Introduction
Gastric cancer was the leading cause of mortality
from gastrointestinal malignancy worldwide in 2012 (1,2). A
number of pathways and genes have been implicated in the
development of gastric cancer. Interleukin-8 (IL-8), a member of
the neutrophil-specific CXC subfamily of chemokines, is important
not only in leukocyte chemotaxis, inflammatory responses and
infectious diseases (3), but also
in the proliferation, invasion and migration of endothelial cells
(4,5). Previous studies suggested that solid
tumors, including those of prostate, breast and ovarian cancer,
express IL-8 (6,7). IL-8, an autocrine growth factor,
promotes tumor growth, tissue invasion and metastasis (5). Upregulation of IL-8 occurs in gastric
cancer (8) and has been associated
with the adhesion, migration and invasion of human gastric cancer
cells (9). Recent studies have
demonstrated that Helicobacter pylori (Hp) infection causes
extensive gastric epithelial cell inflammation, which may result in
atrophic gastritis, intestinal metaplasia and even gastric
adenocarcinoma (10–15). Furthermore, IL-8 levels are higher
in Hp-infected gastric tissue than in Hp-negative tissue (16).
Overexpression of IL-8 has been associated with
invasion and metastasis in gastric cancer; however, the association
between IL-8 and gastric cancer cell proliferation remains unclear.
The present study evaluated whether IL-8 affects the proliferation
of the SGC7901 gastric cancer cell line, and investigated the
effect of IL-8 on the expression levels of proliferating cell
nuclear antigen (PCNA) protein and mRNA.
Materials and methods
Cell culture
The SGC7901 human gastric cancer cell strain was
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China), and the cells were
inoculated in RPMI-1640 medium (Genom Biopharmaceutical Technology
Co., Ltd., Hangzhou, China), supplemented with 10% fetal bovine
serum (Zhejiang Tianhang Biological Technology Co., Ltd., Hangzhou,
China), 1% penicillin/streptomycin and 1% L-glutamine. The cells
were maintained at 37°C in a humidified chamber containing 5%
CO2.
Cell grouping and drug treatment
IL-8 stock solution (Sigma-Aldrich, St. Louis, MO,
USA) was added to each well at a predetermined concentration.
Therefore, based on IL-8 dosage, the following five groups were
experimentally maintained: 0, 0.2, 0.5, 0.8 and 1 ng/ml groups.
Cell proliferation assay
Cell proliferation was assessed by Cell Counting
Kit-8 (Dojindo, Kunamoto, Japan) assay, using cellular DNA labeled
with the fluorescence reagent. SGC7901 cells in logarithmic phase
were inoculated on a 96-well plate at a density of 3×104
cells/well and incubated overnight to allow adherence. Subsequent
to washing, the culture medium and IL-8 at fixed concentrations
were added to the cells. The cells of each group were incubated for
1–7 days. Nine duplicate wells were employed for each group. At the
end of the culture period, WST-8, which produces a water-soluble
formazan, was added to the cells. The cells were incubated for an
additional 4 h. Colorimetric absorbance was measured by a
microplate reader (Multiskan MK3; Thermo Fisher Scientific,
Waltham, MA, USA) at 450 nm to obtain an optical density (OD)
value. The OD values were calculated using the following equation:
OD ultimate value = OD measure value − OD blank value.
Immunofluorescence staining
A total of 2×105 SGC7901 cells were
seeded on a six-well plate and cultured with the fixed
concentrations of IL-8 for 72 h. Subsequently, 7×104
cells were placed on coverslips and cultured in RPMI-1640 medium at
37°C to allow adherence. Following fixation in 4% paraformaldehyde
for 15 min, a 10-min treatment with 0.5% Triton X-100 (Shanghai
Sangon Biotech, Co., Ltd., Shanghai, China) and a 1-h incubation
with 4% bovine serum albumin (Wisent Inc., St Bruno, Quebec,
Canada) at room temperature, the cells of each group were incubated
with PCNA rabbit anti-human monoclonal antibody (Epitomics,
Burlingame, CA, USA) at 4°C overnight. Cy3-conjugated affinipure
goat polyclonal anti-rabbit IgG (H+L;1:1,000 dilution; Proteintech
Group, Wuhan, China) was added for an additional 1-h incubation.
The cell nuclei were then labeled with DAPI. The coverslips were
analyzed with a laser confocal scanning microscope (LSM710; Zeiss,
Oberkochen, Germany).
