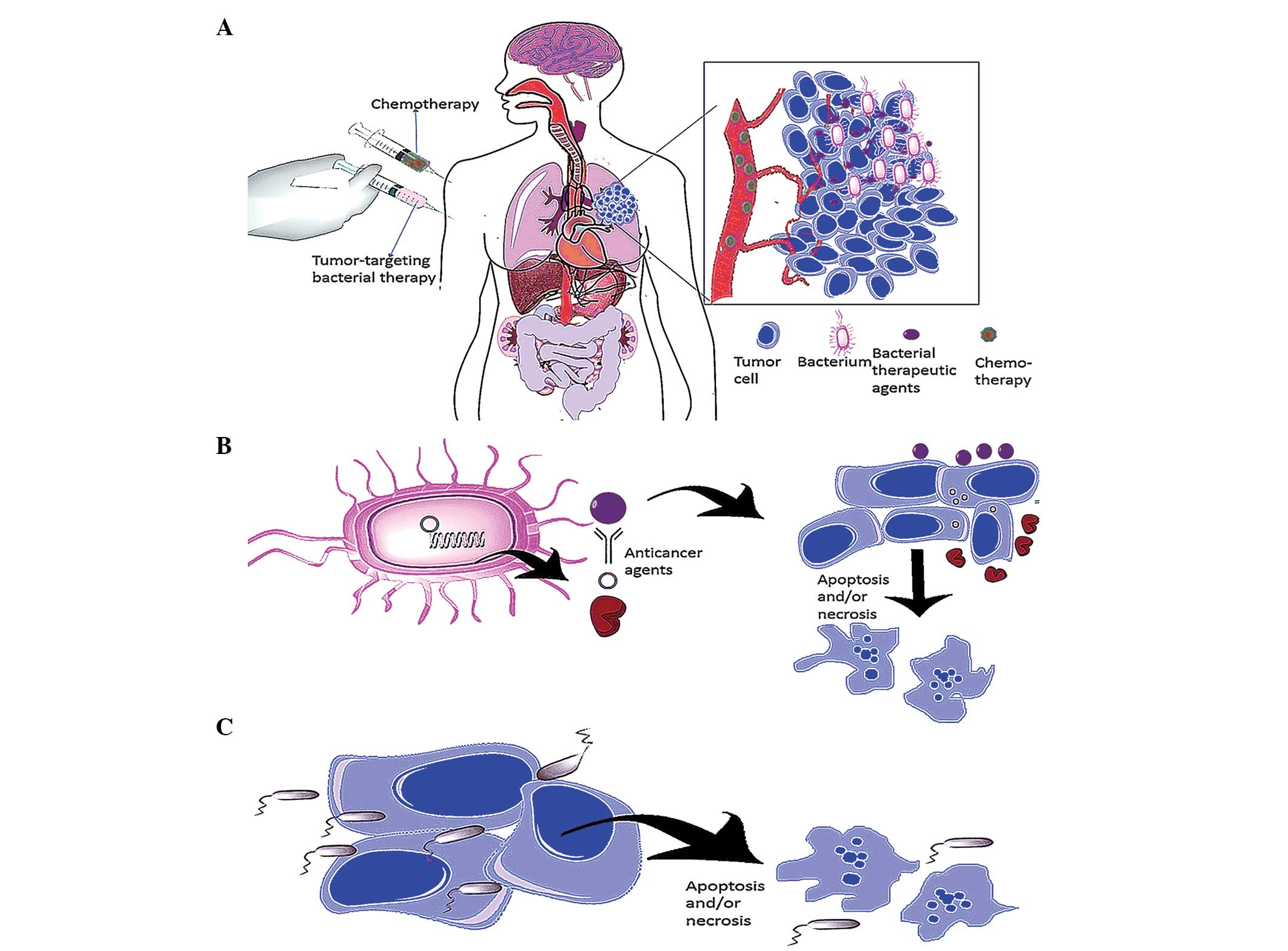
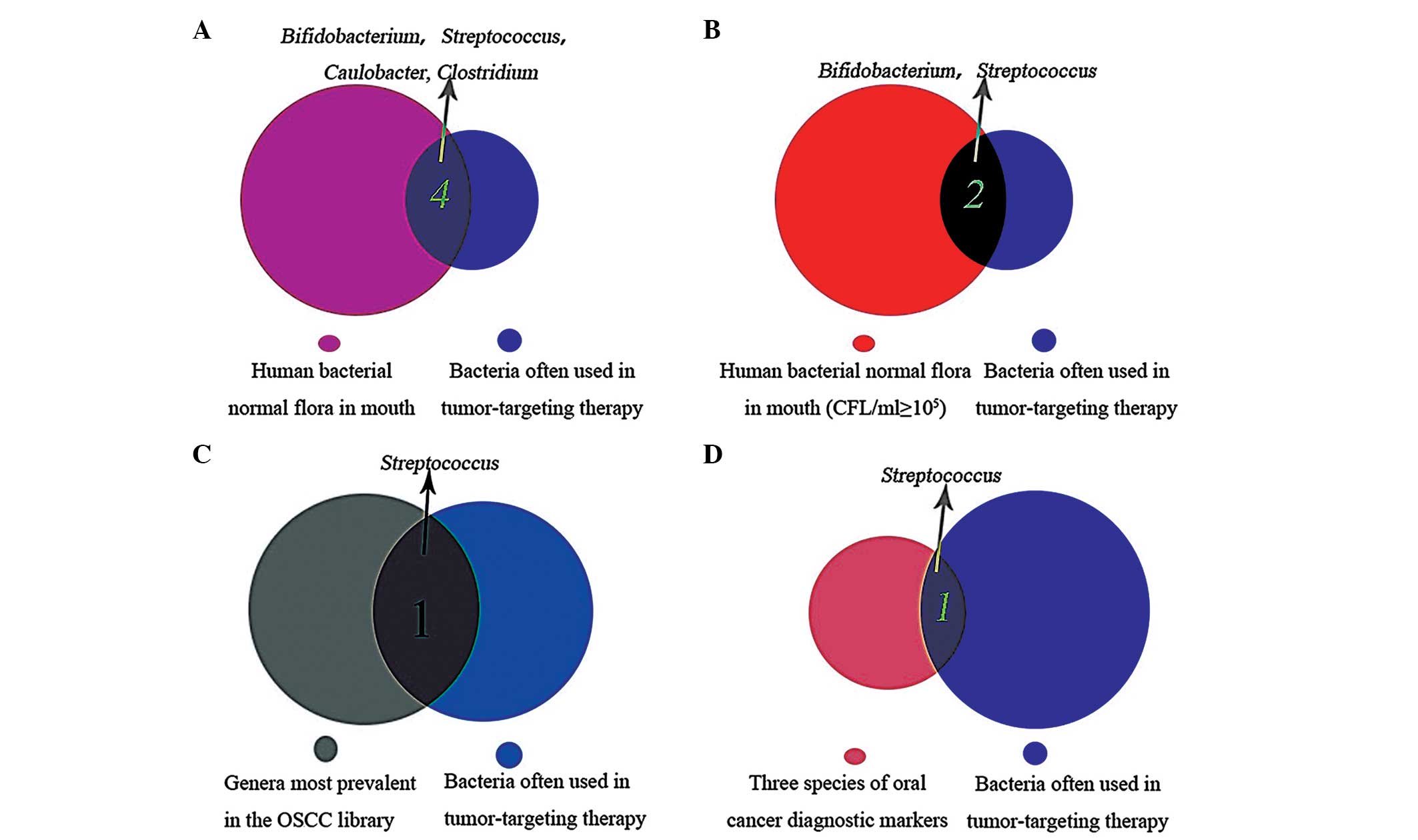
|
1
|
Nowotny A: Antitumor effects of
endotoxins. Handbook of Endotoxin. Berry LJ: 3. Elsevier Science;
Amsterdam: pp. 389–448. 1985
|
|
2
|
Busch W: Aus der Sitzung der medicinischen
Section vom 13 November 1867. Berl Klin Wochenschr. 5:1371868.(In
German).
