Introduction
Colorectal cancer is one of the most common
malignancies worldwide, with >1,000,000 cases reported annually
(1). Several developmental
signaling pathways that are involved in the carcinogenesis of
colorectal cancer, including the Wnt/β-catenin (2), TGF-β/Smad (3) Notch (4) and receptor tyrosine kinase (5) pathways, have been widely investigated.
However, the role of hedgehog (Hh)-glioma-associated oncogene
homolog (GLI) signaling in colorectal cancer remains controversial
(6,7), and certain studies have indicated that
Hh signaling is inactive in colorectal cancer (8–10).
Canonical Hh signaling predominantly consists of Hh,
the Hh receptor, patched homolog 1 (PTCH1), the intermediary
signaling molecule, smoothened (SMO), and the transcription factor,
GLI. The mammalian GLI family has three isoforms, GLI1, GLI2 and
GLI3. GLI1 is an activator of primary transcription, whereas GLI3
is a repressor of transcription and GLI2 has the functions of a
transcriptional activator and repressor (11). In the absence of Hh, PTCH1 interacts
with SMO and inhibits its activity, while GLI is degraded to the
repressor form, which results in the transcriptional inhibition of
Hh target genes. When an Hh ligand binds to the PTCH1 receptor, it
relieves the PTCH1-mediated inhibition of SMO. Subsequently, the
active SMO affects the expression of GLI proteins, which may enter
the nucleus and regulate the expression of the Hh target genes
(including PTCH1, GLI, Wnt, c-MYC and
CCND1) in responding cells (12,13).
Aberrant Hh signaling was initially identified in nevoid basal cell
carcinoma syndrome, also termed Gorlin syndrome (14). Soon afterwards, aberrant Hh
signaling activity was also observed in several types of human
cancer, including medulloblastoma, glioblastoma, rhabdomyosarcoma,
pancreatic and prostate cancer, and hematological malignancies
(15). Cyclopamine, a specific
inhibitor of SMO (16), is
currently under investigation in anticancer studies.
Cyclooxygenase-2 (COX-2) has been reported to be
highly expressed in a number of human cancers and cancer cell
lines, including pancreatic and colon cancer (17). Celecoxib, a selective COX-2
inhibitor, may inhibit the proliferation of cancer cells and
promote apoptosis (18). In
addition, celecoxib has been approved by the Food and Drug
Administration in the USA for the chemoprevention of colorectal
cancer. At present, it is unclear whether celecoxib and its
anticancer effects are associated with Hh signaling. The present
study aimed to investigate Hh signaling in colon cancer cell lines
and the effect of celecoxib on Hh signaling in colon cancer
cells.
Materials and methods
Cell culture and reagents
The human colon cancer HT-29 and LoVo cell lines,
and the pancreatic cancer PANC-1 cell line were obtained from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai Institute of Cell Biology, Chinese Academy of Sciences,
Shanghai, China), while the human colon cancer HCT-116 cell line
was donated by the experimental center of the Shanghai Traditional
Chinese Medicine Hospital (Shanghai, China). The HT-29 cells were
cultured in RPMI 1640 medium with 10% newborn calf serum, the LoVo
and HCT-116 cells were cultured in RPMI 1640 medium with 10% fetal
bovine serum (FBS) and the PANC-1 cells were cultured in Dulbecco’s
modified Eagle’s medium with 10% FBS. All culture media also
contained 100 U/ml penicillin and 100 μg/ml streptomycin. The
PANC-1 cells were used as control cells, as Hh signaling is active
in these cells (9). Cyclopamine was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and celecoxib was
purchased from Pfizer (New York, NY, USA). For the experiments
conducted in the present study, these agents were dissolved in
dimethyl sulfoxide (DMSO) and were then added to the cells in the
corresponding medium with a final DMSO concentration of ≤0.1%.