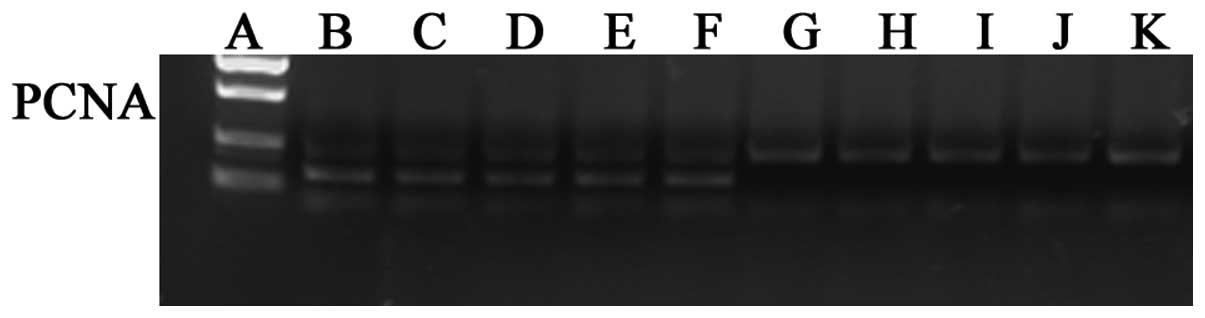
Western blot analysis
The cells of each group were incubated for 72 h. The
cells were collected and decomposed by 150 μl cell lysis buffer,
and the sample was boiled out for 10 min. Subsequent to cooling on
ice, the cell lysate was centrifuged for 1 min at 13,201 × g. The
supernatant fluid was loaded onto SDS-PAGE (10% separation gel, 5%
spacer gel) and electrotransferred to polyvinylidene difluoride
film (Bio-Rad, Hercules, CA, USA). The blotted films were placed in
blocking solution for 1 h at room temperature. Rabbit anti-human
PCNA monoclonal antibody (1:250; Epitomics) was used to probe the
blots overnight at 4°C. The film was washed twice and then
incubated with goat polyclonal anti-rabbit IgG-horse radish
peroxidase secondary antibody (1:1,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) for 1 h at room temperature. The film
was washed three times and the signal determined by the enhanced
chemiluminescence method using an ECL kit (PerkinElmer, Inc.,
Waltham, MA, USA). The blots were subsequently exposed to plain
X-ray film in a darkroom, and the film was scanned by an image
analyzer. Grayscale reconstruction was performed using Image J
software 1.48 (http://rsb.info.nih.gov./ij/), and the expression rate
of PCNA versus that of GAPDH protein, serving as an internal
control protein, was calculated. All experiments were repeated
three times.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
The cells of each group were inoculated on a
six-well plate at a density of 1×105 cells/well and
incubated for 72 h. In brief, total RNA of the SGC7901 cells was
extracted by TRIzol reagent (Takara, Shiga, Japan) according to the
manufacturer’s instructions and reverse-transcribed. RT-qPCR was
performed with SYBR Green in a real-time PCR system (Bio-Rad iQ5;
Bio-Rad), with each sample analyzed in triplicate. The cycling
