1. Brief history of tumor-targeting
bacterial therapy
The possibility of using bacteria in the treatment
of cancer has been recognized for more than a century (1,2).
Although it has potential as a novel treatment, the usage of
bacteria to target tumors has limitations due to potential
biosafety and other deleterious effects, including intrinsic
bacterial toxicity, lowered targeting efficiency, genetic
instability, and complicated interactions with other therapies
(3–7). The original observation of spontaneous
tumor regression from concurrent clostridial infection was reported
in 1813 (8,9). The first patient with cancer to be
purposefully infected with bacteria was possibly cured by German
physician Busch in 1868 (2,10). Over 20 years later, in 1890, Coley,
a New York physician, found that several patients with inoperable
tumors exhibited tumor regression subsequent to being inoculated
with Streptococcus pyogenes. However, the effect was not as
great as to eradicate the disease (11). In 1935, Connell observed tumor
regression in advanced cancer during therapy using sterile
filtrates from Clostridium histolyticum; the author
attributed these results to the production of enzymes (12). In 1947, the first study concerning
the deliberate injection of Clostridium was published
(13). Nonetheless, this field was
stagnant due to certain drawbacks (14). It was not until 1976, when Morales,
Eidinger and Bruce reported successful treatment of bladder cancer
with bacillus Calmette-Guérin (BCG), that this field began to
increase rapidly (15). Since then,
a number of investigative reports, experimental studies and reviews
have been published in this area. Due to these efforts, certain
attenuated and engineered obligatory anaerobic bacteria, such as
Clostridium, Bifidobacterium, Salmonella,
Mycobacterium, Bacillus and Listeria, are
known to specifically act as antitumor agents, and colonize hypoxic
and necrotic regions, which are present in solid tumors while
normally absent in other parts of the body.
2. Strategies using bacteria to target
tumors
The hypothesis that living bacteria may function as
anticancer therapeutic agents was first advanced in the middle of
the twentieth century. Due to the obstacles of hypoxia and
necrosis, accessing tumor tissue with traditional treatments has
proved difficult. However, bacteria may actively migrate away from
the vasculature and penetrate deep into tumor tissue and accumulate
(Fig. 1A). Three classes of
anaerobic and facultative anaerobes have been examined for use in
anticancer therapy (16,17): Bifidobacteria, facultative
intracellular bacteria and strictly anaerobic bacteria.
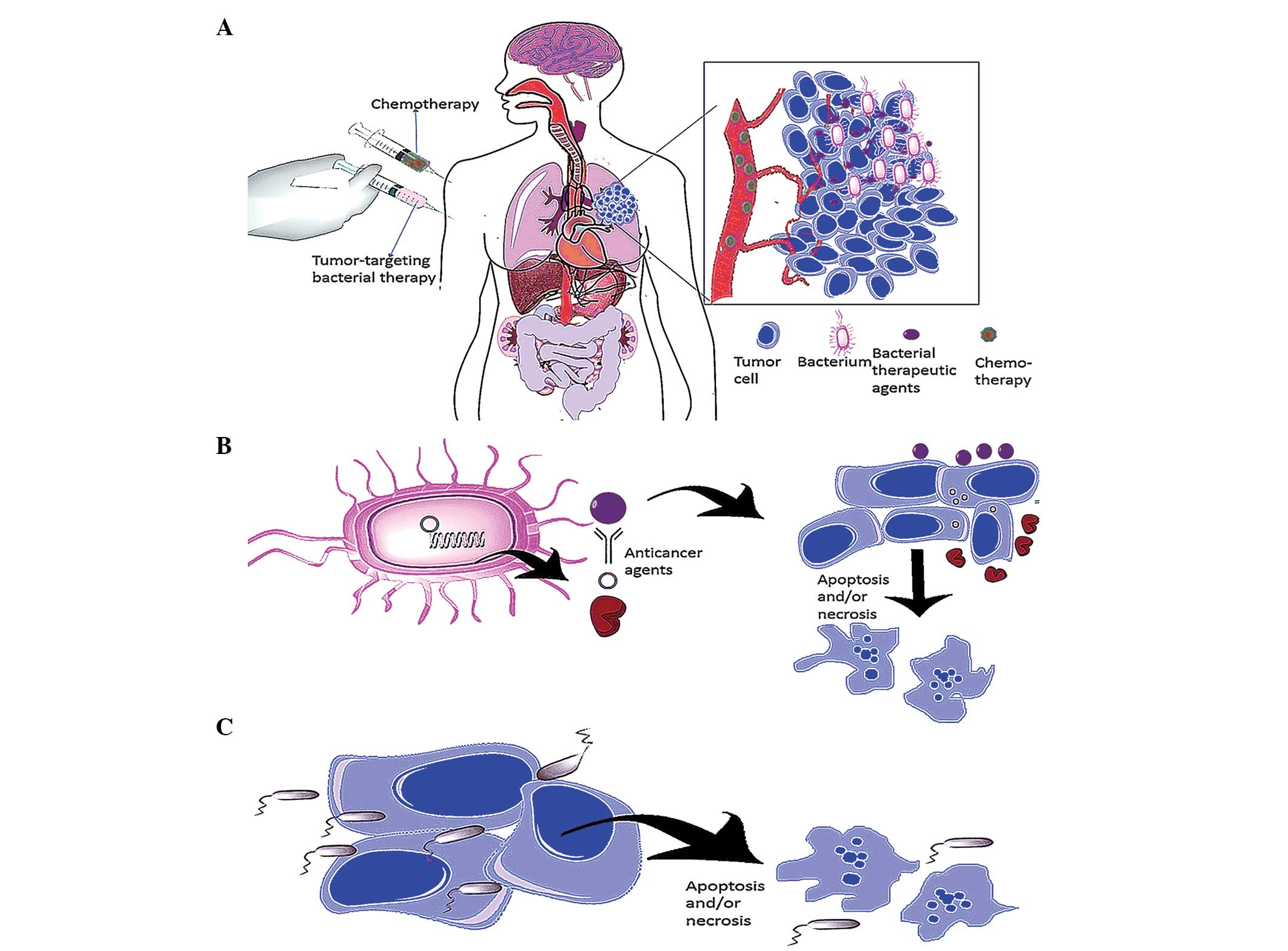 | Figure 1Strategies in tumor-targeting
bacterial therapy. (A) Bacteria have adequate tissue penetrating
ability. Anaerobic bacteria, which only colonize in areas devoid of
oxygen, may actively swim away from the vasculature, penetrate deep
into tumor tissue and accumulate following systematic injection
(pink syringe), a property traditional chemotherapy (green syringe)
does not possess. (B) Delivery of anticancer agents. Bacteria have
the ability to manufacture and deliver specific materials, which
may be coupled with particular anticancer agents. Engineered
bacteria kill cancer cells by expressing proteins that act against
tumors (e.g. cytotoxic agents, cytokines, antibodies, cytotoxins,
antiangiogenic agents and enzymes that convert the nonfunctional
prodrug to an active anticancer drug) and transferring eukaryotic
expression vectors into infected cancer cells. (C) Bacteria in
oncolytic therapy. Anaerobic bacteria swim into tumor tissue,
multiply in the hypoxic/necrotic areas and directly kill tumor
cells. |
The ideal criteria for the selection of therapeutic
bacteria (18–20) are as follows: Non-toxic to the host;
selective for a specific type of tumor; has the ability to
penetrate deeply into the tumor where ordinary treatment does not
reach; non-immunogenic (does not trigger an immune response
immediately but may be cleared by the host); harmless to normal
tissue; able to be manipulated easily; and has a drug carrier that
may be controlled. In addition to studies of bacteria designed to