Cell viability
A total of 5×103 cells per well were
seeded in 96-well plates, and each group consisted of five parallel
wells. Following 24 h of incubation, fresh medium was added to the
cells, with or without cyclopamine or celecoxib. Following the
required period of culture, cell viability was determined by MTT
assay according to the following formula: Cell viability (%) =
[optical density (OD)average dosing group /
ODcontrol group mean] × 100.
Measurement of GLI1 levels
A total of 2×105 cells per well were
seeded in six-well plates, and each group consisted of two parallel
wells. At 24 h after the seeding, the cells were treated with
cyclopamine or celecoxib. The cells were then harvested following
the required period of treatment and nuclear proteins were
subsequently extracted from the treated cells using a nuclear
protein/plasma protein extraction kit (Aidlab Biotechnologies Co.
Ltd., Beijing, China). The protein concentrations were quantified
using the bicinchoninic acid protein quantitative kits (Beyotime
Institute of Biotechnology, Jiangsu, China) according to the
manufacturer’s instructions. The levels of GLI1 were measured by
ELISA (CUSABIO Biotech Co., Ltd., Wuhan, China).
Expression of PTCH1, SMO and GLI1 genes
by quantitative polymerase chain reaction (qPCR)
In total, 2×105 cells per well were
seeded in six-well plates, and treated with cyclopamine or
celecoxib for 36 h. Total RNA was isolated using TRIzol (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Next, the expression of the mRNA was
examined by qPCR using the StepOnePlus™ Real-Time PCR System
(Applied Biosystems, Foster City, CA, USA) and Fast SYBR Green
Master Mix 2X reagent (Applied Biosystems, Foster City, CA, USA) in
a 20 μl reaction volume according to the manufacturer’s
instructions. The thermal cycling conditions were as follows: 20
sec at 95°C, followed by the amplification reaction consisting of
40 cycles of denaturation for 3 sec at 95°C and annealing for 30
sec at 60°C. For sample analysis, the threshold was set based on
the exponential phase of the products, and the cycle threshold (Ct)
value for the sample was determined. The results were analyzed
using the comparative Ct method for relative gene expression
quantification against the housekeeping gene, GAPDH. The
primers were designed using the Oligo Primer Analysis 4.0 software
and the sequences were BLASTed (http://www.ncbi.nlm.nih.gov/BLAST/). The primer
sequences were as follows: Sense, 5′-GGTGGCACAGTCAAGAACA-3′ and
antisense, 5′-TCGTGGTGGTGAAGGAAA-3′ for PTCH1; sense,
5′-CCCTTGGTTCGGACAGACA-3′ and antisense,
5′-AAAGAAGCACGCATTGACG-3′for SMO; sense,
5′-TTCCTACCAGAGTCCCAAGT-3′ and antisense,
5′-CCCTATGTGAAGCCCTATTT-3′ for GLI1; sense,
5′-AACGGATTTGGTCGTATTG-3′ and antisense, 5′-GGA AGATGGTGATGGGATT-3′
for GAPDH.
Statistical analysis
All experiments were performed in duplicate or more.
Data are presented as the mean ± standard deviation and the
difference between two groups was assessed using Student’s
two-tailed t-test. P<0.05 and P<0.01 were considered to
indicate statistically significant differences.
Results
Effect of cyclopamine or celecoxib on the
proliferation of colon cancer cells
As shown in Fig. 1A,
the control PANC-1 cells were sensitive to cyclopamine; the rate of
inhibition was 15.2% (P<0.05) after 24 h and increased to 39.8%
(P<0.01) at 72 h. However, the LoVo cells were more sensitive to
the growth inhibition of cyclopamine compared with the PANC-1
cells; the rate of inhibition was ~38.7% (P<0.01) after 24 h and
increased to 66.1% (P<0.01) after 72 h. The HT-29 cells were not
as sensitive to cyclopamine when compared with the LoVo cells;
following 24 and 72 h of incubation, the rate of inhibition was
25.9 (P<0.05) and 26.2% (P<0.01), respectively. The response
of the HCT-116 cells to cyclopamine was weak, with a maximum
inhibition rate of 12.2% (P>0.05) at 72 h.
