Introduction
In East Asian countries, esophageal squamous cell
carcinoma (ESCC) is the major histological form of esophageal
cancer. The disease is also one of the most lethal digestive tract
malignances (1). In the majority of
cases, the initial diagnosis of ESCC is made when the malignancy
has already progressed to an advanced stage (1). Despite recent improvements in
multi-treatment approaches, including surgery, radiotherapy and
chemotherapy, the prognosis for patients with ESCC remains
unsatisfactory (2). Predicting a
prognosis by examining the clinicopathological characteristics
remains difficult, even when using the tumor-node-metastasis
staging system. This is due to considerable tumor variability and
heterogeneity within the same pathological stage.
The IMP3 gene, also known as the K homology
domain-containing gene (KOC) or L523S, encodes the IMP3 protein
(3). IMP3 is located on chromosome
7p11.5 and encodes a 4350-bp mRNA and a 580-aa protein. IMP3 is
expressed in the developing epithelium, muscle and placenta during
the early stages of human and mouse embryogenesis, and low or
undetectable levels of IMP3 are present in adult tissues (4,5). IMP3
has been shown to be overexpressed in testicular cancer, renal cell
carcinoma, ovarian carcinoma, gastric cancer, colon cancer and
adenocarcinoma of the lung (6–15). The
IMP3 protein, together with IMP-1 and IMP-2, has different
functions in various post-transcriptional processes, including mRNA
localization, mRNA turnover and translational control (16–19).
The IMP3 gene has previously been used as a marker to detect
malignant cells in fine-needle aspirates (20). Additionally, in K562 leukemia cells,
the inhibition of IMP3 has been shown to result in apoptosis,
indicating that it may be vital for cancer cell survival (18). IMP3 is a prognostic biomarker in
patients with endometrial serous carcinoma and renal cell
carcinoma. In such cases, IMP3 expression appears to predict an
increased likelihood of metastasis following surgery and a shorter
metastasis-free survival time (8–11,15).
However, to the best of our knowledge, the clinicopathological and
prognostic significance of IMP3 expression in ESCC remains unknown.
In the present study, the prevalence and clinicopathological
significance of IMP3 expression were investigated with regard to
overall survival (OS) and recurrence-free survival (RFS) in 191
patients.
Materials and methods
Patients and treatments
The present study examined 191 patients with
pathologically confirmed primary ESCC who underwent surgical
resection at the Osaka University Hospital (Osaka, Japan) between
1998 and 2007 (Table I). Approval
for the study was obtained from the Ethics Committee of Osaka
University Hospital. The study population consisted of 24 female
and 167 male patients who ranged between 29 and 85 years of age
(median, 62.7 years). All patients underwent a subtotal
esophagectomy via a right thoracotomy, with a two- or three-field
lymphadenectomy, with curative resection. None of the patients
succumbed to post-operative complications. Of the 104 patients with
lymph node metastases at the initial diagnosis, 86 received
neoadjuvant chemotherapy (NAC), which consisted of two courses of
5-fluorouracil (700 mg/m2 on days one to seven),
cisplatin (70 mg/m2 on day one) and Adriamycin (35
mg/m2 on day one). Following surgery, the patients were
followed up every 3 months by physical examination and an analysis
of serum tumor markers (squamous cell carcinoma antigen and
carcinoembryonic antigen), every 6 months by computed tomography
scanning and abdominal ultrasonography, and every year by endoscopy
until tumor recurrence became evident. Patients exhibiting tumor
recurrence received chemotherapy or chemoradiotherapy for as long
as this regimen was systemically tolerated. The mean OS time was 41
months, and the mean RFS time was 39 months.
 | Table ICharacteristics of patients with
ESCC. |
Table I
Characteristics of patients with
ESCC.