induce immune responses (21) and
mediate antiangiogenesis therapy (22), a recent study has focused on the
usage of bacterial products as anticancer agents (23). Three main strategies in bacterial
cancer treatment are discussed in this review: i) Bacteria as tumor
markers; ii) Bacteria engineered to express anticancer agents
(Fig. 1B); and iii) Bacteria for
oncolytic therapy (Fig. 1C).
Bacteria as tumor markers
As replicating anaerobic bacteria are able to
selectively target tumors, the use of these bacteria may be an
innovative approach for locating tumors that is simple and direct,
but practical and effective. Two types of non-bacterial material
have served as tumor markers: Viral vectors, including adenovirus,
adeno-associated virus, herpes simplex virus (HSV)-1, HSV amplicon,
Sindbis, poliovirus replicon and lentivirus/Moloney murine leukemia
virus; and non-viral vectors, such as therapeutic DNA, microRNA,
short hairpin (sh)RNA, small interfering (si)RNA and
oligodeoxynucleotides (ODNs) (24–27).
However, anaerobic bacteria are preferable to these other two types
of tumor marker due to increased mobility (Table I). Once the marker has been
administered, a number of methods may be used to locate the tumor,
including bioluminescence, fluorescence and magnetic resonance
imaging (MRI), as well as positron emission tomography (6). Bacteria may be detected using light,
MRI or positron emission tomography (28,29).
 | Table IMaterials used as tumor markers. |
Table I
Materials used as tumor markers.
| A, Viral
vectors |
|---|
|
|---|
| Examples | Advantages | Disadvantages |
|---|
| • Adenovirus | • High transfection
efficiency | • Generation of
immune response |
| • Adeno-associated
virus | • Efficient in
initiating gene expression | • Toxicity |
| • HSV-1 | | • Possibility of
proto-oncogene activation |
| • HSV amplicon | • Specific
targeting | |
| • Sindbis | | |
| • Poliovirus
replicon | | • High production
cost |
| •
Lentivirus/MoMLV | | |
| | • Limitations in
deliverable gene size |
|
| B, Non-viral
vectors |
|
| Therapeutic DNA,
RNAsa and ODNs |
-
Easy to prepare and to scale-up
-
Flexible with regard to the size of the DNA
-
Do not elicit an immune response
-
Less immunogenic
-
Ease of chemical modification
-
Low cost
-
Can be used in different combinations
|
|
| Anaerobic
bacteria |
|
-
Toxicity
-
Genetic instability
|
Bacteria engineered to express anticancer
agents
Bacteria exhibit the ability to manufacture and
deliver specific materials; these can be artificially coupled to
certain anticancer agents (Fig. 1B)
(28). The most common current
carriers employed in gene therapy are viral vectors, such as
retrovirus, adenovirus, viral vaccines, herpes simplex virus and
adeno-associated virus. Non-viral delivery systems have been
gradually established with the development of technology;
currently, the gene therapy field has evolved to encompass not only
the delivery of therapeutic DNA, but also of microRNA, shRNA, siRNA
and ODNs (20,30,31).
However, non-viral gene delivery systems exhibit lower transfection
potency, resulting in lowered ability to traverse the various
obstacles encountered during treatment (27). Conversely, bacteria have great
advantages in the drug carrier field. Two predominant mechanisms
have been investigated: The direct expression of antitumor proteins
and the transfer of eukaryotic expression vectors into infected
cancer cells. In direct expression, four categories of anticancer
therapies may be utilized: Proteins with physiological activity
against tumors, cytotoxic agents, antiangiogenic agents or enzymes
that convert the nonfunctional prodrug to an anticancer drug. In
the transfer of eukaryotic expression vectors, gene-silencing
shRNAs (32), cytokines and growth
factors, and tumor antigens have been investigated (Table II) (7). Furthermore, the number of useful
agents is increasing due to new developments in combinatorial
synthesis and the advent of metagenomics, which is an unlimited
source of novel anticancer bacterial products.
 | Table IIMolecules that may be used as
anticancer agents through direct expression by bacteria. |
Table II
Molecules that may be used as
anticancer agents through direct expression by bacteria.