conditions consisted of one cycle of 95°C for 2 min, 95°C for 15
sec, 60°C for 20 sec and 72°C for 20 sec, and then 40 cycles of
72°C for 30 sec. The primer sequences for the genes analyzed are
shown in Table I. The relative
levels of PCNA mRNA expression were normalized to those of GAPDH
mRNA, and were calculated according to the 2−ΔΔCt
method.
 | Table IPrimer sequences used for quantitative
polymerase chain reaction. |
Table I
Primer sequences used for quantitative
polymerase chain reaction.
| mRNA | Sense primer
sequence | bp |
|---|
| hGAPDH-F |
5′-GGGTGTGAACCATGAGAAGTATG-3′ | 145 |
| hGAPDH-R |
5′-GATGGCATGGACTGTGGTCAT-3′ | |
| PCNA-F |
5′-TCATTACACTAAGGGCCGAAGA-3′ | 229 |
| PCNA-R |
5′-GCACAGGAAATTACAACAGCATC-3′ | |
Statistical methods
All data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). All results are presented as the
mean ± standard deviation. Analysis of variance (ANOVA) of repeated
measurement data was used to assess cell proliferation. One-way
ANOVA was used to assess protein and mRNA expression levels. The
least significant difference method was used to analyze multiple
post hoc comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
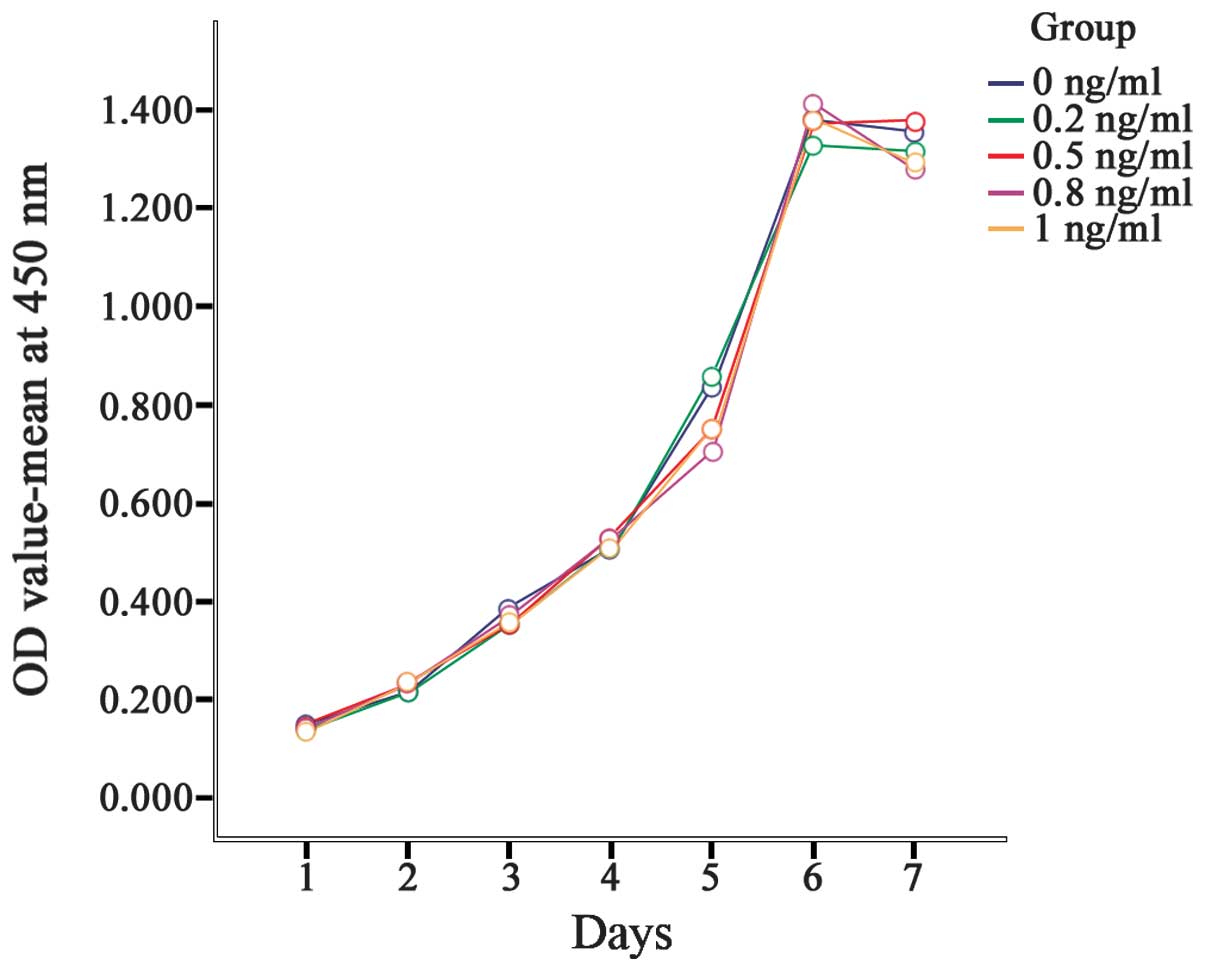
Effect of IL-8 on SGC7901 cell
growth
The SGC7901 cells proliferated rapidly, exhibiting a
fusiform shape, adherence and overlapping growth. The number of
cells increased gradually between the first and the sixth days. On
the seventh day, cell growth was arrested in the plateau phase with
no further increase in the number of cells. The exposure of the
cells to IL-8 at concentrations ranging from 0 to 1 ng/ml did not
exert a significant effect on growth (Fig. 1).
Effect of IL-8 on SGC7901 cell
proliferation
Between the first and the sixth days, the OD values
increased gradually; peak OD values were obtained on the sixth day,
with slight decreases on the seventh day. The OD values between the