| Parameters | Value |
|---|
| Median age, years
(range) | 62.7 (29–85) |
| Gender, n (%) |
| Male | 167 (87.4) |
| Female | 24 (12.6) |
| Histology of SCC, n
(%) |
|
Poorly-differentiated | 45 (23.6) |
|
Moderately-differentiated | 99 (51.8) |
|
Well-differentiated | 47 (24.6) |
| Pathological
classificationa, n (%) |
| pT |
| 0 | 0 (0.0) |
| 1 | 51 (26.7) |
| 2 | 30 (15.7) |
| 3 | 93 (48.7) |
| 4 | 17 (8.9) |
| pN |
| N0 | 68 (35.6) |
| N1 | 53 (27.7) |
| N2 | 35 (18.3) |
| N3 | 35 (18.3) |
| pStage |
| 0 | 0 (0.0) |
| I | 39 (20.4) |
| II | 53 (27.7) |
| III | 63 (33.0) |
| IV | 36 (18.8) |
Immunohistochemical analysis
IMP3 expression was examined in formalin-fixed,
paraffin-embedded ESCC tissue sections by immunohistochemistry
(IHC). One representative slide with the deepest tumor invasion was
selected from each patient and examined by IHC. The tissue sections
were deparaffinized in xylene and then rehydrated through a graded
ethanol series. For antigen retrieval, the slides were incubated by
autoclaving at 110°C in 10 mm Tris and 1 mm EDTA buffer (pH 9.0)
for 20 min. Endogenous peroxidase activity was blocked with 0.3%
H2O2 in methanol for 20 min and non-specific
binding was blocked with 10% normal serum for 20 min. Subsequently,
the tissue slides were incubated overnight with anti-IMP3 antibody
(monoclonal mouse anti-human L523S; dilution, 1:200; Dako
Cytomation, Carpinteria, CA, USA) at 4°C in a humidified chamber.
The bound antibody was visualized using the Avidin/Biotin Complex
Peroxidase Detection System (Vector Laboratories, Burlingame, CA,
USA). Finally, the sections were incubated in 3,3′-diaminobenzidine
tetrahydrochloride with 0.05% H2O2 for 3 min
and counterstained with 0.1% hematoxylin. IMP3 staining for each
ESCC sample was defined as positive when >10% of the cancer
cells in the section were immunoreactive with the anti-IMP3
antibody. Staining was defined as negative when ≤10% of the cancer
cells in the section were positive.
Statistical analysis
Statistical analysis was performed using JMP
software (JMP version 9.0.2; SAS Institute, Cary, NC, USA). The
association between IMP3 expression and the clinicopathological
parameters was assessed using the χ2 test. The RFS and
OS were assessed using the Kaplan-Meier method and compared using
the log-rank test. All the parameters that were found to be
significant in a univariate analysis using the Cox proportional
hazard model were entered into a multivariate survival analysis.
P-values were derived from two-tailed testing and P<0.05 was
considered to indicate a statistically significant difference.
Results
IMP3 expression in ESCC
A total of 191 samples that contained cancerous and
non-cancerous lesions were evaluated for IMP3 expression using IHC.
Of these, 113 (59.2%) showed positive IMP3 expression that was
predominantly localized to the cytoplasm of the tumor cells, along
with faint nuclear staining (Fig.
1A). The remaining 78 (40.8%) were negative for IMP3 expression
(Fig. 1B). The positive staining
was almost homogeneous in individual cancer foci and in different
areas, such as in the surface, central and deepest areas, of the
cancer lesions. By contrast, none of the normal squamous epithelia
exhibited substantial IMP3 staining, although certain basal cells
showed faint nuclear staining (Fig.
1C).
Association between IMP3 expression and
clinicopathological parameters
Table II lists the
associations between IMP3 expression and the clinicopathological
parameters. The IMP3-positive tumors were significantly associated
with deeper tumor invasion and lymph node metastases compared with
the IMP3-negative tumors (P=0.0001 and P=0.026, respectively). No
significant associations were observed between IMP3 expression and
other parameters, including age, gender, histology and use of
NAC.
 | Table IICorrelation between IMP3 expression
and clinicopathological parameters. |
Table II
Correlation between IMP3 expression
and clinicopathological parameters.