| Category | Anticancer
molecule | Refs |
|---|
| Cytotoxic
agents | Cly A | (34,35) |
| FASL | (36) |
| TRAIL | (37) |
| TNFα | (38,39) |
| Cytokines | CCL21 | (41) |
| IL-2 | (41,42,43) |
| IL-18 | (43,44) |
| LIGHT | (44,45) |
| Antigens and
antibodies | CtxB-PSA fusion
protein | (46) |
| CPV-OmpA fusion
protein | (47) |
| NY-ESO-1 tumor
antigen | (48) |
| RAF1 | (49) |
| Single chain HIF1α
antibodies | (50) |
| DNA transfer | Endostatin | (53,57) |
|
Thrombospondin-1 | (54) |
| TRAIL and SMAC | (53) |
| Stat3 | (54,55,57) |
| Bcl2 | (56,57,58) |
| FLT3L | (58) |
| GM-CSF | (57) |
| IL-12 | (58,61) |
| AFP | (62) |
| VEGFR2 | (63) |
| Enzymes | E. coli
CD | (64,65) |
| HSV-TK | (66) |
Bacterial oncolytic therapy
The employment of bacteria in oncolytic therapy is
the initial treatment and most direct method to kill tumor cells.
Clostridial spores are the main components in oncolytic therapy and
have been thoroughly analyzed (22,33,67).
Bacterial-based cancer therapies using Clostridium spores
have the advantage of overcoming the obstacles of hypoxia and
necrosis (68). Clostridium
spp. are strictly anaerobic and only colonize areas devoid of
oxygen; therefore, when Clostridium spp. are systematically
injected into solid tumors, spores germinate and multiply in the
hypoxic/necrotic regions. Parker et al were the first to
demonstrate clostridial oncolysis and tumor regression in mouse
tumors by injecting a Clostridium spore suspension into
transplanted mouse sarcomas 69). However, during follow-up studies,
spore treatment with wild-type Clostridium was not
sufficient to eradicate solid tumors (17,70,71).
Thus, genetic engineering and repetitive screens are required to
enhance the tumor oncolytic capacity of Clostridium. M-55,
which was isolated from a non-pathogenic Clostridium
oncolyticum strain by Carey et al (72,73),
broke this impasse. Since then, multitudinous recombinant
Clostridium strains have been used in tumor treatment. Among these,
C. histolyticium, C. tetani, C. oncolyticum,
C. oncolyticum (sporogenes), C. beijerinckii
(acetobutylicum) and C. novyi-NT have been the most commonly
investigated (9,74).
3. Advantages and problems of
tumor-targeting bacterial therapy
As novel tumor-targeting therapies are introduced,
tumor-targeting bacteria have an irreplaceable status due to their
unique traits (3). Firstly, it is
unsuitable for various types of tumor. Solid tumors are seldom
homogeneous; however, almost all tumors have the same
microenvironment of low oxygen tension or hypoxia, an environment
obligate anaerobes prefer. Furthermore, as bacteria may be easily
manipulated, bacteria may be engineered to overcome the limitations
that hamper current cancer therapies. In addition, bacteria are
highly mobile and actively move away from the vasculature,
penetrate deeply and accumulate in tumor tissue. Bacterial therapy
achieves adequate tissue penetration, which other treatments,
including chemotherapy and radiation, do not (Fig. 1A).
However, certain human trials have shown that the
flaws of bacterial therapy cannot be ignored (3–7). As
mentioned above, the investigation of bacteria for tumor targeting
was stagnant for a long time due to intrinsic bacterial toxicity.
In addition, the wild-type bacteria used for therapy, such as
Bifidobacterium longum, Salmonella, Listeria and
Escherichia coli, exerted no marked targeting efficiency or
oncolytic effect, which reduces the effect of cancer therapy.
Furthermore, bacteria exhibit intrinsic genetic instability.
Although advanced recombinant DNA technology has rendered it
possible to overcome numerous hurdles, bacterial plasmids are not
stable and may be lost during bacterial growth.
4. Methods and tools used to overcome
treatment issues
Several approaches have been employed as attempts to
overcome the difficulties mentioned above. Considerable efforts
have been recently invested, and synthetic biology techniques are
being improved to optimize bacterial therapy and to resolve key
challenges. The use of live, attenuated and engineered bacterial
strains may abate toxicity (75).
The antitumor effects of bacterial treatment were found to be
increased by the application of engineered Clostridium
strains. Saccharolytic Clostridium, C. novyi-NT and E.
coli have been repeatedly screened to become non-toxic with
higher tumor colonization (76).
C. oncolyticum M-55 engineered by Carey et al
(77), was the first bacterial
strain to be genetically manipulated to express an exotic gene, and
was used without any side effects. However, the recombinant strains
did not function as expected. A non-toxic strain of Clostridium
nocyi was developed by deleting the virulence gene through heat
treatment (78). In addition, a
number of genes have been successfully expressed (33–50),
which has improved bacterial targeting efficiency and oncolytic
effects, as explained previously. A recent study examined and
characterized the dynamics of plasmid instability using attenuated
strains of S. typhimurium in vivo, which produced good
results (79).
Bacterial treatment has also achieved gratifying
outcomes when administered in combination with other treatments,
including antivascular agents, chemotherapeutic drugs, heat shock
proteins, heavy metals and radiation. Combined bacteriolytic
therapy is a proposed method for cancer treatment that has been
relatively successful thus far (68). The combination of particular species
with low-dose radiotherapy dampened the tumor immune escape
mechanism (79). In addition,
Salmonella with a modified lipid A (strain VNP20009) was
found to be non-toxic and successfully colonized the tumors
(81). Although the precise
immunological mechanism of BCG therapy remains unclear, increasing
numbers of reaction types have been found to be induced by BCG
complexes, including infections of urothelial cells or bladder
cancer cells, induction of immune responses and induction of
antitumor effects (82).
5. Analyzing potential OSCC tumor-targeting
bacteria groups
Oral cancer, a subtype of head and neck cancer, is
defined as cancerous tissue growth located in the oral cavity.