|
|
3
|
Mengesha A: Use of non-pathogenic bacteria
as vectors for targeted gene expression in cancer gene therapy
(unpublished PhD thesis). Maastricht University. 2009.
|
|
4
|
Theys J, Barbé S, Landuyt W, et al:
Tumor-specific gene delivery using genetically engineered bacteria.
Curr Gene Ther. 3:207–221. 2003.
|
|
5
|
Lavigne MD and Górecki DC: Emerging
vectors and targeting methods for nonviral gene therapy. Expert
Opin Emerg Drugs. 11:541–557. 2006.
|
|
6
|
Ptak C and Petronis A: Epigenetics and
complex disease: from etiology to new therapeutics. Annu Rev
Pharmacol Toxicol. 48:257–276. 2008.
|
|
7
|
Patyar S, Joshi R, Byrav DS, et al: Review
bacteria in cancer therapy: a novel experimental strategy. J Biomed
Sci. 17:212010.
|
|
8
|
Van Mellaert L, Barbé S and Anné J:
Clostridium spores as anti-tumour agents. Trends Microbiol.
14:190–196. 2006.
|
|
9
|
Wei MQ, Mengesha A, Good D and Anné J:
Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett.
259:16–27. 2008.
|
|
10
|
Linnebacher M, Maletzki C, Klier U and
Klar E: Bacterial immunotherapy of gastrointestinal tumors.
Langenbecks Arch Surg. 397:557–568. 2012.
|
|
11
|
Coley WB: The treatment of malignant
tumors by repeated inoculations of erysipelas: with a report of ten
original cases. Am J Med Sci. 105:487–510. 1893.
|
|
12
|
Connell HC: The Study and Treatment of
Cancer by Proteolytic Enzymes: Preliminary Report. Can Med Assoc J.
33:364–370. 1935.
|
|
13
|
Bhat JV and Barker HA: Clostridium
lacto-acetophilum Nov. Spec and the Role of Acetic Acid in the
Butyric Acid Fermentation of Lactate. J Bacteriol. 54:381–391.
1947.
|
|
14
|
Takícs J and Imreh E: Incidence of
clostridia in meat in the complementary bacteriological meat
examinations. Journal. 421:1977.
|
|
15
|
Morales A, Eidinger D and Bruce AW:
Intracavitary Bacillus Calmette-Guerin in the treatment of
superficial bladder tumors. J Urol. 116:180–183. 1976.
|
|
16
|
Bernardes N, Chakrabarty AM and Fialho AM:
Engineering of bacterial strains and their products for cancer
therapy. Appl Microbiol Biotechnol. 97:5189–5199. 2013.
|
|
17
|
Xu J, Liu XS, Zhou SF and Wei MQ:
Combination of immunotherapy with anaerobic bacteria for immunogene
therapy of solid tumours. Gene Ther Mol Biol. 13:36–52. 2009.
|
|
18
|
Inoue M, Mukai M, Hamanaka Y, et al:
Targeting hypoxic cancer cells with a protein prodrug is effective
in experimental malignant ascites. Int J Oncol. 25:713–720.
2004.
|
|
19
|
Forbes NS: Profile of a bacterial tumor
killer. Nat Biotechnol. 24:1484–1485. 2006.
|
|
20
|
Schmidt-Wolf GD and Schmidt-Wolf IG:
Non-viral and hybrid vectors in human gene therapy: an update.
Trends Mol Med. 9:67–72. 2003.
|
|
21
|
Cebra JJ: Influences of microbiota on
intestinal immune system development. Am J Clin Nutr.