| IMP3 expression, n
(%) | |
|---|
|
| |
|---|
| Parameters | Positive | Negative | P-value |
|---|
| Age, years | | | |
| <65 | 64 (33.5) | 47 (24.6) | 0.6179 |
| ≥65 | 49 (25.7) | 31 (16.2) | |
| Gender | | | |
| Male | 97 (50.8) | 70 (36.6) | 0.4191 |
| Female | 16 (8.4) | 8 (4.2) | |
| Histologya | | | |
| Poor/moderate | 89 (46.6) | 55 (28.8) | 0.1955 |
| Well | 24 (12.6) | 23 (12.0) | |
| Neoadjuvant
chemotherapy | | | |
| Yes | 48 (25.1) | 38 (19.9) | 0.4654 |
| No | 65 (34.0) | 40 (20.9) | |
| Depth of tumor
invasionb | | | |
| pT1–2 | 35 (18.3) | 46 (24.1) | 0.0010 |
| pT3–4 | 78 (40.8) | 32 (16.8) | |
| Lymph node
metastasisb | | | |
| pN0 | 33 (17.3) | 35 (18.3) | 0.0267 |
| pN1–3 | 80 (41.9) | 43 (22.5) | |
| pStageb | | | |
| I, II | 67 (35.1) | 46 (24.1) | 0.0003 |
| III, IV | 46 (24.1) | 32 (16.8) | |
Association between IMP3 expression and
survival
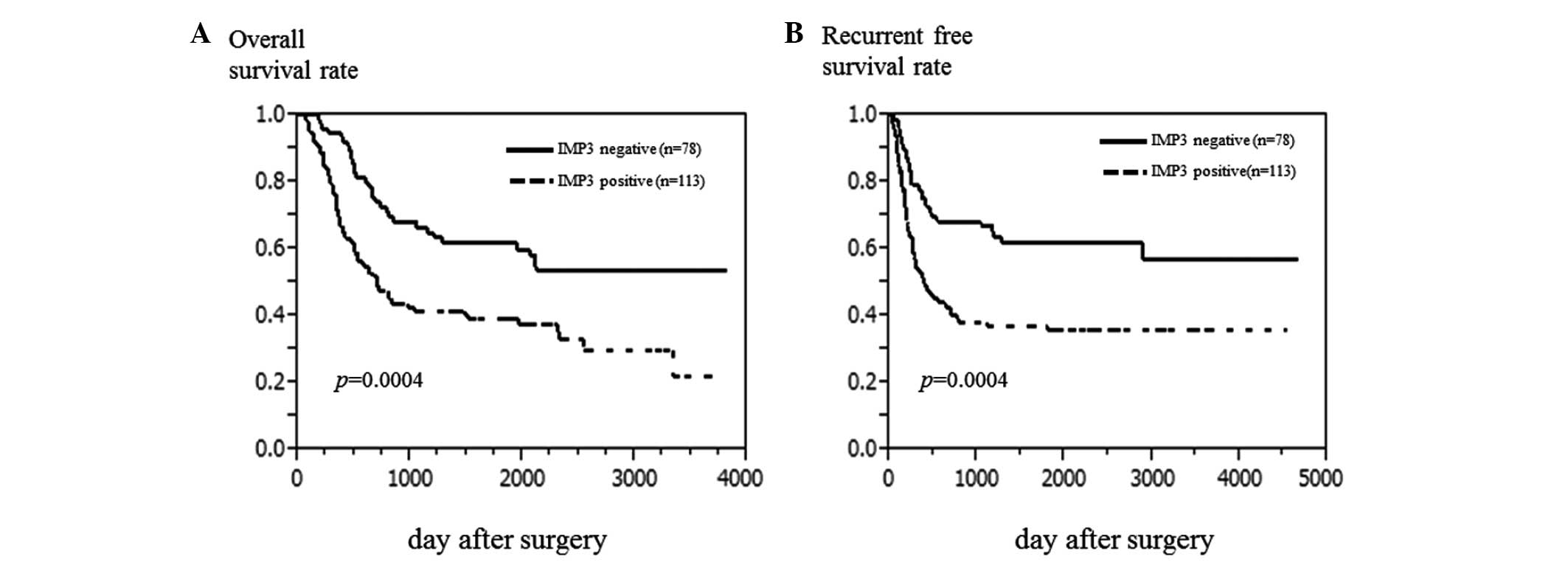
The 5-year OS rate of the population was 48.5%.