Several types of oral cancers have been classified, but 90% of
cases worldwide are oral squamous cell carcinoma (OSCC) (83). When the tumor is small enough, a
commonly recommended treatment is surgical removal if the outcome
would be functionally satisfactory. However, in circumstances in
which the tumor is inoperable, radiation therapy with or without
chemotherapy is a common treatment option (84). Despite recent advances in diagnosis
and therapy, the five-year survival rate of patients with OSCC is
only 50% (85). Oral cancer is
unusual in conferring a high risk of second primary tumors. This
heightened risk may last 5–10 years or occasionally longer
(86). Therefore, novel targeting
strategies are required to prevent and treat oral cancer. Among the
candidate methods of postoperative treatments, tumor-targeting
bacterial therapy is expected to have the greatest potential and
may even become the main method due to the tumor-targeting
specificity.
Specific bacterial species colonize different host
locations (87). However, the
different roles of the majority of these bacteria have not been
determined (88), and may be
causal, coincidental or potentially protective. In the human mouth,
~500–1,000 types of bacteria have been detected with various
functions; ~110 types constitute the vast majority of oral bacteria
(data not shown) (89,90). Three species, Capnocytophaga
gingivalis, Pevotella melaninogenica and
Streptococcus mitis, have been found to act as diagnostic
markers, predicting 80% of oral cancers (86). Although considerable progress has
been achieved in elucidating the etiology of oral cancer, the
mechanism underlying the association between oral bacteria and oral
cancer remains unknown. Further investigation is certainly
warranted, but in terms of tumor-targeting therapy, as long as
bacteria thrive in OSCC, modern molecular techniques using bacteria
may be applied. In addition, artificial modification may further
optimize bacteria to meet specific treatment requirements.
Bacteria commonly used in tumor-targeting therapy
include Bifidobacterium, Caulobacter,
Clostridium, Escherichia, Listeria,
Proteus, Salmonella, Streptococcus,
Mycobacterium and Shigella. As a vector in
tumor-targeting treatment, Salmonella typhimurium VNP2009,
an attenuated mutant of S. typhimurium, was first considered
due to its significant native toxicity against murine tumors
(91). In addition, S.
typhimurium was analyzed in a first-in-man phase I clinical
trial for toxicity and anticancer activity (92). However, S. typhimurium is not
considered part of the native oral microbiota, which indicates that
this species may have a poor OSCC tumor-targeting effect. Six
prevalent genera in the OSCC library (93) have been identified:
Streptococcus, Gemella, Rothia,
Peptostreptococcus, Porphyromonas and
Lactobacillus (94). In the
present review, the bacteria commonly used in tumor-targeting
therapy were compared with the following: Human bacterial flora in
the mouth, bacteria with colony-forming units (CFU)/ml
≥105 flora in the human mouth, the genera most prevalent
in the OSCC library and three species of oral cancer diagnostic
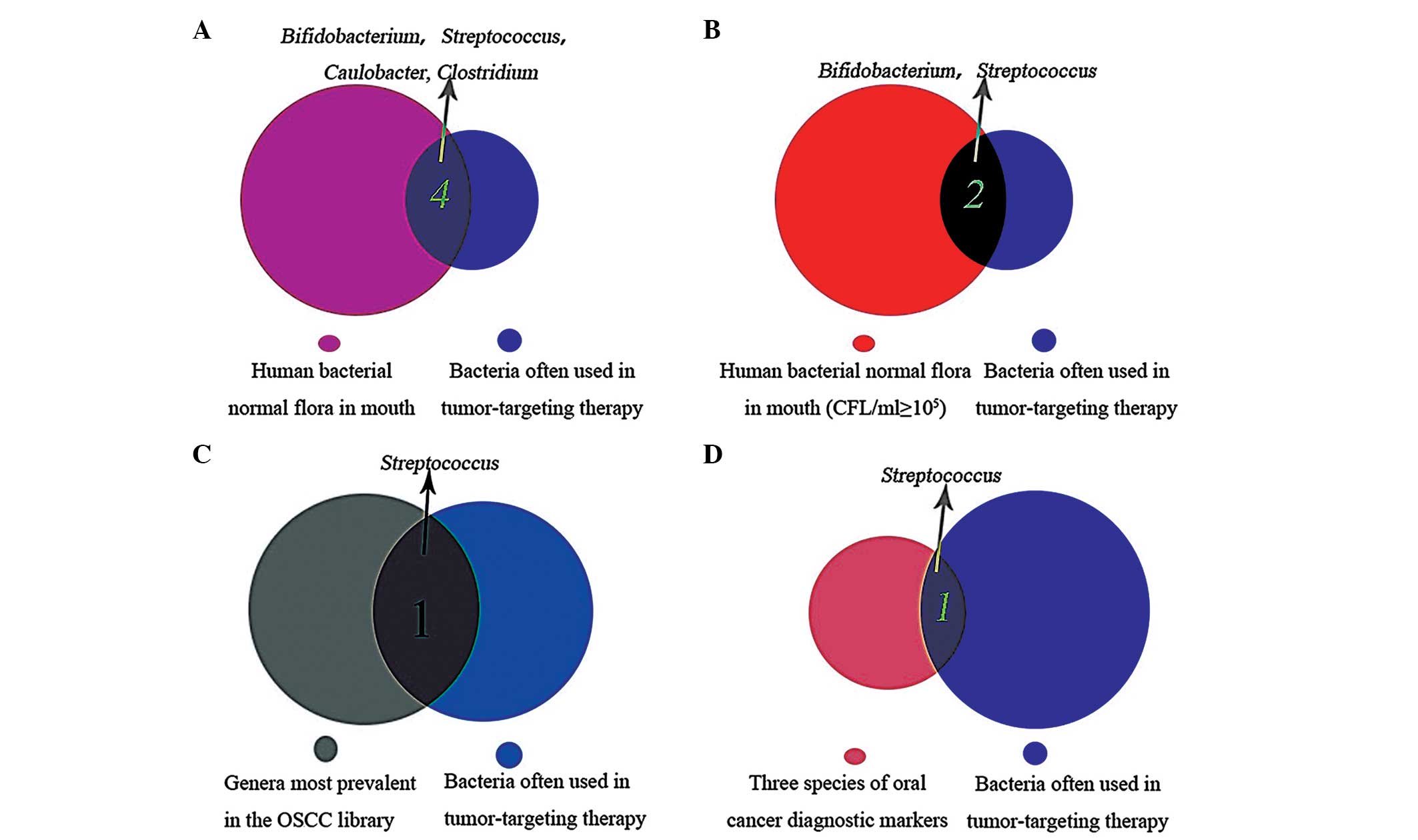
markers (86), respectively.