69:1046S–1051S. 1999.
|
|
22
|
Gardlik R, Behuliak M, Palffy R, Celec P
and Li C: Gene therapy for cancer: bacteria-mediated
anti-angiogenesis therapy. Gene Ther. 18:425–431. 2011.
|
|
23
|
Jain KK: Use of bacteria as anticancer
agents. Expert Opin Biol Ther. 1:291–300. 2001.
|
|
24
|
Thomas CE, Ehrhardt A and Kay MA: Progress
and problems with the use of viral vectors for gene therapy. Nat
Rev Genet. 4:346–358. 2003.
|
|
25
|
Morille M, Passirani C, Vonarbourg A,
Clavreul A and Benoit JP: Progress in developing cationic vectors
for non-viral systemic gene therapy against cancer. Biomaterials.
29:3477–3496. 2008.
|
|
26
|
Ji SR, Liu C, Zhang B, et al: Carbon
nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta.
1806:29–35. 2010.
|
|
27
|
McCrudden CM and McCarthy HO: Cancer gene
therapy - key biological concepts in the design of multifunctional
non-viral delivery systems. Gene Therapy - Tools and Potential
Applications. Martin Molina F: InTech; Rijeka: pp. 213–248.
2013
|
|
28
|
Forbes NS: Engineering the perfect
(bacterial) cancer therapy. Nat Rev Cancer. 10:785–794. 2010.
|
|
29
|
van der Meel R, Gallagher WM, Oliveira S,
et al: Recent advances in molecular imaging biomarkers in cancer:
application of bench to bedside technologies. Drug Discov Today.
15:102–114. 2010.
|
|
30
|
Kay MA, Glorioso JC and Naldini L: Viral
vectors for gene therapy: the art of turning infectious agents into
vehicles of therapeutics. Nat Med. 7:33–40. 2001.
|
|
31
|
Atkinson H and Chalmers R: Delivering the
goods: viral and non-viral gene therapy systems and the inherent
limits on cargo DNA and internal sequences. Genetica. 138:485–498.
2010.
|
|
32
|
Xu DQ, Zhang L, Kopecko DJ, et al:
Bacterial delivery of siRNAs: a new approach to solid tumor
therapy. siRNA and miRNA Gene Silencing. Sioud M: Springer; New
York, NY: pp. 1–27. 2009
|
|
33
|
Barbé S, Van Mellaert L and Anné J: The
use of clostridial spores for cancer treatment. J Appl Microbiol.
101:571–578. 2006.
|
|
34
|
Ganai S, Arenas RB, Sauer JP, Bentley B
and Forbes NS: In tumors Salmonella migrate away from
vasculature toward the transition zone and induce apoptosis. Cancer
Gene Ther. 18:457–466. 2011.
|
|
35
|
Jiang SN, Phan TX, Nam TK, et al:
Inhibition of tumor growth and metastasis by a combination of
Escherichia coli-mediated cytolytic therapy and
radiotherapy. Mol Ther. 18:635–642. 2010.
|
|
36
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Inhibition of tumor growth using Salmonella
expressing Fas ligand. J Natl Cancer Inst. 100:1113–1116. 2008.
|
|
37
|
Schrama D, Reisfeld RA and Becker JC:
Antibody targeted drugs as cancer therapeutics. Nat Rev Drug
Discovery. 5:147–159. 2006.
|
|
38
|
Theys J, Nuyts S, Landuyt W, et al: Stable
Escherichia coli-Clostridium acetobutylicum shuttle vector for
secretion of murine tumor necrosis factor alpha. Appl Environ
Microbiol. 65:4295–4300. 1999.
|
|
39
|
Nuyts S, Theys J, Landuyt W, van Mellaert
L, Lambin P and Anné J: Increasing specificity of anti-tumor
therapy: cytotoxic protein delivery by non-pathogenic clostridia
under regulation of radio-induced promoters. Anticancer Res.
21:857–861. 2001.
|
|
40
|
Saltzman DA: Cancer immunotherapy based on
the killing of Salmonella typhimurium-infected tumour cells.