different days were significantly different (P<0.001). However,
no significant differences in the OD value between treatment groups
were identified (P=0.162). This result indicated that IL-8 exerted
no significant effect on gastric cancer cell proliferation
(Table II, Fig. 2).
 | Table IIEffect of interleukin-8 on SGC7901
gastric cancer cell proliferation (optical density). |
Table II
Effect of interleukin-8 on SGC7901
gastric cancer cell proliferation (optical density).
| Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|
| 0 ng/ml | 0.150±0.052 | 0.216±0.012 | 0.387±0.060 | 0.507±0.017 | 0.838±0.024 | 1.381±0.019 | 1.356±0.030 |
| 0.2 ng/ml | 0.141±0.001 | 0.214±0.012 | 0.352±0.020 | 0.514±0.006 | 0.860±0.016 | 1.331±0.060 | 1.318±0.047 |
| 0.5 ng/ml | 0.148±0.020 | 0.233±0.006 | 0.354±0.001 | 0.530±0.035 | 0.751±0.036 | 1.371±0.064 | 1.380±0.003 |
| 0.8 ng/ml | 0.139±0.005 | 0.232±0.016 | 0.368±0.009 | 0.531±0.035 | 0.705±0.016 | 1.414±0.042 | 1.281±0.033 |
| 1 ng/ml | 0.133±0.007 | 0.236±0.010 | 0.354±0.006 | 0.512±0.024 | 0.752±0.046 | 1.386±0.034 | 1.293±0.039 |
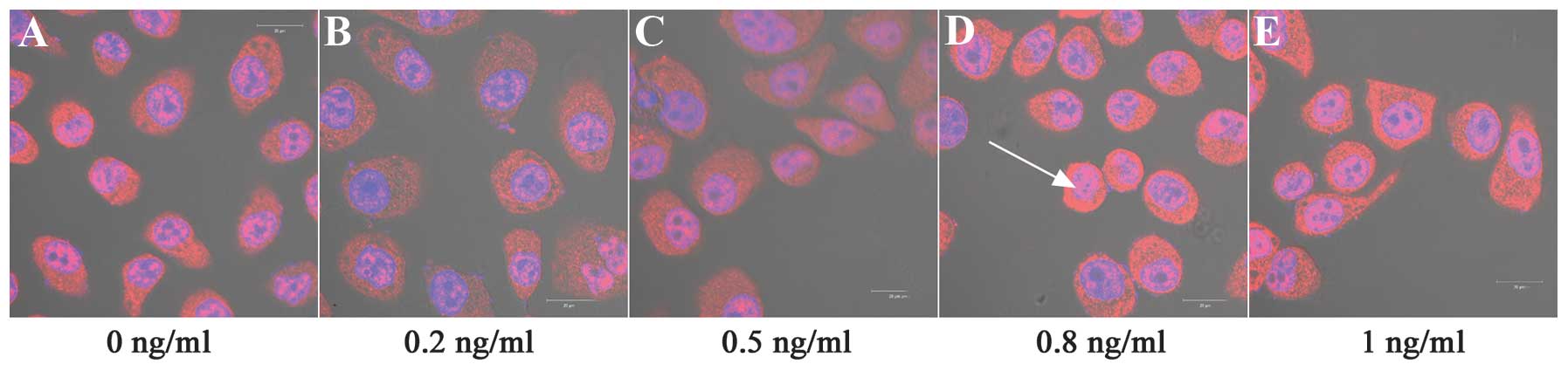
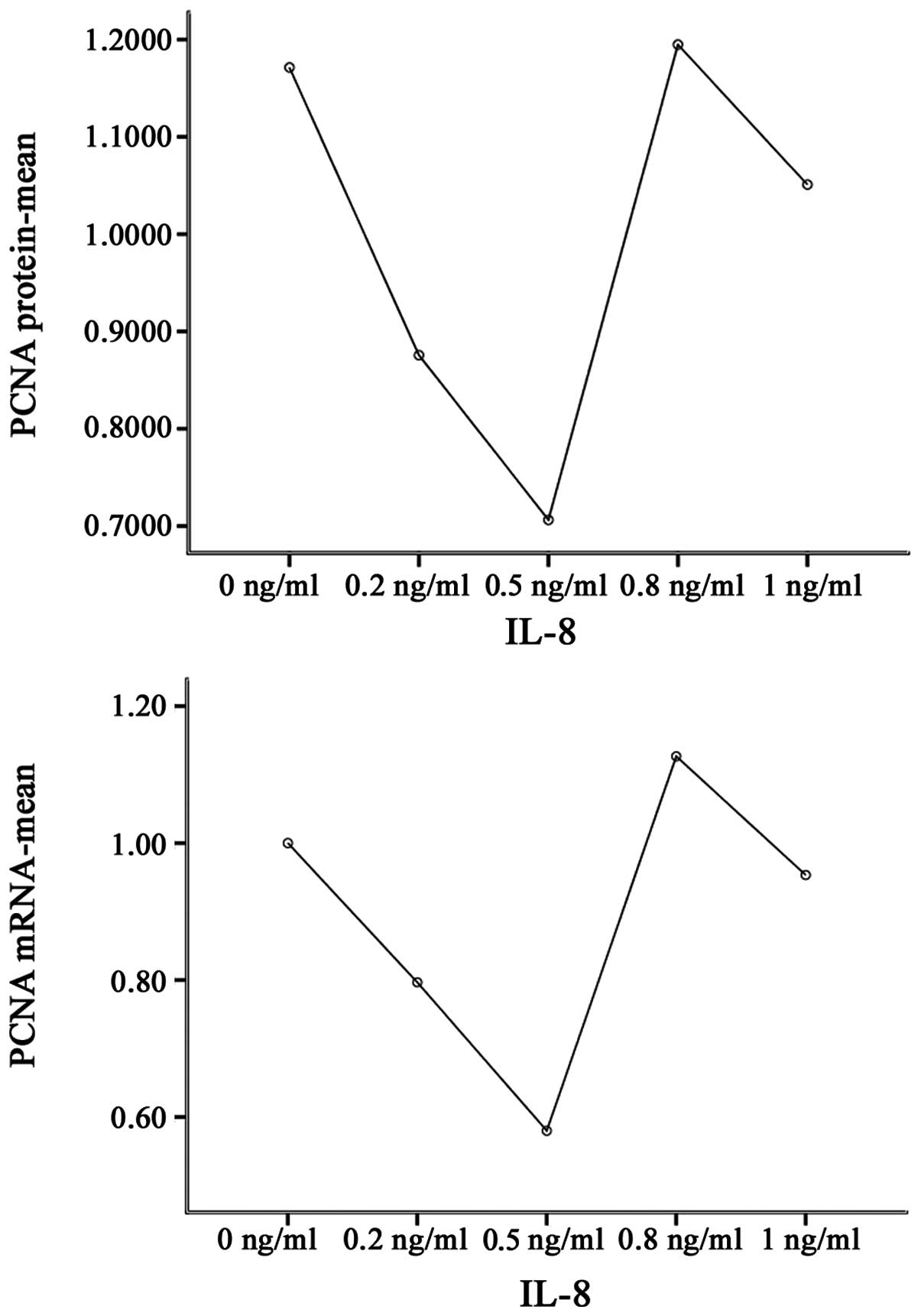
Effect of IL-8 on PCNA protein expression
levels in SGC7901 cells
Cell nuclei were detected with DAPI (blue) staining
and PCNA protein was counterstained with Cy3-Conjugated Affinipure
Goat Anti-Rabbit IgG (red). Fig. 3
reveals that PCNA immunostaining was restricted to the cell
cytoplasm and nuclei. Notably, IL-8 significantly affected the PCNA
protein expression levels under the experimental conditions
compared with the control cells (P<0.001). IL-8 at 0.2, 0.5 and
1 ng/ml concentrations significantly downregulated the expression
of PCNA protein, compared with no treatment (P<0.01). By
contrast, 0.8 ng/ml IL-8 significantly upregulated the expression
levels of PCNA protein (P<0.01; Table III, Figs. 3–5).