Through this analysis, Bifidobacterium,
Streptococcus, Caulobacter and Clostridium
species were found to have potential for use in OSCC therapy, as
these bacteria are part of the normal flora of the mouth, and have
previously been used in tumor-targeting therapy (Fig. 2A); Bifidobacterium and
Streptococcus were present at CFU/ml ≥105
commensal flora in the mouth (Fig.
2B). Streptococcus may have the most promising OSCC
tumor-targeting therapeutic effect, as it is one of the genera most
prevalent in the OSCC library and is used as an oral cancer
diagnostic marker (Fig. 2C and
D).
Promising bacteria used as part of the three main
strategies in oral cancer therapy have been discussed in the
present review. In order to identify suitable bacteria as
diagnostic tools to predict oral cancer, the available information
was searched and three of the six most prevalent genera were found
in the OSCC library. C. gingivalis, P. melaninogenica
and S. mitis predict >80% of oral cancers. In addition,
Candida spp., which is commonly detected in oral cancer, has
been reported to serve as a precancerous diagnostic marker
(95). Among the three genera,
S. mitis may be the best candidate for application as an
OSCC tumor-targeting vector due to previous analysis (Fig. 2C and D).
The strategy of employing bacteria engineered to
express anticancer agents may be easily used in oral cancer therapy
if the correct carriers are selected. Bifidobacterium,
Streptococcus and Caulobacter are all suitable, but
Streptococcus exhibits the most promising therapeutic
capacity in this strategy (Fig. 2C and
D). Although Clostridium is not part of the human
bacterial flora of the mouth whose presense is not
<105 CFU/ml, it is present in the oral cavity
(Fig. 2A), which suggests that
Clostridia spp. may also be used in OSCC bacterial oncolytic
therapy.
However, this is only conjecture according to
analysis of the existing data; further experiments are required to
verify these hypotheses.
6. Conclusion and future perspectives
In the field of cancer treatment, bacterial
therapies show great promise, due to the potential tumor-targeting
antitumor capability and the ability to deliver therapeutic genes.
Currently, one issue in tumor-targeting therapy is selecting the
appropriate carrier. The most commonly used carriers are viral
vectors, such as retrovirus, adenovirus, viral vaccines, herpes
simplex virus and adeno-associated virus. However, the safety,
immunogenicity and the limitations of viral vectors are not yet
fully understood, and there appears to be no perfect solution for
these problems. Thus, as a novel method, bacterial therapy may aid
in cancer treatment.
Through this review, Bifidobacterium,
Streptococcus, Caulobacter and Clostridium
spp. were found to be suitable for application in OSCC
tumor-targeting therapy. Streptococcus exhibited the most
promising therapeutic application. Engineered bacteria may further
alter mutant bacterial strains to express anticancer agents. Thus,
tumor-targeting bacterial therapy has the greatest potential of all
candidate methods for oral cancer postoperative treatments.
Acknowledgements
This review was supported by the National Natural
Science Foundation of China (grant nos. 81302371 and 81072218), the
Foundation for Innovative Research Groups of the National Natural
Science Foundation of China (grant no. 81321002), Projects of
International Cooperation and Exchanges National Natural Science
Foundation of China (grant no. 2012DFA31370) and the Doctoral
Program of the Ministry of Education of China (grant no.
20110181110055).
References
|
1
|
Nowotny A: Antitumor effects of
endotoxins. Handbook of Endotoxin. Berry LJ: 3. Elsevier Science;
Amsterdam: pp. 389–448. 1985
|
|
2
|
Busch W: Aus der Sitzung der medicinischen
Section vom 13 November 1867. Berl Klin Wochenschr. 5:1371868.(In
German).
|
|
3
|
Mengesha A: Use of non-pathogenic bacteria
as vectors for targeted gene expression in cancer gene therapy
(unpublished PhD thesis). Maastricht University. 2009.
|
|
4
|
Theys J, Barbé S, Landuyt W, et al:
Tumor-specific gene delivery using genetically engineered bacteria.
Curr Gene Ther. 3:207–221. 2003.
|
|
5
|
Lavigne MD and Górecki DC: Emerging
vectors and targeting methods for nonviral gene therapy. Expert
Opin Emerg Drugs. 11:541–557. 2006.
|
|
6
|
Ptak C and Petronis A: Epigenetics and
complex disease: from etiology to new therapeutics. Annu Rev
Pharmacol Toxicol. 48:257–276. 2008.
|
|
7
|
Patyar S, Joshi R, Byrav DS, et al: Review
bacteria in cancer therapy: a novel experimental strategy. J Biomed
Sci. 17:212010.
|
|
8
|
Van Mellaert L, Barbé S and Anné J:
Clostridium spores as anti-tumour agents. Trends Microbiol.
14:190–196. 2006.
|
|
9
|
Wei MQ, Mengesha A, Good D and Anné J:
Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett.
259:16–27. 2008.
|
|
10
|
Linnebacher M, Maletzki C, Klier U and
Klar E: Bacterial immunotherapy of gastrointestinal tumors.