Expert Opin Biol Ther. 5:443–449. 2005.
|
|
41
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Salmonella typhimurium engineered to produce CCL21
inhibit tumor growth. Cancer Immunol Immunother. 58:769–775.
2009.
|
|
42
|
Theys J, Pennington O, Dubois L, et al:
Repeated cycles of Clostridium-directed enzyme prodrug
therapy result in sustained antitumour effects in vivo. Br J
Cancer. 95:1212–1219. 2006.
|
|
43
|
Barbé S, Van Mellaert L, Theys J, et al:
Secretory production of biologically active rat interleukin-2 by
Clostridium acetobutylicum DSM792 as a tool for anti-tumor
treatment. FEMS Microbiol Lett. 246:67–73. 2005.
|
|
44
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: IL-18-producing Salmonella inhibit tumor growth.
Cancer Gene Ther. 15:787–794. 2008.
|
|
45
|
Loeffler M, Le’Negrate G, Krajewska M and
Reed JC: Attenuated Salmonella engineered to produce human cytokine
LIGHT inhibit tumor growth. Proc Natl Acad Sci USA.
104:12879–12883. 2007.
|
|
46
|
Fensterle J, Bergmann B, Yone C, et al:
Cancer immunotherapy based on recombinant Salmonella
enterica serovar Typhimurium aro A strains secreting
prostate-specific antigen and cholera toxin subunit B. Cancer Gene
Ther. 15:85–93. 2008.
|
|
47
|
Lee SR, Kim SH, Jeong KJ, et al:
Multi-immunogenic outer membrane vesicles derived from an
MsbB-deficient Salmonella enterica serovar typhimurium
mutant. J Microbiol Biotechnol. 19:1271–1279. 2009.
|
|
48
|
Karbach J, Neumann A, Brand K, et al:
Phase I Clinical Trial of Mixed Bacterial Vaccine (Coley’s Toxins)
in Patients with NY-ESO-1 Expressing Cancers: Immunological Effects
and Clinical Activity. Clin Cancer Res. 18:5449–5459. 2012.
|
|
49
|
Gentschev I, Fensterle J, Schmidt A, et
al: Use of a recombinant Salmonella enterica serovar Typhimurium
strain expressing C-Raf for protection against C-Raf induced lung
adenoma in mice. BMC Cancer. 5:152005.
|
|
50
|
Groot AJ, Mengesha A, van der Wall E, van
Diest PJ, Theys J and Vooijs M: Functional antibodies produced by
oncolytic clostridia. Biochem Biophys Res Commun. 364:985–989.
2007.
|
|
51
|
Ao A, Wang H, Kamarajugadda S and Lu J:
Involvement of estrogen-related receptors in transcriptional
response to hypoxia and growth of solid tumors. Proc Natl Acad Sci
USA. 105:7821–7826. 2008.
|
|
52
|
Salyers AA and Shoemaker NB: Resistance
gene transfer in anaerobes: new insights, new problems. Clin Infect
Dis. 23(Suppl 1): S36–S43. 1996.
|
|
53
|
Lee CH, Wu CL and Shiau AL: Endostatin
gene therapy delivered by Salmonella choleraesuis in murine
tumor models. J Gene Med. 6:1382–1393. 2004.
|
|
54
|
Lee CH, Wu CL and Shiau AL: Systemic
administration of attenuated Salmonella choleraesuis
carrying thrombospondin-1 gene leads to tumor-specific transgene
expression, delayed tumor growth and prolonged survival in the
murine melanoma model. Cancer Gene Ther. 12:175–184. 2004.
|
|
55
|
Hoffman RM: Tumor-seeking
Salmonella amino acid auxotrophs. Curr Opin Biotechnol.
22:917–923. 2011.
|
|
56
|
Leschner S and Weiss S: Salmonella
- allies in the fight against cancer. J Mol Med (Berl). 88:763–773.