 | Table IIIEffect of interleukin-8 on the
expression levels of PCNA protein and mRNA in SGC7901 gastric
cancer cells. |
Table III
Effect of interleukin-8 on the
expression levels of PCNA protein and mRNA in SGC7901 gastric
cancer cells.
| Group | PCNA protein | PCNA mRNA |
|---|
| 0 ng/ml | 1.171±0.003 | 1.00±0.09 |
| 0.2 ng/ml | 0.876±0.006b | 0.80±0.02b |
| 0.5 ng/ml | 0.706±0.011b,c | 0.58±0.03b,c |
| 0.8 ng/ml | 1.195±0.006b,c,d | 1.13±0.06a,c,d |
| 1 ng/ml | 1.051±0.001b,c,d,e | 0.95±0.06c,d,e |
Effect of IL-8 on PCNA mRNA expression
levels in SGC7901 cells
A statistically significant difference between mRNA
expression levels in all groups was observed when compared with
that of the control group (P<0.001), with PCNA mRNA expression
levels exhibiting a similar pattern to the PCNA protein expression
levels. IL-8 at concentrations of 0.2 and 0.5 ng/ml significantly
downregulated the expression of PCNA mRNA, compared with no
treatment (P<0.01). By contrast, treatment with 0.8 ng/ml IL-8
significantly upregulated the expression of PCNA mRNA, compared
with no treatment (P<0.05; Table
III, Figs. 5 and 6).
Discussion
Hp, a Gram-negative spiral bacterium, is a
predominant stomach pathogen associated with chronic gastric
disease that infects >50% of the population worldwide (17). Hp colonizes the human stomach and
causes extensive gastric epithelial cell inflammation (10,18).
Once Hp adheres to the host gastric epithelial cells, signal
transduction is activated through virulence factors, such as
cytotoxin-associated antigen (CagA). The inflammatory cascade is
immediately initiated, with increased secretion of various
inflammatory cytokines, including IL-1, IL-6, IL-8, intercellular
adhesion molecule-1, cyclooxygenase-2 and tumor necrosis factor α
(19–24). Therefore, infection by CagA-positive
Hp is a known risk factor for the development of gastric disease
due not only to marked changes in cellular morphology but also the
release of cytokines from the gastric epithelium (25).
IL-8 is a multifunctional pro-inflammatory cytokine
released through the nuclear factor-κB signaling pathway, which
includes extracellular signal-regulated kinase activity (26,27)
and mitogen-activated protein kinase (28). IL-8 has been shown, through whole
genome analysis, to be the most markedly upregulated gene, and to
exert an important role in numerous epithelial cellular responses
to Hp infection and in the pathological processes resulting in
gastric disease (29). IL-8
production in vitro and in vivo induced by Hp has
been recognized as a host response to microbes. Hp directly
increases gastric epithelial IL-8 protein secretion and IL-8 mRNA
expression levels (30,31).
Thus far, several studies have identified the
association between Hp infection and gastric cancer. Hp infection
is known to exert a predominant role in gastric carcinogenesis, a
process that commences with chronic gastritis and results in a
sequence of atrophic gastritis, metaplasia, dysplasia and
subsequently, gastric cancer (32,33).
Therefore, WHO classified Hp as a group I carcinogen in 1994
(34).
Increased IL-8 expression levels have been detected
in numerous types of cancer cell, suggesting that IL-8 may function
as a significant regulatory factor within the tumor
microenvironment (35). As the
overexpression of IL-8 is induced by Hp infection, IL-8 has been
associated with gastric cancer. In vitro, IL-8 is produced
by gastric cancer cells in response to exposure to the cytotoxic
strain of Hp (36), and IL-8 is
involved in the progressive growth of gastric cancer by autocrine
or paracrine mechanisms (8). In
vivo, IL-8 produced by gastric tumor cells may regulate the
neovascularization, growth and spread of human gastric cancer
(37). IL-8 levels have also been
significantly correlated with depth of invasion, venous invasion
and lymphatic invasion, and may be an independent prognostic factor
in human gastric carcinomas (38).