The MTT assay results for celecoxib are shown in
Fig. 1B. Significant inhibition was
observed in the HCT-116 cells; following 24 and 48 h of treatment,
the rate of inhibition was 22.2 (P<0.05) and 47.3% (P<0.01),
respectively. However, the inhibition of celecoxib was weaker in
the HT-29 and PANC-1 cells than in the HCT-116 cells; the rate of
inhibition in the HT-29 and PANC-1 cells was 27.6 (P<0.01) and
21.2% (P<0.05), respectively, after 72 h of treatment. The LoVo
cells were resistant to the growth inhibition of celecoxib and the
maximum inhibition rate was only 11.6% (P>0.05) following 72 h
of treatment.
Effect of cyclopamine or celecoxib on
GLI1 level in colon cancer cells
When the PANC-1 cells were treated with cyclopamine,
the GLI1 level significantly decreased. Following 24 and 72 h of
treatment, the level of GLI1 had decreased by 15.5 (P<0.05) and
42.3% (P<0.01), respectively (Fig.
2A). When the colon cancer cells were treated with cyclopamine,
changes were observed in the GLI1 levels between the cell lines
(Fig. 2A). The effect of
cyclopamine on GLI1 was pronounced in the LoVo cells, consistent
with the MTT results (Fig. 1A).
Following 24 and 72 h of treatment, the level of GLI1 had declined
by 31.6 (P<0.05) and 59.9% (P<0.01), respectively. The HT-29
cells were the second most sensitive to cyclopamine from the three
colon cancer cell lines; the GLI1 levels were decreased by 36.4%
(P<0.05) after 72 h. However, the HCT-116 cells were not
sensitive to cyclopamine; the GLI1 levels were only decreased by
6.4% (P>0.05), consistent with the MTT results (Fig. 1A).
When the colon cancer cells were treated with
celecoxib, changes in the GLI1 levels in the three cell lines were
evident (Fig. 2B). The level of
GLI1 was significantly decreased in the HCT-116 cells, consistent
with the MTT results (Fig. 1B);
following 24 and 72 h of treatment, the GLI1 levels had decreased
by 14.8 (P<0.05) and 55.5% (P<0.01), respectively. The
response of the HT-29 cells to celecoxib was similar to the MTT
results (Fig. 1B); at 72 h, the
level of GLI1 had decreased by 38.1% (P<0.05). However, the LoVo
cells were resistant to the anticancer effect of celecoxib, which
was also consistent with MTT results (Fig. 1B); following 72 h of treatment, GLI1
was decreased by only 9.6% (P>0.05). When the PANC-1 cells were
treated with celecoxib, the GLI1 level also decreased by 15.5%
(P<0.05) following 48 h of treatment.
