Introduction
The incidence of primary squamous cell carcinoma
(SCC) of the stomach ranges between 0.04 and 0.09%, making the
disease an extremely rare entity (1). SCC was first identified in 1895, and
<100 cases have been reported worldwide (2–15).
Although theories exist regarding its development in the stomach
(2,6,7), the
pathogenesis of SCC remains unclear. Primary SCC of the stomach is
often diagnosed at a late stage, and its prognosis is generally
poor. Furthermore, no effective adjuvant chemotherapy has been
identified.
Case report
A 61-year-old male was admitted to the Pusan
National University Yangsan Hospital (Yangsan, Korea) with a
six-month history of weight loss, totaling ~6 kg. The patient
exhibited no symptoms, including abdominal pain, nausea, vomiting
or melena. Furthermore, a physical examination and routine
laboratory tests on admission revealed no specific abnormalities.
The patient underwent an abdominal computed tomography (CT) scan,
which demonstrated a heterogeneously-enhanced tumor mass of 6.5 cm
(Fig. 1). Endoscopic examination
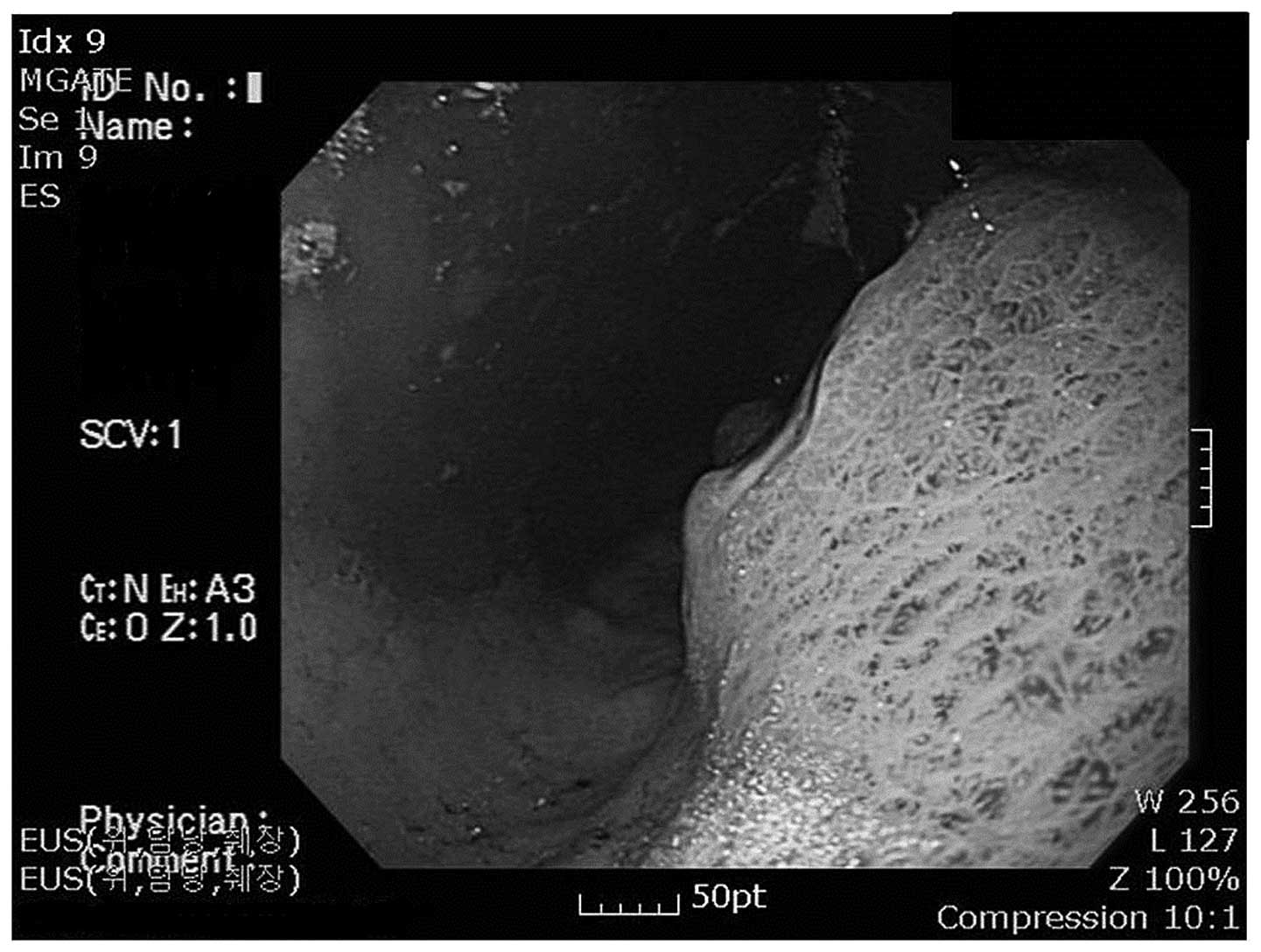
and endoscopic ultrasonography of the gastrointestinal tract
revealed a submucosal tumor mass of 6 cm, with surface ulceration
on the fundus and cardia of the stomach (Fig. 2). These observations indicated that
the tumor was derived from non-epithelial cells, such as those that
derive a gastrointestinal stroma tumor or malignant lymphoma.