In a previous study using recombinant IL-8 treatment, IL-8 was
reported to be associated with the adhesion, migration and invasion
of SGC-7901 human gastric cancer cells (39). Similarly, using cDNA and small
interfering (si)RNA transfectants, Kuai et al (9) reported that IL-8 was essential in
human gastric cancer cell adhesion, migration, invasion and
chemosensitivity.
Cell proliferation is a key process in the growth of
carcinoma. Several recent studies have indicated that upregulated
IL-8 mediates tumorigenic and mitogenic effects (40), and stimulates proliferation in a
variety of human cancer cell types, including human melanoma
(41), squamous cell carcinoma
(42,43), ovarian cancer (44), non-small cell lung cancer (45) and colon cancer cells (46). By contrast, downregulation of IL-8
via siRNA inhibited proliferation and delayed G1 to S phase cell
cycle progression in several types of cancer, including ER-negative
breast cancer (47). However,
whether IL-8 influences cell proliferation of gastric cancer
remains unclear. As determined by the associations amongst IL-8, Hp
and gastric cancer, IL-8 was hypothesized to promote cell
proliferation in gastric cancer. The present study aimed to
determine the effects of IL-8 on gastric cancer cell proliferation.
A previous study indicated that treatment of SGC7901 cells with
recombinant IL-8 at concentrations ranging between 0 and 100 ng/ml
did not exert a significant effect on cancer cell proliferation
(39). Similarly, using cDNA and
siRNA transfectants, overexpression of IL-8 in the MKN-45 gastric
cancer cell line and silencing IL-8 expression in the KATO-III
gastric cancer cell line was not significantly associated with cell
proliferation (9). The IL-8 level
produced by gastric cancer cells is marginal. For example, in
vitro, the highest levels of IL-8 were 0.17 ng/ml in the IM95
gastric cancer line cultured for three days (48). Therefore, the effect of IL-8 on
gastric cancer cell proliferation may have been associated with
IL-8 dosage. However, in the present study, the proliferation rate
of SGC7901 cells treated with IL-8 at concentrations ranging
between 0 and 1 ng/ml was not significantly different from that of
the control cells. This result indicated that IL-8 exerted no
significant effect on the proliferation of SGC7901 gastric cancer
cells, although IL-8 was considered to promote invasion, migration
and adhesion of gastric cancer cells (9). In previous studies, no significant
effect of IL-8 on cell proliferation in several types of
hepatocellular and breast cancer cells was observed (49,50).
PCNA, an auxiliary protein of DNA polymerase δ
located in the nuclei of tumor cells, is known as a cell
cycle-related nuclear antigen and is synthesized in late G1 and S
phase. PCNA levels therefore correlate with the cell proliferative
state (51,52). Although no effect of IL-8 on the
proliferation of SGC7901 gastric cancer cells was identified in the
present study, notably, the data revealed that PCNA expression
levels were associated with IL-8. Immunofluorescence staining and
western blot analysis were used to observe the expression levels of
PCNA protein, and the qPCR method was employed to assay the levels
of PCNA mRNA. The data demonstrated that IL-8 had a significant
effect on PCNA protein and mRNA expression levels in the SGC7901
cells, in a dose-dependent manner. At a 0.8-ng/ml dosage, IL-8
significantly increased the expression levels of PCNA protein and
mRNA. However, IL-8 significantly inhibited the expression of PCNA
at the other dosages. This may be associated with the IL-8
regulatory mechanism of PCNA expression, however, this regulatory
effect does not appear to be involved in gastric cancer cell
proliferation. Other potential regulatory mechanisms of IL-8 in
gastric cancer require investigation.
In conclusion, the present study demonstrated that
IL-8 exerts no direct effect on the proliferation of gastric cancer
cells, but influences the expression levels of PCNA protein and
mRNA, depending on the IL-8 dosage. The findings suggest that IL-8
is a potent pro-inflammatory cytokine with multiple effects on the
development of gastric cancer.
Acknowledgements
The authors would like to thank Shanghai R&S
Biotechnology Co., Ltd. for supplying the iQ5 PCR detection system
and iQ5 optical system software. This study was supported by a
grant from the three-year action plan fund of Traditional Chinese
Medicine, Shanghai City Health Administration (grant no.
ZYSNXD-CC-ZDYJ024).
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, et al:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014.
|
|
2
|
Ferlay J, Soerjomataram I, Ervik M, Forman
D, Bray F, Dikshit R, Elser S, Mathers C, Rebelo M and Parkin DM:
GLOBOCAN 2012 v10, Cancer Incidence and Mortality Worldwide. IARC
CancerBase No. 11 Lyon, France: International Agency for Research
on Cancer; 2013, Available from: http://globocan.iarc.fr.
accessed on August 30, 2014
|
|
3
|
Harada A, Sekido N, Akahoshi T, et al:
Essential involvement of interleukin-8 (IL-8) in acute
inflammation. J Leukoc Biol. 56:559–564. 1994.
|
|
4
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007.
|
|
5
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001.
|
|
6
|
Xu L and Fidler IJ: Interleukin-8: an
autocrine growth factor for human ovarian cancer. Oncol Res.