Effect of cyclopamine or celecoxib on the
expression of PTCH1, SMO and GLI1 genes in colon cancer cells
Based on the aforementioned results, the expression
of the PTCH1, SMO and GLI1 genes in the four
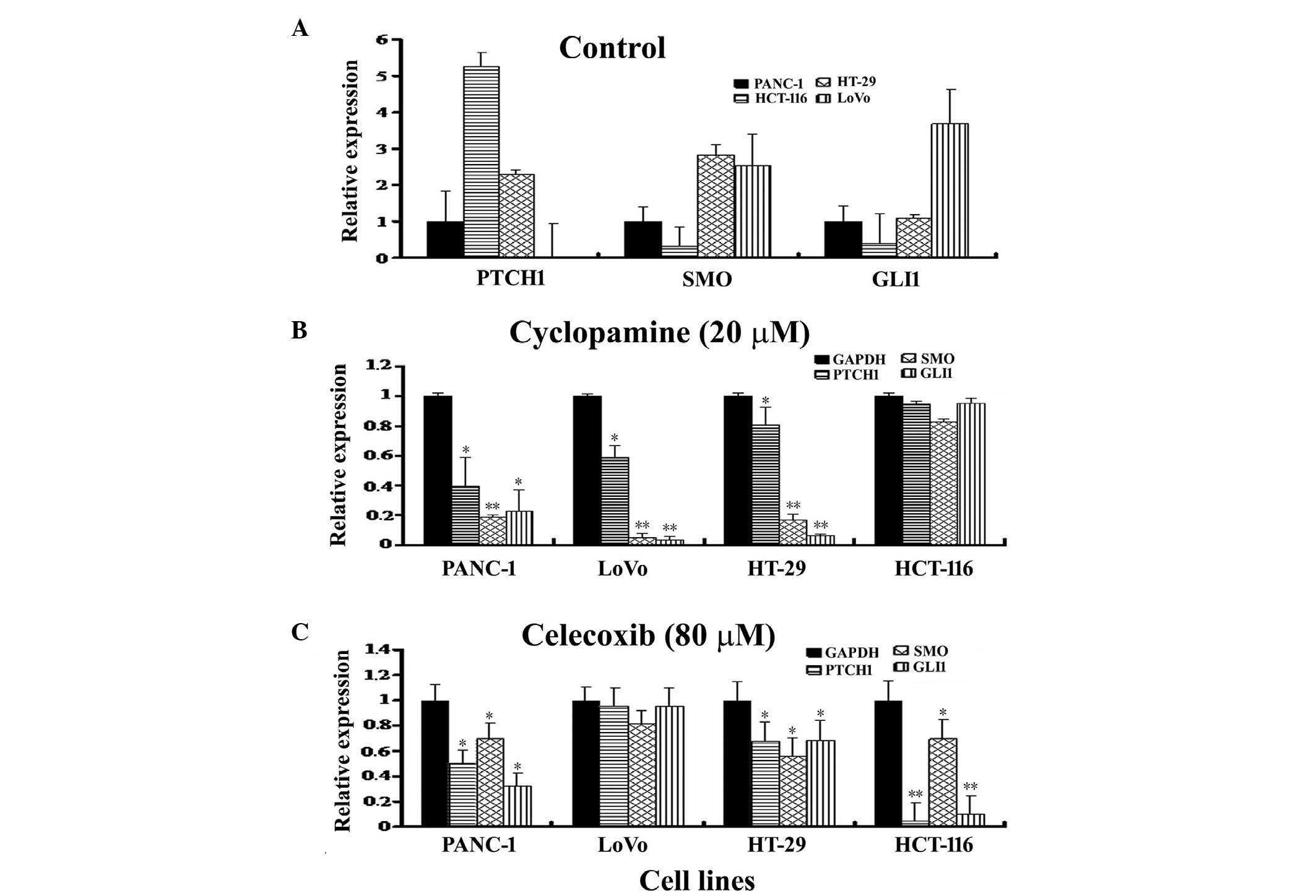
cell lines was measured using qPCR (Fig. 3A). PTCH1 was highly expressed
in the HCT-116 cells, moderately expressed in the HT-29 and PANC-1
cells and poorly expressed in the LoVo cells. The mRNA levels were
recorded as 5.26 (HCT-116), 2.29 (HT-29) and 0.03 (LoVo) comparerd
with the internal control, GAPDH, when normalized against
the PANC-1 cells. The SMO gene was highly expressed in the
HT-29 and LoVo cells, however, a low expression level was observed
in the HCT-116 cells, with mRNA levels of 2.81, 2.55 and 0.32,
respectively, when normalized against the PANC-1 cells. GLI1
expression was observed to be relatively low in the HCT-116 cells,
moderate in the HT-29 and PANC-1 cells and high in the LoVo cells.
The mRNA level for GLI1 was 0.38 (HCT-116), 1.09 (HT-29) and
3.68 (LoVo) compared with the internal control, GAPDH, when
normalized against the PANC-1 cells.