Notably, a biopsy specimen revealed the diagnosis of SCC.
The patient underwent a total gastrectomy. The
resected stomach exhibited a 7.0×6.7×4.5-cm tumor in the cardia, in
the vicinity of the gastroesophageal junction (GEJ). The tumor
exhibited a superiorly located ulcer and infiltrated the stomach
wall (Fig. 3). A 0.7-cm tumor-free
section was identified between the GEJ and the tumor.
Histologically, the tumor was composed of moderately-differentiated
squamous cells with keratinization and intercellular bridges in
certain sections (Fig. 4). No
evidence of glandular differentiation was identified within the
tumor. SCC in situ without squamous metaplasia was observed
adjacent to the tumor. The tumor was not contiguous with the
squamous mucosa of the distal esophagus and was growing
predominantly in the submucosal and muscle layers. The serosal
layer exposed to the tumor and the gastric mucosa exhibited focal
invasion by the tumor. One section of the lymph nodes of the lesser
curvature exhibited metastatic SCC.
To investigate the role of human papillomavirus
(HPV) and Epstein-Barr virus (EBV) infection in the carcinogenesis
of SCC arising in the stomach, DNA microarray and in situ
hybridization, respectively, were used for detection. However, no
evidence of HPV or EBV infection was identified in the patient.
One month after the surgery, combination
chemotherapy consisting of cisplatin (90 mg every 15 min) and
5-flourouracil (1,500 mg every 10 h) was administered for five days
every 21 days. The patient received two cycles of chemotherapy. A
follow-up abdominal CT scan four months later revealed tumor
invasion of the jejunum and pancreas, enlargement of abdominal
lymph nodes and multiple foci of liver metastasis. The patient
succumbed to the disease six months later.
Discussion
Primary SCC of the stomach consists of only SCC as
opposed to one consisting of adenocarcinoma, the most common
malignancy in the stomach. The clinicopathological features of 90
patients with SCC are shown in Table
I. Data were selected from previous studies in the English
literature (2–15). The incidence of SCC was three times
higher in the male patients compared with the females. The mean age
of the patients was 59.7 years and the tumor size ranged between
2.1 and 17 cm (mean size, 7.1 cm).
 | Table ICharacteristics of patients with
primary squamous cell carcinoma of the stomach (n=90). |
Table I
Characteristics of patients with
primary squamous cell carcinoma of the stomach (n=90).
| Parameter | Value |
|---|
| Mean patient age,
years | 59.7 |
| Gender, n (%) |
| Male | 63 (70.0) |
| Female | 18 (20.0) |
| Not stated | 9 (10.0) |
| Tumor location, n
(%) |
| Proximal | 22 (24.4) |
| Middle | 22 (24.4) |
| Distal | 18 (20.0) |
| Not stated | 28 (31.1) |
| Mean tumor size,
cm | 7.1 |
In SCC proximal to the GEJ, evidence of its gastric
origin must be present. The Japanese Gastric Cancer Association
proposed the following criteria for the diagnosis of primary SCC
(16): The tumor must consist of
only SCC without adenocarcinoma, and any tumor close to the GEJ
must not be diagnosed as gastric SCC without evidence that it
originated in the stomach. The diagnosis of primary gastric SCC
requires that normal gastric mucosa is present between the GEJ and
SCC of the stomach. The present case matched these criteria and
thus was diagnosed as primary SCC of the stomach cardia. However,
Parks (2) proposed that SCC
occurring in the cardia must not be considered as a primary gastric
carcinoma, as it may arise from the distal esophagus or misplaced
islands of squamous cells in the cardia. A number of previous
studies have proposed that it may be referred to as gastric SCC
when the lesion is completely separate from the esophageal
epithelium (3–5).
A number of theories regarding the origin of SCC of
the stomach have been proposed, including totipotent stem cells,
squamous metaplasia, foci of heterotopic squamous epithelium and
the overgrowth of a squamous epithelium element in a primary
adenocarcinoma. However, the pathogenesis of SCC remains unclear.
We favor the theory that SCC originates from the squamous
metaplastic epithelium or heterotopic squamous epithelium, as the
presence of squamous epithelium in the cardia has been reported
(6), squamous metaplasia has been
observed with SCC (7) and evidence
of SCC in situ was identified during pathological
examination in the present case.