2010.
|
|
57
|
Li X, Li Y, Wang B, et al: Delivery of the
co-expression plasmid pEndo-Si-Stat3 by attenuated
Salmonella serovar typhimurium for prostate cancer
treatment. J Cancer Res Clin Oncol. 139:971–980. 2013.
|
|
58
|
Basu D and Herlyn M: Salmonella
typhimurium as a novel RNA interference vector for cancer gene
therapy. Cancer Biol Ther. 7:151–152. 2008.
|
|
59
|
Hawkins LK, Lemoine NR and Kirn D:
Oncolytic biotherapy: a novel therapeutic platform. Lancet Oncol.
3:17–26. 2002.
|
|
60
|
Fux CA, Costerton JW, Stewart PS and
Stoodley P: Survival strategies of infectious biofilms. Trends
Microbiol. 13:34–40. 2005.
|
|
61
|
Fujimori M, Taniguchi SI, Amano J, et al:
Anaerobic bacterium as a drug for cancer gene therapy US Patent
7,740,835. Filed February 23, 2004; issued June 22, 2010.
|
|
62
|
Fu W, Lan H, Li S, et al: Synergistic
antitumor efficacy of suicide/ePNP gene and 6-methylpurine
2′-deoxyriboside via Salmonella against murine tumors.
Cancer Gene Ther. 15:474–484. 2008.
|
|
63
|
Wei MQ, Ellem KA, Dunn P, et al:
Facultative or obligate anaerobic bacteria have the potential for
multimodality therapy of solid tumours. Eur J Cancer. 43:490–496.
2007.
|
|
64
|
Liu SC, Minton NP, Giaccia AJ and Brown
JM: Anticancer efficacy of systemically delivered anaerobic
bacteria as gene therapy vectors targeting tumor hypoxia/necrosis.
Gene Ther. 9:291–296. 2002.
|
|
65
|
Fuchita M, Ardiani A, Zhao L, et al:
Bacterial cytosine deaminase mutants created by molecular
engineering show improved 5-fluorocytosine-mediated cell killing in
vitro and in vivo. Cancer Res. 69:4791–4799. 2009.
|
|
66
|
Bermudes D, Low B and Pawelek J:
Tumor-targeted Salmonella. Adv Exp Med Biol. 57–63. 2002.
|
|
67
|
Minton NP, Mauchline ML, Lemmon MJ, et al:
Chemotherapeutic tumour targeting using clostridial spores. FEMS
Microbiol Rev. 17:357–364. 1995.
|
|
68
|
Umer B, Good D, Anné J, Duan W and Wei MQ:
Clostridial spores for cancer therapy: targeting solid tumour
microenvironment. J Toxicol. 2012:8627642012.
|
|
69
|
Parker RC, Plummer HC, et al: Effect of
histolyticus infection and toxin on transplantable mouse tumors.
Proc Soc Exp Biol Med. 66:461–467. 1947.
|
|
70
|
Gericke D and Engelbart K: Oncolysis by
Clostridia. II Experiments on a tumor spectrum with a
variety of Clostridia in combination with heavy metal.
Cancer Res. 24:217–221. 1964.
|
|
71
|
Dietzel F and Gericke D: Intensification
of the oncolysis by Clostridia by means of radio-frequency
hyperthermy in experiments on animals-dependence on dosage and on
intervals (author’s transl). Strahlentherapie. 153:263–266.
1977.(In German).
|
|
72
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.
|
|
73
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
|
|
74
|
Mengesha A, Dubois L, Paesmans K, et al:
Clostridia in anti-tumour therapy. Clostridia: Molecular Biology in
the Post-Genomic Era. Brüggemann H and Gottschalk G: Caister
Academic Press; Norfolk, UK: pp. 199–214. 2009
|
|
75
|
Vassaux G, Nitcheu J, Jezzard S and
Lemoine NR: Bacterial gene therapy strategies. J Pathol.
208:290–298. 2006.
|
|
76
|
Dang LH, Bettegowda C, Agrawal N, et al:
Targeting vascular and avascular compartments of tumors with C.
novyi-NT and anti-microtubule agents. Cancer Biol Ther. 3:326–337.