12:97–106. 2000.
|
|
7
|
Huang S, Mills L, Mian B, et al: Fully
humanized neutralizing antibodies to interleukin-8 (ABX-IL8)
inhibit angiogenesis, tumor growth, and metastasis of human
melanoma. Am J Pathol. 161:125–134. 2002.
|
|
8
|
Kitadai Y, Haruma K, Mukaida N, et al:
Regulation of disease-progression genes in human gastric carcinoma
cells by interleukin-8. Clin Cancer Res. 6:2735–2740. 2000.
|
|
9
|
Kuai WX, Wang Q, Yang XZ, et al:
Interleukin-8 associates with adhesion, migration, invasion and
chemosensitivity of human gastric cancer cells. World J
Gastroenterol. 18:979–985. 2012.
|
|
10
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev
Cancer. 2:28–37. 2002.
|
|
11
|
Ren Z, Pang G, Clancy R, et al: Shift of
the gastric T-cell response in gastric carcinoma. J Gastroenterol
Hepatol. 16:142–148. 2001.
|
|
12
|
Sheh A, Chaturvedi R, Merrell DS, et al:
Phylogeographic origin of Helicobacter pylori determines
host-adaptive responses upon coculture with gastric epithelial
cells. Infect Immun. 81:2468–2477. 2013.
|
|
13
|
Wang YC and Huang KM: In vitro
anti-inflammatory effect of apigenin in the Helicobacter
pylori-infected gastric adenocarcinoma cells. Food Chem
Toxicol. 53:376–383. 2013.
|
|
14
|
Yamaoka Y, Kita M, Kodama T, et al:
Induction of various cytokines and development of severe mucosal
inflammation by cagA gene positive Helicobacter pylori
strains. Gut. 41:442–451. 1997.
|
|
15
|
D’Elios MM and Andersen LP:
Helicobacter pylori inflammation, immunity, and vaccines.
Helicobacter. 12(Suppl 1): 15–19. 2007.
|
|
16
|
D’Elios MM and Andersen LP: Inflammation,
immunity, and vaccines for Helicobacter pylori.
Helicobacter. 14(Suppl 1): 21–28. 2009.
|
|
17
|
Montecucco C and Rappuoli R: Living
dangerously; How Helicobacter pylori survives in the human
stomach. Nat Rev Mol Cell Biol. 2:457–466. 2001.
|
|
18
|
Naito Y and Yoshikawa T: Molecular and
cellular mechanisms involved in Helicobacter pylori-induced
inflammation and oxidative stress. Free Radic Biol Med. 33:323–336.
2002.
|
|
19
|
Peek RM Jr, Fiske C and Wilson KT: Role of
innate immunity in Helicobacter pylori-induced gastric
malignancy. Physiol Rev. 90:831–858. 2010.
|
|
20
|
Chiba T, Marusawa H, Seno H and Watanabe
N: Mechanism for gastric cancer development by Helicobacter
pylori infection. J Gastroenterol Hepatol. 23:1175–1181.
2008.
|
|
21
|
Nozawa Y, Nishihara K, Peek RM, et al:
Identification of a signaling cascade for interleukin-8 production
by Helicobacter pylori in human gastric epithelial cells.
Biochem Pharmacol. 64:21–30. 2002.
|
|
22
|
Sharma SA, Tummuru MK, Blaser MJ and Kerr
LD: Activation of IL-8 gene expression by Helicobacter
pylori is regulated by transcription factor nuclear
factor-kappa B in gastric epithelial cells. J Immunol.
160:2401–2407. 1998.
|
|
23
|
Keates S, Keates AC, Warny M, et al:
Differential activation of mitogen-activated protein kinases in AGS
gastric epithelial cells by cag+ and cag− Helicobacter
pylori. J Immunol. 163:5552–5559. 1999.
|
|
24
|
Eftang LL, Esbensen Y, Tannæs TM, Bukholm
IR and Bukholm G: Interleukin-8 is the single most up-regulated
gene in whole genome profiling of H. Pylori exposed gastric
epithelial cells. BMC Microbiol. 12:92012.
|
|
25
|
Crabtree JE, Farmery SM, Lindley IJ, et
al: CagA/cytotoxic strains of Helicobacter pylori and
interleukin-8 in gastric epithelial cell lines. J Clin Pathol.
47:945–950. 1994.
|
|
26
|
Sharma SA, Tummuru MK, Miller GG and
Blaser MJ: Interleukin-8 response of gastric epithelial cell lines
to Helicobacter pylori stimulation in vitro. Infect Immun.
63:1681–1687. 1995.
|
|
27
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001.
|
|
28
|
Correa P and Houghton J: Carcinogenesis of
Helicobacter pylori. Gastroenterology. 133:659–672.
2007.
|
|
29
|
World Health Organization; International
Agency for Research on Cancer (IARC). IARC monographs on the
evaluation of carcinogenic risks to humans. 61. World Health
Organization; Geneva, Switzerland: pp. 177–240. 1994
|
|
30
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008.
|
|
31
|
Takagi A, Kamiya S, Koga Y, et al:
Analysis of interleukin-8 secretion induced by Helicobacter
pylori from the gastric epithelial cell line MKN45: a mechanism
independent of the intensity of cytotoxicity. J Gastroenterol
Hepatol. 12:368–372. 1997.
|
|
32
|
Kitadai Y, Haruma K, Sumii K, et al:
Expression of interleukin-8 correlates with vascularity in human
gastric carcinomas. Am J Pathol. 152:93–100. 1998.
|
|
33
|
Kido S, Kitadai Y, Hattori N, et al:
Interleukin-8 and vascular endothelial growth factor - prognostic
factors in human gastric carcinomas? Eur J Cancer. 37:1482–1487.