Patients with IMP3-positive tumors experienced a poorer 5-year OS
rate compared with those with IMP3-negative tumors (39.3 vs. 61.7%,
P=0.0004; Fig. 2A). Similarly,
patients with IMP3-positive tumors experienced a poorer RFS rate
compared with those with IMP3-negative tumors (35.7 vs. 61.9%,
P=0.0004; Fig. 2B). By univariate
analyses, histology [hazard ratio (HR), 1.94; 95% confidence
interval (CI), 1.18–3.49; P=0.0082], pathological T stage (pT; HR,
2.34; 95% CI, 1.55–3.62; P<0.0001), pathological N stage (pN;
HR, 2.85; 95% CI, 1.81–4.69; P<0.0001), lymphatic invasion (HR,
2.08; 95% CI, 1.26–3.70; P=0.0036), venous invasion (HR, 1.79; 95%
CI, 1.21–2.64; P=0.0039), NAC (HR, 2.01; 95% CI, 1.35–3.00;
P=0.0005), and IMP expression (HR, 2.12; 95% CI, 1.40–3.29;
P=0.0003) were significantly correlated with OS (Table III). The seven parameters that
demonstrated statistical significance (P<0.05) by univariate
analysis were further analyzed by multivariate analysis.
Multivariate analysis showed that pathological lymph node
metastasis was the poorest prognostic factor (HR, 2.19; 95% CI,
1.36–3.66; P=0.0010), followed by NAC (HR, 1.88; 95% CI, 1.24–2.86;
P=0.0028), histology (HR, 1.87; 95% CI, 1.13–3.49; P=0.014), and
IMP3 expression (HR, 1.84; 95% CI, 1.18–2.93; P=0.0064) (Table III).
 | Table IIIUnivariate and multivariate analysis
of OS using Cox’s proportional hazard model. |
Table III
Univariate and multivariate analysis
of OS using Cox’s proportional hazard model.
| | Univariate | Multivariate |
|---|
| |
|
|
|---|
| Parameter | Number of cases | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>65
years) | 78/113 | 1.24 (0.84–1.84) | 0.2766 | | |
| Gender
(female/male) | 24/167 | 1.05 (0.56–1.82) | 0.8591 | | |
| Histology
(poor-moderate/well)a | 144/47 | 1.94
(1.18–3.49) | 0.0082 | 1.87
(1.13–3.29) | 0.0134 |
| pT
(T3,4/T1,2)b | 110/81 | 2.34
(1.55–3.62) | <0.0001 | 1.28
(0.79–2.10) | 0.3303 |
| pN (N1–3,
N0)b | 123/68 | 2.85
(1.81–4.69) | <0.0001 | 2.19
(1.36–3.66) | 0.0010 |
| Lympathic invasion
(present/absent) | 148/43 | 2.08
(1.26–3.70) | 0.0036 | 1.11
(0.62–2.08) | 0.7354 |
| Venous invasion
(present/absent) | 79/112 | 1.79
(1.21–2.64) | 0.0039 | 1.22
(0.79–1.91) | 0.3740 |
| NAC (yes/no) | 86/105 | 2.01
(1.35–3.00) | 0.0005 | 1.88
(1.24–2.86) | 0.0028 |
| IMP3 expression
(positive/negative) | 113/78 | 2.12
(1.40–3.29) | 0.0003 | 1.84
(1.18–2.93) | 0.0064 |
Discussion
IMP3 is an RNA-binding protein and a KH
domain-containing member of the IMP family. In mice, IMPs are
primarily expressed during early embryogenesis and at
mid-gestation, but they are not expressed in the majority of adult
human tissues (3,4,22).
IMP3 has been reported to function by regulating tumor cell
proliferation, migration and metastasis. IMP3 has been shown to
promote tumor cell proliferation through the upregulation of IGF2,
a potent mitogenic factor previously shown to exert effects in a
number of diseases (18,23,24).
Studies have additionally found that IMP3 can exert a marked effect
on cellular adhesion and invasion during normal development and
during the development of cancers (25). For these reasons, strong IMP3
expression is regarded as an indicator of a poor prognosis
(6,9,10,26,27).
However, to the best of our knowledge, the clinicopathological and
prognostic significance of IMP3 expression in ESCC has not been
reported.
The present study demonstrated the positive
immunoreactivity to IMP3 of 59.2% of ESCC surgical samples.
Positive IMP3 expression was significantly associated with
pathological factors associated with tumor progression [pT, pN and
pathological stage (pStage)]. IMP3 was identified as a prognostic
factor for OS. Although pT is generally considered to be an
independent prognostic factor, this was not the case in the present
series. In the present study, patients with advanced ESCC received
NAC. Hence, the effect of pT was canceled by the effect of NAC in
the multivariate analysis. This result was similar to that reported
in other cancers (6,9–11,26,27).