2004.
|
|
77
|
Carey R, Holland J, Whang H, Neter E and
Bryant B: Clostridial oncolysis in man. Eur J Cancer. 3:37–46.
1967.
|
|
78
|
St Jean AT, Zhang M and Forbes NS:
Bacterial therapies: completing the cancer treatment toolbox. Curr
Opin Biotechnol. 19:511–517. 2008.
|
|
79
|
Danino T, Lo J, Prindle A, Hasty J and
Bhatia SN: In Vivo Gene Expression Dynamics of Tumor-Targeted
Bacteria. ACS Synth Biol. 1:465–470. 2012.
|
|
80
|
Avogadri F, Mittal D, Saccheri F, et al:
Intra-tumoral Salmonella typhimurium induces a systemic
anti-tumor immune response that is directed by low-dose radiation
to treat distal disease. Eur J Immunol. 38:1937–1947. 2008.
|
|
81
|
Jia LJ, Wei DP, Sun QM, et al: Oral
delivery of tumor-targeting Salmonella for cancer therapy in murine
tumor models. Cancer Sci. 98:1107–1112. 2007.
|
|
82
|
Kawai K, Miyazaki J, Joraku A, Nishiyama H
and Akaza H: Bacillus Calmette-Guerin (BCG) immunotherapy for
bladder cancer: Current understanding and perspectives on
engineered BCG vaccine. Cancer Sci. 104:22–27. 2013.
|
|
83
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: implications for
therapy. J Dent Res. 87:14–32. 2008.
|
|
84
|
Subramani K and Ahmed W: Emerging
nanotechnologies in dentistry: Processes, materials and
applications. William Andrew; Norwich, NY, USA: 2011
|
|
85
|
Vira D, Basak SK, Lai C, et al:
Immunohistochemical variations in the expression of cancer stem
cell and macrophage markers in primary and recurrent oral squamous
cell carcinomas. Cancer Res. 72:53552012.
|
|
86
|
Mager DL: Bacteria and cancer: cause,
coincidence or cure? A review. J Transl Med. 4:142006.
|
|
87
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009.
|
|
88
|
Kurago ZB, Lam-ubol A, Stetsenko A, et al:
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions
induce cancer-supporting factors leading to rapid STAT3 activation.
Head Neck Pathol. 2:1–12. 2008.
|
|
89
|
Gunn HD: Tissue targeted antigenic
activation of the immune response to cancers US Patent 8,034,359.
Filed September 19, 2008; issued October 11, 2011.
|
|
90
|
Hu Y-J, Wang Q, Jiang Y-T, et al:
Characterization of oral bacterial diversity of irradiated patients
by high-throughput sequencing. Int J Oral Sci. 5:21–25. 2013.
|
|
91
|
Chen G, Tang B, Yang BY, et al:
Tumor-targeting Salmonella typhimurium, a natural tool for
activation of prodrug 6MePdR and their combination therapy in
murine melanoma model. Appl Microbiol Biotechnol. 97:4393–4401.
2013.
|
|
92
|
Chorobik P, Czaplicki D, Ossysek K and
Bereta J: Salmonella and cancer: from pathogens to
therapeutics. Acta Biochim Pol. 60:285–297. 2013.
|
|
93
|
Hooper SJ, Crean SJ, Lewis MA, Spratt DA,
Wade WG and Wilson MJ: Viable bacteria present within oral squamous
cell carcinoma tissue. J Clin Microbiol. 44:1719–1725. 2006.
|
|
94
|
Pushalkar S, Mane SP, Ji X, et al:
Microbial diversity in saliva of oral squamous cell carcinoma. FEMS
Immunol Med Microbiol. 61:269–277. 2011.
|
|
95
|
Gall F, Colella G, Di Onofrio V, et al:
Candida spp. in oral cancer and oral precancerous lesions.
New Microbiol. 36:283–288. 2013.
|