Following cyclopamine treatment, the LoVo cells were
the most sensitive to cyclopamine treatment, as shown in Fig. 3B. The expression of PTCH1,
SMO and GLI1 mRNA was reduced to 58.9, 4.59 and 3.25%
in the LoVo cells, respectively, compared with the control. These
findings were consistent with the results shown in Figs. 1A and 2A. Cyclopamine effectively reduced the
expression of PTCH1, SMO and GLI1 mRNA to
39.7, 18.8 and 22.5% in the PANC-1 cells, and to 80.7, 16.5 and
6.37% in the HT-29 cells, respectively, compared with the control.
However, the effect of cyclopamine on the expression of the genes
in the HCT-116 cells was weak; the expression of PTCH1,
SMO and GLI1 mRNA was reduced to 94.5, 82.7 and 95.3%
(P>0.05), respectively, compared with the control. This was
consistent with the results shown in Figs. 1A and 2A.
Conversely, the HCT-116 cells were observed to be
extremely sensitive to celecoxib treatment; the expression of
PTCH1, SMO and GLI1 mRNA was reduced to 4.0,
69.8 and 9.4% in the HCT-116 cells (Fig. 3C), respectively, compared with the
control, consistent with the results presented in Figs. 1B and 2B. Celecoxib reduced PTCH1,
SMO and GLI1 mRNA expression to 67.3, 55.8 and 68.5%
in the HT-29 cells and to 50.2, 69.9 and 32.2% in the PANC-1 cells,
respectively, compared with the control. However, the changes in
gene expression were minor in the LoVo cells, with the expression
of PTCH1, SMO and GLI1 mRNA reduced to 95.1,
81.1 (P>0.05) and 95.1%, respectively, compared with the
control, consistent with the results shown in Figs. 1B and 2B.
Discussion
Although aberrant Hh signaling is indicated to be
involved in endodermally-derived human cancers that account for 25%
of human cancer-related mortalities (19), the role of Hh signaling in human
colorectal cancers is not fully understood (6,7), and
several studies have indicated that Hh signaling is inactive in
colorectal cancer (8–10). The results of the present study
showed that Hh signaling activity varies between colon cancer
HT-29, LoVo and HCT-116 cells. When the colon cancer cells were
treated with cyclopamine, the LoVo cells were the most sensitive to
the drug among the three cell lines, and compared with the control
PANC-1 cells. Examination of the cells under the microscope and
analysis of the MTT assay confirmed these results, indicating that
Hh signaling was highly active in the LoVo cells. To the best of
our knowledge, this is the first study to report Hh signaling
activity in LoVo cells. Aberrant Hh signaling in the LoVo cells was
evidently associated with the absent expression of PTCH1 in
these cells, which is consistent with the results of a previous
study (20). The results of the
current study demonstrated that the absent expression of
PTCH1 in LoVo cells is associated with epigenetic changes,
as the expression of PTCH1 was present in these cells
following treatment with cyclopamine or celecoxib. The HT-29 cells
showed a certain level of response to cyclopamine treatment
according to microscopic examination and MTT assay. The ELISA
results also indicated that cyclopamine downregulated the
expression of GLI1 in the HT-29 cells, suggesting that Hh
signaling is active in HT-29 cells. In addition, the results
indicated that the Hh signaling activity in the HT-29 cells was
similar to that in the PANC-1 cells, but lower than that in the
LoVo cells. Several studies have also shown that HT-29 cells
possess Hh signaling activity and respond to cyclopamine (7,21,22),
however, contrasting results have been presented (9). In the present study, the HCT-116 cells
lacked sensitivity to cyclopamine, a result that was confirmed by
GLI1 ELISA, suggesting that Hh signaling activity is low in HCT-116
cells. The results revealed that the low Hh signaling levels
observed in HCT-116 cells are associated with a high expression
level of PTCH1 and a low expression level of SMO,
which was methylated (23). Several
studies have shown that HCT-116 cells exhibit low Hh signaling
activity and lack a significant response to cyclopamine (8,9). The
present study results indicated that cyclopamine may be used as an
adjuvant treatment agent for Hh signaling-positive colon
cancer.