Langenbecks Arch Surg. 397:557–568. 2012.
|
|
11
|
Coley WB: The treatment of malignant
tumors by repeated inoculations of erysipelas: with a report of ten
original cases. Am J Med Sci. 105:487–510. 1893.
|
|
12
|
Connell HC: The Study and Treatment of
Cancer by Proteolytic Enzymes: Preliminary Report. Can Med Assoc J.
33:364–370. 1935.
|
|
13
|
Bhat JV and Barker HA: Clostridium
lacto-acetophilum Nov. Spec and the Role of Acetic Acid in the
Butyric Acid Fermentation of Lactate. J Bacteriol. 54:381–391.
1947.
|
|
14
|
Takícs J and Imreh E: Incidence of
clostridia in meat in the complementary bacteriological meat
examinations. Journal. 421:1977.
|
|
15
|
Morales A, Eidinger D and Bruce AW:
Intracavitary Bacillus Calmette-Guerin in the treatment of
superficial bladder tumors. J Urol. 116:180–183. 1976.
|
|
16
|
Bernardes N, Chakrabarty AM and Fialho AM:
Engineering of bacterial strains and their products for cancer
therapy. Appl Microbiol Biotechnol. 97:5189–5199. 2013.
|
|
17
|
Xu J, Liu XS, Zhou SF and Wei MQ:
Combination of immunotherapy with anaerobic bacteria for immunogene
therapy of solid tumours. Gene Ther Mol Biol. 13:36–52. 2009.
|
|
18
|
Inoue M, Mukai M, Hamanaka Y, et al:
Targeting hypoxic cancer cells with a protein prodrug is effective
in experimental malignant ascites. Int J Oncol. 25:713–720.
2004.
|
|
19
|
Forbes NS: Profile of a bacterial tumor
killer. Nat Biotechnol. 24:1484–1485. 2006.
|
|
20
|
Schmidt-Wolf GD and Schmidt-Wolf IG:
Non-viral and hybrid vectors in human gene therapy: an update.
Trends Mol Med. 9:67–72. 2003.
|
|
21
|
Cebra JJ: Influences of microbiota on
intestinal immune system development. Am J Clin Nutr.
69:1046S–1051S. 1999.
|
|
22
|
Gardlik R, Behuliak M, Palffy R, Celec P
and Li C: Gene therapy for cancer: bacteria-mediated
anti-angiogenesis therapy. Gene Ther. 18:425–431. 2011.
|
|
23
|
Jain KK: Use of bacteria as anticancer
agents. Expert Opin Biol Ther. 1:291–300. 2001.
|
|
24
|
Thomas CE, Ehrhardt A and Kay MA: Progress
and problems with the use of viral vectors for gene therapy. Nat
Rev Genet. 4:346–358. 2003.
|
|
25
|
Morille M, Passirani C, Vonarbourg A,
Clavreul A and Benoit JP: Progress in developing cationic vectors
for non-viral systemic gene therapy against cancer. Biomaterials.
29:3477–3496. 2008.
|
|
26
|
Ji SR, Liu C, Zhang B, et al: Carbon
nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta.
1806:29–35. 2010.
|
|
27
|
McCrudden CM and McCarthy HO: Cancer gene
therapy - key biological concepts in the design of multifunctional
non-viral delivery systems. Gene Therapy - Tools and Potential
Applications. Martin Molina F: InTech; Rijeka: pp. 213–248.
2013
|
|
28
|
Forbes NS: Engineering the perfect
(bacterial) cancer therapy. Nat Rev Cancer. 10:785–794. 2010.
|
|
29
|
van der Meel R, Gallagher WM, Oliveira S,
et al: Recent advances in molecular imaging biomarkers in cancer:
application of bench to bedside technologies. Drug Discov Today.
15:102–114. 2010.
|
|
30
|
Kay MA, Glorioso JC and Naldini L: Viral
vectors for gene therapy: the art of turning infectious agents into
vehicles of therapeutics. Nat Med. 7:33–40. 2001.
|
|
31
|
Atkinson H and Chalmers R: Delivering the
goods: viral and non-viral gene therapy systems and the inherent
limits on cargo DNA and internal sequences. Genetica. 138:485–498.
2010.
|
|
32
|
Xu DQ, Zhang L, Kopecko DJ, et al:
Bacterial delivery of siRNAs: a new approach to solid tumor
therapy. siRNA and miRNA Gene Silencing. Sioud M: Springer; New
York, NY: pp. 1–27. 2009
|
|
33
|
Barbé S, Van Mellaert L and Anné J: The
use of clostridial spores for cancer treatment. J Appl Microbiol.
101:571–578. 2006.
|
|
34
|
Ganai S, Arenas RB, Sauer JP, Bentley B
and Forbes NS: In tumors Salmonella migrate away from
vasculature toward the transition zone and induce apoptosis. Cancer
Gene Ther. 18:457–466. 2011.
|
|
35
|
Jiang SN, Phan TX, Nam TK, et al:
Inhibition of tumor growth and metastasis by a combination of
Escherichia coli-mediated cytolytic therapy and
radiotherapy. Mol Ther. 18:635–642. 2010.
|
|
36
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Inhibition of tumor growth using Salmonella
expressing Fas ligand. J Natl Cancer Inst. 100:1113–1116. 2008.
|
|
37
|
Schrama D, Reisfeld RA and Becker JC:
Antibody targeted drugs as cancer therapeutics. Nat Rev Drug
Discovery. 5:147–159. 2006.
|
|
38
|
Theys J, Nuyts S, Landuyt W, et al: Stable
Escherichia coli-Clostridium acetobutylicum shuttle vector for
secretion of murine tumor necrosis factor alpha. Appl Environ
Microbiol. 65:4295–4300. 1999.
|
|
39
|
Nuyts S, Theys J, Landuyt W, van Mellaert
L, Lambin P and Anné J: Increasing specificity of anti-tumor
therapy: cytotoxic protein delivery by non-pathogenic clostridia
under regulation of radio-induced promoters. Anticancer Res.