Takita et al (17) proposed that EBV infection may be
involved in the pathogenesis of certain cases of gastric SCC. In
this study, a liquid hybridization assay for HPV infection and a
polymerase chain reaction for EBV infection was performed and
revealed the presence of EBV infection in surgical specimens of the
tumor. However, no evidence of HPV or EBV infection was identified
in the present case when using DNA microarray for HPV infection and
in situ hybridization for EBV infection.
A standard chemotherapy regimen for this disease has
not yet been established, and only one previous study has
demonstrated the efficacy of chemotherapy against the tumor
(4). The prognosis of primary SCC
of the stomach has not been clearly defined. However, previous
studies have indicated a poor prognosis for this disease, as the
majority of lesions are detected at an advanced stage, with marked
infiltrative growth. The present case exhibited disease progression
despite chemotherapy administration.
Acknowledgements
This study was supported by a two-year research
grant from Pusan National University (Yangsan, Gyeongsangnam-do,
Republic of Korea).
References
|
1
|
Straus R, Henschel S and Fortman DJ:
Primary adenosquamous carcinoma of the stomach. A case report and
review. Cancer. 24:985–995. 1969.
|
|
2
|
Parks RE: Squamous neoplasms of the
stomach. Am J Roentgenol Radium Ther Nucl Med. 101:447–449.
1967.
|
|
3
|
Schwab G, Wetscher G, Dietze O, Schmid K
and Pointner R: Primary squamous cell carcinoma of the stomach in a
seventeen-year-old boy. Surg Today. 22:561–564. 1992.
|
|
4
|
Marubashi S, Yano H, Monden T, et al:
Primary squamous cell carcinoma of the stomach. Gastric Cancer.
2:136–141. 1999.
|
|
5
|
Tokuhara K, Nakano T, Inoue K, Nakane Y
and Kwon AH: Primary squamous cell carcinoma in the gastric
remnant. Surg Today. 42:666–669. 2012.
|
|
6
|
Fass R and Sampliner RE: Extension of
squamous epithelium into the proximal stomach: a newly recognized
mucosal abnormality. Endoscopy. 32:27–32. 2000.
|
|
7
|
Oono Y, Fu K, Nagahisa E, et al: Primary
gastric squamous cell carcinoma in situ originating from gastric
squamous metaplasia. Endoscopy. 42(Suppl 2): E290–E291. 2010.
|
|
8
|
Raju GC, Barton EN, Marchack D and
Naraynsingh V: Hypercalcaemia in primary squamous cell carcinoma of
the stomach. J R Soc Med. 80:587–588. 1987.
|
|
9
|
Lee WA, Woo DK, Kim YI and Kim WH: p53,
p16 and RB expression in adenosquamous and squamous cell carcinomas
of the stomach. Pathol Res Pract. 195:747–752. 1999.
|
|
10
|
Dursun M, Yaldiz M, Işikdoğan A, Yilmaz G,
Canoruç F, Ormeci N and Yilmaz S: Primary squamous cell carcinoma
of the stomach: a case report and review of the literature. Eur J
Gastroenterol Hepatol. 15:329–330. 2003.
|
|
11
|
Hara J, Masuda H, Ishii Y, et al:
Exophytic primary squamous cell carcinoma of the stomach. J
Gastroenterol. 39:299–300. 2004.
|
|
12
|
Yildirim Y, Akcali Z, Bilezikci B and
Ozyilkan O: Primary squamous cell carcinoma of the stomach: a case
report. Tumori. 91:440–442. 2005.
|
|
13
|
Choi SB, Park SS, Oh SY, et al: Primary
squamous cell carcinoma of the stomach that developed with
Menetrier’s disease. Dig Dis Sci. 52:1722–1724. 2007.
|
|
14
|
Callacondo D, Ganoza-Salas A, Anicama-Lima
W, Quispe-Mauricio A and Longacre TA: Primary squamous cell
carcinoma of the stomach with paraneoplastic leukocytosis: a case
report and review of literature. Hum Pathol. 40:1494–1498.
2009.
|
|
15
|
Karaca G, Pekcici MR, Özer H, Köklü S,
Kavlakoğlu B, Astarci M and Güler O: Primary squamous cell
carcinoma of the stomach in a 68-years-old man. Geriatr Gerontol
Int. 11:119–120. 2011.
|
|
16
|
Japanese Gastric Cancer Association.
Japanese Classification of Gastric Carcinoma. 13th edition. Tokoyo:
Kanchara; 1999
|
|
17
|
Takita J, Kato H, Miyazaki T, et al:
Primary squamous cell carcinoma of the stomach: a case report with
immunohistochemical and molecular biologic studies.
Hepatogastroenterology. 52:969–974. 2005.
|