The differing Hh signaling activities reflect the
various malignant potentials in these colon cancer cell lines.
Among the cell lines under investigation, LoVo cells possess high
metastatic potential, whereas HT-29 cells exhibit low metastatic
potential, and HCT-116 cells have low invasive capacity (24). You et al (20) identified PTCH1 expression in
HT-29 cells, while the expression of PTCH1 was absent in
LoVo cells, indicating that the expression of PTCH1 is
inversely correlated with the metastatic potential of colon cancer
cell lines. The results presented in the present study support the
view that Hh signaling is closely correlated with the malignant
behaviors of colorectal cancer.
In the present study, in order to study the effects
of celecoxib on Hh signaling in colon cancer cells, the cancer cell
lines were treated with celecoxib. The results demonstrated that
colon cancer and PANC-1 cells exhibit different sensitivities to
celecoxib. When the PANC-1 cells were treated with celecoxib, cell
growth was inhibited and the levels of GLI1 were significantly
decreased. When the three colon cancer cell lines were treated with
celecoxib, the HCT-116 cells were the most sensitive. This result
was further confirmed by GLI1 assay, suggesting that celecoxib may
target HCT-116 cells via the SMO-independent modulation of GLI1
activity, as the HCT-116 cells were not sensitive to the SMO
inhibitor, cyclopamine. A previous study also showed that celecoxib
may widely regulate the expression of proteins in HCT-116 cells
based on proteomic profiles, and degrade GLI1 by downregulating
molecular chaperone activities, activating tumor suppressors and
regulating the expression of peroxiredoxin I and creatine kinase,
among others (25). In another
study, a similar result showed that celecoxib induces the
proteasome-dependent degradation of T-cell factor-1 and −4 in
HCT-116 cells (26). In the present
study, the LoVo cells were resistant to the anticancer effect of
celecoxib, and the change in GLI1 levels was mild following
celecoxib treatment, suggesting that Hh signaling is essential for
maintaining the malignant behavior of LoVo cells. The HT-29 cells
showed a certain level of response to celecoxib treatment,
according to microscopic examination and MTT assay. The GLI1 assay
also revealed that celecoxib downregulated the expression of
GLI1 in the HT-29 cells, suggesting that the anticancer
effects of celecoxib on HT-29 cells may be due to interference with
Hh signaling.
Wnt/β-catenin signaling is important in the
carcinogenesis of colorectal cancers (2). The canonical Wnt/β-catenin signaling
pathway is composed of Wnt, the Wnt receptor, frizzled, and the
signaling molecule, β-catenin. In the absence of Wnt, β-catenin
forms a destruction complex containing the tumor suppressor APC
protein. This complex leads to the destruction of β-catenin and
therefore, the Wnt target genes are not expressed. When Wnt binds
to its receptor, frizzled, it leads to the disintegration of the
destruction complex and the accumulation of β-catenin in the
cytoplasm. Subsequently, β-catenin translocates to the nucleus,
where it interacts with Tcf/Lef transcription factors to promote
the transcription of Wnt target genes. Therefore, APC and β-catenin
are the basic components involved in Wnt signaling. The association
between Wnt/β-catenin and Hh signaling is complex, as well as
cooperative and competitive (27).
The different responses to cyclopamine or celecoxib
may reflect a variety of genetic backgrounds in the colon cancer
cell lines. In HCT-116 cells, APC is the wild-type and the
CTNNB1 gene, which encodes β-catenin, is the mutant-type,
while in LoVo and HT-29 cells, APC is the mutant-type and
CTNNB1 is the wild-type (28). HCT-116 cells are often used as a
representative of constitutive Wnt signaling. We believe that LoVo
cells may be used as a representative of constitutive Hh signaling
in colon cancer cells, as Hh signaling is highly active in LoVo
cells. Furthermore, HCT-116 cells are COX-2 deficient, while HT-29
and LoVo cells exhibit COX-2 activities (29,30).