21:857–861. 2001.
|
|
40
|
Saltzman DA: Cancer immunotherapy based on
the killing of Salmonella typhimurium-infected tumour cells.
Expert Opin Biol Ther. 5:443–449. 2005.
|
|
41
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Salmonella typhimurium engineered to produce CCL21
inhibit tumor growth. Cancer Immunol Immunother. 58:769–775.
2009.
|
|
42
|
Theys J, Pennington O, Dubois L, et al:
Repeated cycles of Clostridium-directed enzyme prodrug
therapy result in sustained antitumour effects in vivo. Br J
Cancer. 95:1212–1219. 2006.
|
|
43
|
Barbé S, Van Mellaert L, Theys J, et al:
Secretory production of biologically active rat interleukin-2 by
Clostridium acetobutylicum DSM792 as a tool for anti-tumor
treatment. FEMS Microbiol Lett. 246:67–73. 2005.
|
|
44
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: IL-18-producing Salmonella inhibit tumor growth.
Cancer Gene Ther. 15:787–794. 2008.
|
|
45
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Attenuated Salmonella engineered to produce human cytokine
LIGHT inhibit tumor growth. Proc Natl Acad Sci USA.
104:12879–12883. 2007.
|
|
46
|
Fensterle J, Bergmann B, Yone C, et al:
Cancer immunotherapy based on recombinant Salmonella
enterica serovar Typhimurium aro A strains secreting
prostate-specific antigen and cholera toxin subunit B. Cancer Gene
Ther. 15:85–93. 2008.
|
|
47
|
Lee SR, Kim SH, Jeong KJ, et al:
Multi-immunogenic outer membrane vesicles derived from an
MsbB-deficient Salmonella enterica serovar typhimurium
mutant. J Microbiol Biotechnol. 19:1271–1279. 2009.
|
|
48
|
Karbach J, Neumann A, Brand K, et al:
Phase I Clinical Trial of Mixed Bacterial Vaccine (Coley’s Toxins)
in Patients with NY-ESO-1 Expressing Cancers: Immunological Effects
and Clinical Activity. Clin Cancer Res. 18:5449–5459. 2012.
|
|
49
|
Gentschev I, Fensterle J, Schmidt A, et
al: Use of a recombinant Salmonella enterica serovar Typhimurium
strain expressing C-Raf for protection against C-Raf induced lung
adenoma in mice. BMC Cancer. 5:152005.
|
|
50
|
Groot AJ, Mengesha A, van der Wall E, van
Diest PJ, Theys J and Vooijs M: Functional antibodies produced by
oncolytic clostridia. Biochem Biophys Res Commun. 364:985–989.
2007.
|
|
51
|
Ao A, Wang H, Kamarajugadda S and Lu J:
Involvement of estrogen-related receptors in transcriptional
response to hypoxia and growth of solid tumors. Proc Natl Acad Sci
USA. 105:7821–7826. 2008.
|
|
52
|
Salyers AA and Shoemaker NB: Resistance
gene transfer in anaerobes: new insights, new problems. Clin Infect
Dis. 23(Suppl 1): S36–S43. 1996.
|
|
53
|
Lee CH, Wu CL and Shiau AL: Endostatin
gene therapy delivered by Salmonella choleraesuis in murine
tumor models. J Gene Med. 6:1382–1393. 2004.
|
|
54
|
Lee CH, Wu CL and Shiau AL: Systemic
administration of attenuated Salmonella choleraesuis
carrying thrombospondin-1 gene leads to tumor-specific transgene
expression, delayed tumor growth and prolonged survival in the
murine melanoma model. Cancer Gene Ther. 12:175–184. 2004.
|
|
55
|
Hoffman RM: Tumor-seeking
Salmonella amino acid auxotrophs. Curr Opin Biotechnol.
22:917–923. 2011.
|
|
56
|
Leschner S and Weiss S: Salmonella
- allies in the fight against cancer. J Mol Med (Berl). 88:763–773.
2010.
|
|
57
|
Li X, Li Y, Wang B, et al: Delivery of the
co-expression plasmid pEndo-Si-Stat3 by attenuated
Salmonella serovar typhimurium for prostate cancer
treatment. J Cancer Res Clin Oncol. 139:971–980. 2013.
|
|
58
|
Basu D and Herlyn M: Salmonella
typhimurium as a novel RNA interference vector for cancer gene
therapy. Cancer Biol Ther. 7:151–152. 2008.
|
|
59
|
Hawkins LK, Lemoine NR and Kirn D:
Oncolytic biotherapy: a novel therapeutic platform. Lancet Oncol.
3:17–26. 2002.
|
|
60
|
Fux CA, Costerton JW, Stewart PS and
Stoodley P: Survival strategies of infectious biofilms. Trends
Microbiol. 13:34–40. 2005.
|
|
61
|
Fujimori M, Taniguchi SI, Amano J, et al:
Anaerobic bacterium as a drug for cancer gene therapy US Patent
7,740,835. Filed February 23, 2004; issued June 22, 2010.
|
|
62
|
Fu W, Lan H, Li S, et al: Synergistic
antitumor efficacy of suicide/ePNP gene and 6-methylpurine
2′-deoxyriboside via Salmonella against murine tumors.
Cancer Gene Ther. 15:474–484. 2008.
|
|
63
|
Wei MQ, Ellem KA, Dunn P, et al:
Facultative or obligate anaerobic bacteria have the potential for
multimodality therapy of solid tumours. Eur J Cancer. 43:490–496.
2007.
|
|
64
|
Liu SC, Minton NP, Giaccia AJ and Brown
JM: Anticancer efficacy of systemically delivered anaerobic
bacteria as gene therapy vectors targeting tumor hypoxia/necrosis.