However, the clinical association between IMP3 and a worse
prognosis of ESCC remains poorly defined. Yoshino et al
(28) reported that IMP3 mRNA
expression was associated with resistance to radiation therapy in
ESCC cell lines. Further studies to investigate this should
therefore be performed in the future.
Several characteristics of IMP3 indicate that it may
be a potentially attractive prognostic marker. First, IMP3 IHC
staining is a simple and reliable assay to perform (9). In the majority of cases, carcinomas
are treated surgically, allowing chemotherapy and radiation therapy
to be combined. Tumor tissues are thus routinely available for IHC
staining using the monoclonal L523 antibody. The present study
found that IMP3 IHC was reproducible and could be readily performed
on ESCC tissues. The simplicity of this assay will enable a
pre-operative diagnosis from the analysis of biopsy tissue.
Regarding the polymerase chain reaction (PCR)-based method, IMP3
has been used as a molecular marker to predict peritoneal
recurrence following curative surgery for gastric cancer (11), and PCR amplification of IMP3 from
biliary structure specimens have been useful to distinguish between
benign and malignant lesions (29).
Furthermore, IMP3 has been considered a potential target for
immunotherapy. A phase II study using a peptide vaccine therapy,
which included IMP3, has been performed for patients with advance
ESCC who failed to respond to standard therapies (30). It has been reported that the immune
response induced by the vaccination may improve the prognosis for
patients with advanced ESCC.
In conclusion, in the present study, IMP3, a novel
mRNA-binding protein, was shown to be frequently expressed in ESCC.
IMP3 expression was more commonly observed in ESCC patients with
poor prognostic factors. IMP3 may be a potential IHC biomarker that
can be used to evaluate the tumor progression and prognosis of
ESCC.
References
|
1
|
Shimada H, Nabeya Y, Okazumi S, et al:
Prediction of survival with squamous cell carcinoma antigen in
patients with resectable esophageal squamous cell carcinoma.
Surgery. 133:486–494. 2003.
|
|
2
|
Tamoto E, Tada M, Murakawa K, et al:
Gene-expression profile changes correlated with tumor progression
and lymph node metastasis in esophageal cancer. Clin Cancer Res.
10:3629–3638. 2004.
|
|
3
|
Nielsen J, Christiansen J, Lykke-Andersen
J, et al: A family of insulin-like growth factor II mRNA-binding
proteins represses translation in late development. Mol Cell Biol.
19:1262–1270. 1999.
|
|
4
|
Mueller-Pillasch F, Pohl B, Wilda M, et
al: Expression of the highly conserved RNA binding protein KOC in
embryogenesis. Mech Dev. 88:95–99. 1999.
|
|
5
|
Yaniv K and Yisraeli JK: The involvement
of a conserved family of RNA binding proteins in embryonic
development and carcinogenesis. Gene. 287:49–54. 2002.
|
|
6
|
Gu L, Shigemasa K and Ohama K: Increased
expression of IGF II mRNA-binding protein 1 mRNA is associated with
an advanced clinical stage and poor prognosis in patients with
ovarian cancer. Int J Oncol. 24:671–678. 2004.
|
|
7
|
Hammer NA, Hansen T, Byskov AG, et al:
Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and
testicular cancer. Reproduction. 130:203–212. 2005.
|
|
8
|
Hoffmann NE, Sheinin Y, Lohse CM, et al:
External validation of IMP3 expression as an independent prognostic
marker for metastatic progression and death for patients with clear
cell renal cell carcinoma. Cancer. 112:1471–1479. 2008.
|
|
9
|
Jiang Z, Chu PG, Woda BA, et al: Analysis
of RNA-binding protein IMP3 to predict metastasis and prognosis of
renal-cell carcinoma: a retrospective study. Lancet Oncol.
7:556–564. 2006.
|
|
10
|
Li D, Yan D, Tang H, et al: IMP3 is a
novel prognostic marker that correlates with colon cancer
progression and pathogenesis. Ann Surg Oncol. 16:3499–3506.