In conclusion, the results reported in the present
study indicated that Hh signaling is activated in LoVo cells and,
to a lesser degree, in HT-29 cells, but that it is inactive in
HCT-116 cells. The highly activated Hh signaling in LoVo cells is
associated with the absence of PTCH1 expression in these
cells. Celecoxib may inhibit the growth of HCT-116 cells via the
SMO-independent modulation of GLI1 activity. However, LoVo cells
are resistant to the growth inhibition of celecoxib, which has
little effect on Hh signaling in this cell line. Celecoxib inhibits
the growth of HT-29 cells by partly inhibiting the activity of Hh
signaling. These results suggest that cyclopamine and celecoxib are
potential treatment options for the targeted therapy of colon
cancer.
Acknowledgements
This study was in part supported by the Ministry of
Education, China (grant no. 20110092110043).
References
|
1
|
Globocan. 2012, Estimated Cancer
Incidence, Mortality and Prevalance Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed August 7, 2014
|
|
2
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.
|
|
3
|
Lampropoulos P, Zizi-Sermpetzoglou A,
Rizos S, Kostakis A, Nikiteas N and Papavassiliou AG: TGF-beta
signalling in colon carcinogenesis. Cancer Lett. 314:1–7. 2012.
|
|
4
|
Koduru S, Kumar R, Srinivasan S, Evers MB
and Damodaran C: Notch-1 inhibition by Withaferin-A: a therapeutic
target against colon carcinogenesis. Mol Cancer Ther. 9:202–210.
2010.
|
|
5
|
Lièvre A, Blons H and Laurent-Puig P:
Oncogenic mutations as predictive factors in colorectal cancer.
Oncogene. 29:3033–3043. 2010.
|
|
6
|
Gulino A, Ferretti E and De Smaele E:
Hedgehog signalling in colon cancer and stem cells. EMBO Mol Med.
1:300–302. 2009.
|
|
7
|
Mazumdar T, DeVecchio J, Shi T, Jones J,
Agyeman A and Houghton JA: Hedgehog signaling drives cellular
survival in human colon carcinoma cells. Cancer Res. 71:1092–1102.
2011.
|
|
8
|
Berman DM, Karhadkar SS, Maitra A, et al:
Widespread requirement for Hedgehog ligand stimulation in growth of
digestive tract tumours. Nature. 425:846–851. 2003.
|
|
9
|
Chatel G, Ganeff C, Boussif N, Delacroix
L, Briquet A, Nolens G and Winkler R: Hedgehog signaling pathway is
inactive in colorectal cancer cell lines. Int J Cancer.
121:2622–2627. 2007.
|
|
10
|
Fu X, Deng H, Zhao L, Li J, Zhou Y and
Zhang Y: Distinct expression patterns of hedgehog ligands between
cultured and primary colorectal cancers are associated with
aberrant methylation of their promoters. Mol Cell Biochem.
337:185–192. 2010.
|
|
11
|
Maloverjan A and Piirsoo M: Mammalian
homologues of Drosophila fused kinase. Vitam Horm. 88:91–113.
2012.
|
|
12
|
Pasca di Magliano M and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003.
|
|
13
|
Jenkins D: Hedgehog signalling: emerging
evidence for non-canonical pathways. Cell Signal. 21:1023–1034.
2009.
|
|
14
|
Hahn H, Wicking C, Zaphiropoulous PG, et
al: Mutations of the human homolog of Drosophila patched in the
nevoid basal cell carcinoma syndrome. Cell. 85:841–851. 1996.
|
|
15
|
Teglund S and Toftgård R: Hedgehog beyond
medulloblastoma and basal cell carcinoma. Biochim Biophys Acta.