Gene Ther. 9:291–296. 2002.
|
|
65
|
Fuchita M, Ardiani A, Zhao L, et al:
Bacterial cytosine deaminase mutants created by molecular
engineering show improved 5-fluorocytosine-mediated cell killing in
vitro and in vivo. Cancer Res. 69:4791–4799. 2009.
|
|
66
|
Bermudes D, Low B and Pawelek J:
Tumor-targeted Salmonella. Adv Exp Med Biol. 57–63. 2002.
|
|
67
|
Minton NP, Mauchline ML, Lemmon MJ, et al:
Chemotherapeutic tumour targeting using clostridial spores. FEMS
Microbiol Rev. 17:357–364. 1995.
|
|
68
|
Umer B, Good D, Anné J, Duan W and Wei MQ:
Clostridial spores for cancer therapy: targeting solid tumour
microenvironment. J Toxicol. 2012:8627642012.
|
|
69
|
Parker RC, Plummer HC, et al: Effect of
histolyticus infection and toxin on transplantable mouse tumors.
Proc Soc Exp Biol Med. 66:461–467. 1947.
|
|
70
|
Gericke D and Engelbart K: Oncolysis by
Clostridia. II Experiments on a tumor spectrum with a
variety of Clostridia in combination with heavy metal.
Cancer Res. 24:217–221. 1964.
|
|
71
|
Dietzel F and Gericke D: Intensification
of the oncolysis by Clostridia by means of radio-frequency
hyperthermy in experiments on animals-dependence on dosage and on
intervals (author’s transl). Strahlentherapie. 153:263–266.
1977.(In German).
|
|
72
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.
|
|
73
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
|
|
74
|
Mengesha A, Dubois L, Paesmans K, et al:
Clostridia in anti-tumour therapy. Clostridia: Molecular Biology in
the Post-Genomic Era. Brüggemann H and Gottschalk G: Caister
Academic Press; Norfolk, UK: pp. 199–214. 2009
|
|
75
|
Vassaux G, Nitcheu J, Jezzard S and
Lemoine NR: Bacterial gene therapy strategies. J Pathol.
208:290–298. 2006.
|
|
76
|
Dang LH, Bettegowda C, Agrawal N, et al:
Targeting vascular and avascular compartments of tumors with C.
novyi-NT and anti-microtubule agents. Cancer Biol Ther. 3:326–337.
2004.
|
|
77
|
Carey R, Holland J, Whang H, Neter E and
Bryant B: Clostridial oncolysis in man. Eur J Cancer. 3:37–46.
1967.
|
|
78
|
St Jean AT, Zhang M and Forbes NS:
Bacterial therapies: completing the cancer treatment toolbox. Curr
Opin Biotechnol. 19:511–517. 2008.
|
|
79
|
Danino T, Lo J, Prindle A, Hasty J and
Bhatia SN: In Vivo Gene Expression Dynamics of Tumor-Targeted
Bacteria. ACS Synth Biol. 1:465–470. 2012.
|
|
80
|
Avogadri F, Mittal D, Saccheri F, et al:
Intra-tumoral Salmonella typhimurium induces a systemic
anti-tumor immune response that is directed by low-dose radiation
to treat distal disease. Eur J Immunol. 38:1937–1947. 2008.
|
|
81
|
Jia LJ, Wei DP, Sun QM, et al: Oral
delivery of tumor-targeting Salmonella for cancer therapy in murine
tumor models. Cancer Sci. 98:1107–1112. 2007.
|
|
82
|
Kawai K, Miyazaki J, Joraku A, Nishiyama H
and Akaza H: Bacillus Calmette-Guerin (BCG) immunotherapy for
bladder cancer: Current understanding and perspectives on
engineered BCG vaccine. Cancer Sci. 104:22–27. 2013.
|
|
83
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008.
|
|
84
|
Subramani K and Ahmed W: Emerging
nanotechnologies in dentistry: Processes, materials and
applications. William Andrew; Norwich, NY, USA: 2011
|
|
85
|
Vira D, Basak SK, Lai C, et al:
Immunohistochemical variations in the expression of cancer stem
cell and macrophage markers in primary and recurrent oral squamous
cell carcinomas. Cancer Res. 72:53552012.
|
|
86
|
Mager DL: Bacteria and cancer: cause,
coincidence or cure? A review. J Transl Med. 4:142006.
|
|
87
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009.
|
|
88
|
Kurago ZB, Lam-ubol A, Stetsenko A, et al:
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions
induce cancer-supporting factors leading to rapid STAT3 activation.
Head Neck Pathol. 2:1–12. 2008.
|
|
89
|
Gunn HD: Tissue targeted antigenic
activation of the immune response to cancers US Patent 8,034,359.
Filed September 19, 2008; issued October 11, 2011.
|
|
90
|
Hu Y-J, Wang Q, Jiang Y-T, et al:
Characterization of oral bacterial diversity of irradiated patients
by high-throughput sequencing. Int J Oral Sci. 5:21–25. 2013.
|
|
91
|
Chen G, Tang B, Yang BY, et al:
Tumor-targeting Salmonella typhimurium, a natural tool for
activation of prodrug 6MePdR and their combination therapy in
murine melanoma model. Appl Microbiol Biotechnol. 97:4393–4401.
2013.
|
|
92
|
Chorobik P, Czaplicki D, Ossysek K and
Bereta J: Salmonella and cancer: from pathogens to
therapeutics. Acta Biochim Pol. 60:285–297. 2013.
|
|
93
|
Hooper SJ, Crean SJ, Lewis MA, Spratt DA,
Wade WG and Wilson MJ: Viable bacteria present within oral squamous
cell carcinoma tissue. J Clin Microbiol. 44:1719–1725. 2006.
|
|
94
|
Pushalkar S, Mane SP, Ji X, et al:
Microbial diversity in saliva of oral squamous cell carcinoma. FEMS
Immunol Med Microbiol. 61:269–277. 2011.
|
|
95
|
Gall F, Colella G, Di Onofrio V, et al:
Candida spp. in oral cancer and oral precancerous lesions.
New Microbiol. 36:283–288. 2013.
|