.
|
|
46
|
Caruso RA, Fedele F, Crisafulli C, et al:
Abnormal nuclear structures (micronuclei, nuclear blebs, strings,
and pockets) in a case of anaplastic giant cell carcinoma of the
thyroid: an immunohistochemical and ultrastructural study.
Ultrastruct Pathol. 35:14–18. 2011.
|
|
47
|
Caruso RA, Rigoli L, Fedele F, et al:
Modifications of nuclear envelope in tumour cells of human gastric
carcinomas: an ultrastructural study. Anticancer Res. 30:699–702.
2010.
|
|
48
|
Caruso RA, Fedele F, Consolo P, Luigiano
C, Venuti A and Cavallari V: Abnormal nuclear structures
(micronuclei, nucleoplasmic bridges, and nuclear buds) in a
pleomorphic giant cell carcinoma of the stomach. Ultrastruct
Pathol. 32:11–15. 2008.
|
|
49
|
Morse DL, Gray H, Payne CM and Gillies RJ:
Docetaxel induces cell death through mitotic catastrophe in human
breast cancer cells. Mol Cancer Ther. 4:1495–1504. 2005.
|
|
50
|
Swanson PE, Carroll SB, Zhang XF and
Mackey MA: Spontaneous premature chromosome condensation
micronucleus formation, and non-apoptotic cell death in heated HeLa
S3 cells. Ultrastructural observations. Am J Pathol. 146:963–971.
1995.
|
|
51
|
McCluggage WG, Lyness RW, Atkinson RJ, et
al: Morphological effects of chemotherapy on ovarian carcinoma. J
Clin Pathol. 55:27–31. 2002.
|
|
52
|
Honkoop AH, Pinedo HM, De Jong JS, et al:
Effects of chemotherapy on pathologic and biologic characteristics
of locally advanced breast cancer. Am J Clin Pathol. 107:211–218.
1997.
|
|
53
|
Carder P: Typing breast cancer following
primary chemotherapy. Histopathology. 35:584–585. 1999.
|
|
54
|
Spivak JL: The anaemia of cancer: death by
a thousand cuts. Nat Rev Cancer. 5:543–555. 2005.
|
|
55
|
Caro JJ, Salas M, Ward A and Goss G:
Anemia as an independent prognostic factor for survival in patients
with cancer: a systemic, quantitative review. Cancer. 91:2214–2221.
2001.
|
|
56
|
Leek RD, Landers RJ, Harris AL and Lewis
CE: Necrosis correlates with high vascular density and focal
macrophage infiltration in invasive carcinoma of the breast. Br J
Cancer. 79:991–995. 1999.
|
|
57
|
Tollefson MK, Thompson RH, Sheinin Y, et
al: Ki-67 and coagulative tumour necrosis are independent
predictors of poor outcome for patients with clear cell renal cell
carcinoma and not surrogates for each other. Cancer. 110:783–790.
2007.
|
|
58
|
Proskuryakov SY and Gabai VL: Mechanisms
of tumour cell necrosis. Curr Pharm Des. 16:56–68. 2010.
|