2001.
|
|
34
|
Ju D, Sun D, Xiu L, et al: Interleukin-8
is associated with adhesion, migration and invasion in human
gastric cancer SCG-7901 cells. Med Oncol. 29:91–99. 2012.
|
|
35
|
Zhu YM and Woll PJ: Mitogenic effects of
interleukin-8/CXCL8 on cancer cells. Future Oncol. 1:699–704.
2005.
|
|
36
|
Gabellini C, Trisciuoglio D, Desideri M,
et al: Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on
human malignant melanoma progression. Eur J Cancer. 45:2618–2627.
2009.
|
|
37
|
Christofakis EP, Miyazaki H, Rubink DS and
Yeudall WA: Roles of CXCL8 in squamous cell carcinoma proliferation
and migration. Oral Oncol. 44:920–926. 2008.
|
|
38
|
Wu S, Shang H, Cui L, et al: Targeted
blockade of interleukin-8 abrogates its promotion of cervical
cancer growth and metastasis. Mol Cell Biochem. 375:69–79.
2013.
|
|
39
|
Wang Y, Xu RC, Zhang XL, et al:
Interleukin-8 secretion by ovarian cancer cells increases
anchorage-independent growth, proliferation, angiogenic potential,
adhesion and invasion. Cytokine. 59:145–155. 2012.
|
|
40
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007.
|
|
41
|
Ning Y, Manegold PC, Hong YK, et al:
Interleukin-8 is associated with proliferation, migration,
angiogenesis and chemosensitivity in vitro and in vivo in colon
cancer cell line models. Int J Cancer. 128:2038–2049. 2011.
|
|
42
|
Shao N, Chen LH, Ye RY, Lin Y and Wang SM:
The depletion of interleukin-8 causes cell cycle arrest and
increases the efficacy of docetaxel in breast cancer cells. Biochem
Biophys Res Commun. 431:535–541. 2013.
|
|
43
|
Iwai M, Matsuda M and Iwai Y: Cloning of a
cancer cell-producing hepatocyte growth factor, vascular
endothelial growth factor, and interleukin-8 from gastric cancer
cells. In Vitro Cell Dev Biol Anim. 39:288–290. 2003.
|
|
44
|
Kubo F, Ueno S, Hiwatashi K, et al:
Interleukin-8 in human hepatocellular carcinoma correlates with
cancer cell invasion of vessels but not with tumor angiogenesis.
Ann Surg Oncol. 12:800–807. 2005.
|
|
45
|
Lin Y, Wang SM, Lü WM and Huang RP: Effect
of interleukin-8 in cell invasion and proliferation of human breast
cancer. Zhonghua Wai Ke Za Zhi. 43:1541–1544. 2005.(In
Chinese).
|
|
46
|
Jain S, Filipe MI, Hall PA, et al:
Prognostic value of proliferating cell nuclear antigen in gastric
carcinoma. J Clin Pathol. 44:655–659. 1991.
|
|
47
|
Li N: Proliferating cell nuclear antigen
(PCNA/cyclin) in gastric carcinoma in relation to its prognosis.
Zhonghua Zhong Liu Za Zhi. 15:34–36. 1993.(In Chinese).
|
|
48
|
Shibata W and Maeda S: Mechanism of H.
pylori-induced gastric inflammation and carcinogenesis. Nihon
Rinsho. 71:1346–1351. 2013.(In Japanese).
|
|
49
|
Song H, Michel A, Nyrén O, Ekström AM,
Pawlita M and Ye W: A CagA-independent cluster of antigens related
to the risk of noncardia gastric cancer: associations between
Helicobacter pylori antibodies and gastric adenocarcinoma explored
by multiplex serology. Int J Cancer. 134:2942–2950. 2014.
|
|
50
|
Yoshida T, Kato J, Inoue I, Yoshimura N,
Deguchi H, Mukoubayashi C, Oka M, Watanabe M, Enomoto S, Niwa T, et
al: Cancer development based on chronic active gastritis and
resulting gastric atrophy as assessed by serum levels of pepsinogen
and Helicobacter pylori antibody titer. Int J Cancer.
134:1445–1457. 2014.
|
|
51
|
Berber U, Yılmaz I, Erkul BE and Kaplan M:
Peptic ulcer and intestinal metaplasia associated with Helicobacter
pylori colonization in gastric heterotopia of the tongue. Turk J
Gastroenterol. 25:224–225. 2014.
|
|
52
|
Kara N, Urganci N, Kalyoncu D and Yilmaz
B: The association between Helicobacter pylori gastritis and
lymphoid aggregates, lymphoid follicles and intestinal metaplasia
in gastric mucosa of children. J Paediatr Child Health. 50:605–609.
2014.
|