2009.
|
|
11
|
Okada K, Fujiwara Y, Nakamura Y, et al:
Oncofetal protein, IMP3, a potential marker for prediction of
postoperative peritoneal dissemination in gastric adenocarcinoma. J
Surg Oncol. 105:780–785. 2012.
|
|
12
|
Simon R, Bourne PA, Yang Q, et al:
Extrapulmonary small cell carcinomas express K homology domain
containing protein overexpressed in cancer, but carcinoid tumors do
not. Hum Pathol. 38:1178–1183. 2007.
|
|
13
|
Xu H, Bourne PA, Spaulding BO and Wang HL:
High-grade neuroendocrine carcinomas of the lung express K homology
domain containing protein overexpressed in cancer but carcinoid
tumors do not. Hum Pathol. 38:555–563. 2007.
|
|
14
|
Yantiss RK, Woda BA, Fanger GR, et al: KOC
(K homology domain containing protein overexpressed in cancer): a
novel molecular marker that distinguishes between benign and
malignant lesions of the pancreas. Am J Surg Pathol. 29:188–195.
2005.
|
|
15
|
Zheng W, Yi X, Fadare O, et al: The
oncofetal protein IMP3: a novel biomarker for endometrial serous
carcinoma. Am J Surg Pathol. 32:304–315. 2008.
|
|
16
|
Doyle GA, Betz NA, Leeds PF, et al: The
c-myc coding region determinant-binding protein: a member of a
family of KH domain RNA-binding proteins. Nucleic Acids Res.
26:5036–5044. 1998.
|
|
17
|
Gress TM, Müller-Pillasch F, Geng M, et
al: A pancreatic cancer-specific expression profile. Oncogene.
13:1819–1830. 1996.
|
|
18
|
Liao B, Hu Y, Herrick DJ and Brewer G: The
RNA-binding protein IMP3 is a translational activator of
insulin-like growth factor II leader-3 mRNA during proliferation of
human K562 leukemia cells. J Biol Chem. 280:18517–18524. 2005.
|
|
19
|
Runge S, Nielsen FC, Nielsen J, et al: H19
RNA binds four molecules of insulin-like growth factor II
mRNA-binding protein. J Biol Chem. 275:29562–29569. 2000.
|
|
20
|
Mueller F, Bommer M, Lacher U, et al: KOC
is a novel molecular indicator of malignancy. Br J Cancer.
88:699–701. 2003.
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: Oesophagus including oesophagogastric junction. International
Union Against Cancer (UICC) TNM classification of Malignant Tumors.
7th edition. Wiley-Blackwell; New York, NY: pp. 66–72. 2009
|
|
22
|
Hansen TV, Hammer NA, Nielsen J, et al:
Dwarfism and impaired gut development in insulin-like growth factor
II mRNA-binding protein 1-deficient mice. Mol Cell Biol.
24:4448–4464. 2004.
|
|
23
|
Chao W and D’Amore PA: IGF2: epigenetic
regulation and role in development and disease. Cytokine Growth
Factor Rev. 19:111–120. 2008.
|
|
24
|
Foulstone E, Prince S, Zaccheo O, et al:
Insulin-like growth factor ligands, receptors, and binding proteins
in cancer. J Pathol. 205:145–153. 2005.
|
|
25
|
Vikesaa J, Hansen TV, Jønson L, et al:
RNA-binding IMPs promote cell adhesion and invadopodia formation.
EMBO J. 25:1456–1468. 2006.
|
|
26
|
Jeng YM, Wang TH, Lu SH, Yuan RH and Hsu
HC: Prognostic significance of insulin-like growth factor II
mRNA-binding protein 3 expression in gastric adenocarcinoma. Br J
Surg. 96:66–73. 2009.
|
|
27
|
Pryor JG, Bourne PA, Yang Q, et al: IMP3
is a novel progression marker in malignant melanoma. Mod Pathol.
21:431–437. 2008.
|
|
28
|
Yoshino K, Motoyama S, Koyota S, et al:
Identification of insulin-like growth factor 2 mRNA-binding protein
3 as a radioresistance factor in squamous esophageal cancer cells.
Dis Esophagus. Sep 18–2012.(Epub ahead of print).
|
|
29
|
Nischalke HD, Schmitz V, Luda C, et al:
Detection of IGF2BP3, HOXB7, and NEK2 mRNA expression in brush
cytology specimens as a new diagnostic tool in patients with
biliary strictures. PLoS One. 7:e421412012.
|
|
30
|
Kono K, Iinuma H, Akutsu Y, et al:
Multicenter, phase II clinical trial of cancer vaccination for
advanced esophageal cancer with three peptides derived from novel
cancer-testis antigens. J Transl Med. 10:1412012.
|