1805:181–208. 2010.
|
|
16
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
|
|
17
|
Al-Wadei HA, Al-Wadei MH, Ullah MF and
Schuller HM: Celecoxib and GABA cooperatively prevent the
progression of pancreatic cancer in vitro and in xenograft models
of stress-free and stress-exposed mice. PLoS One. 7:e433762012.
|
|
18
|
Jin CH, Wang AH, Chen JM, Li RX, Liu XM,
Wang GP and Xing Q: Observation of curative efficacy and prognosis
following combination chemotherapy with celecoxib in the treatment
of advanced colorectal cancer. J Int Med Res. 39:2129–2140.
2011.
|
|
19
|
Lum L and Beachy PA: The Hedgehog response
network: sensors, switches, and routers. Science. 304:1755–1759.
2004.
|
|
20
|
You S, Zhou J, Chen S, Zhou P, Lv J, Han X
and Sun Y: PTCH1, a receptor of Hedgehog signaling pathway, is
correlated with metastatic potential of colorectal cancer. Ups J
Med Sci. 115:169–175. 2010.
|
|
21
|
Qualtrough D, Buda A, Gaffield W, Williams
AC and Paraskeva C: Hedgehog signalling in colorectal tumour cells:
induction of apoptosis with cyclopamine treatment. Int J Cancer.
110:831–837. 2004.
|
|
22
|
Yoshimoto AN, Bernardazzi C, Carneiro AJ,
et al: Hedgehog pathway signaling regulates human colon carcinoma
HT-29 epithelial cell line apoptosis and cytokine secretion. PLoS
One. 7:e453322012.
|
|
23
|
Zhu Y, James RM, Peter A, Lomas C, Cheung
F, Harrison DJ and Bader SA: Functional Smoothened is required for
expression of GLI3 in colorectal carcinoma cells. Cancer Lett.
207:205–214. 2004.
|
|
24
|
Wang TP, Hsu SH, Feng HC and Huang RF:
Folate deprivation enhances invasiveness of human colon cancer
cells mediated by activation of sonic hedgehog signaling through
promoter hypomethylation and cross action with transcription
nuclear factor-kappa B pathway. Carcinogenesis. 33:1158–1168.
2012.
|
|
25
|
Lou J, Fatima N, Xiao Z, Stauffer S,
Smythers G, Greenwald P and Ali IU: Proteomic profiling identifies
cyclooxygenase-2-independent global proteomic changes by celecoxib
in colorectal cancer cells. Cancer Epidemiol Biomarkers Prev.
15:1598–1606. 2006.
|
|
26
|
Takahashi-Yanaga F, Yoshihara T, Jingushi
K, Miwa Y, Morimoto S, Hirata M and Sasaguri T: Celecoxib-induced
degradation of T-cell factors-1 and −4 in human colon cancer cells.
Biochem Biophys Res Commun. 377:1185–1190. 2008.
|
|
27
|
Wilson NH and Stoeckli ET: Sonic Hedgehog
regulates Wnt activity during neural circuit formation. Vitam Horm.
88:173–209. 2012.
|
|
28
|
Ilyas M, Tomlinson IP, Rowan A, Pignatelli
M and Bodmer WF: Beta-catenin mutations in cell lines established
from human colorectal cancers. Proc Natl Acad Sci USA.
94:10330–10334. 1997.
|
|
29
|
van Erk MJ, Krul CA, Caldenhoven E,
Stierum RH, Peters WH, Woutersen RA and van Ommen B: Expression
profiling of colon cancer cell lines and colon biopsies: towards a
screening system for potential cancer-preventive compounds. Eur J
Cancer Prev. 14:439–457. 2005.
|
|
30
|
Shida D, Kitayama J, Yamaguchi H,
Yamashita H, Mori K, Watanabe T and Nagawa H: Lysophosphatidic acid
transactivates both c-Met and epidermal growth factor receptor, and
induces cyclooxygenase-2 expression in human colon cancer LoVo
cells. World J Gastroenterol. 11:5638–5643. 2005.
|