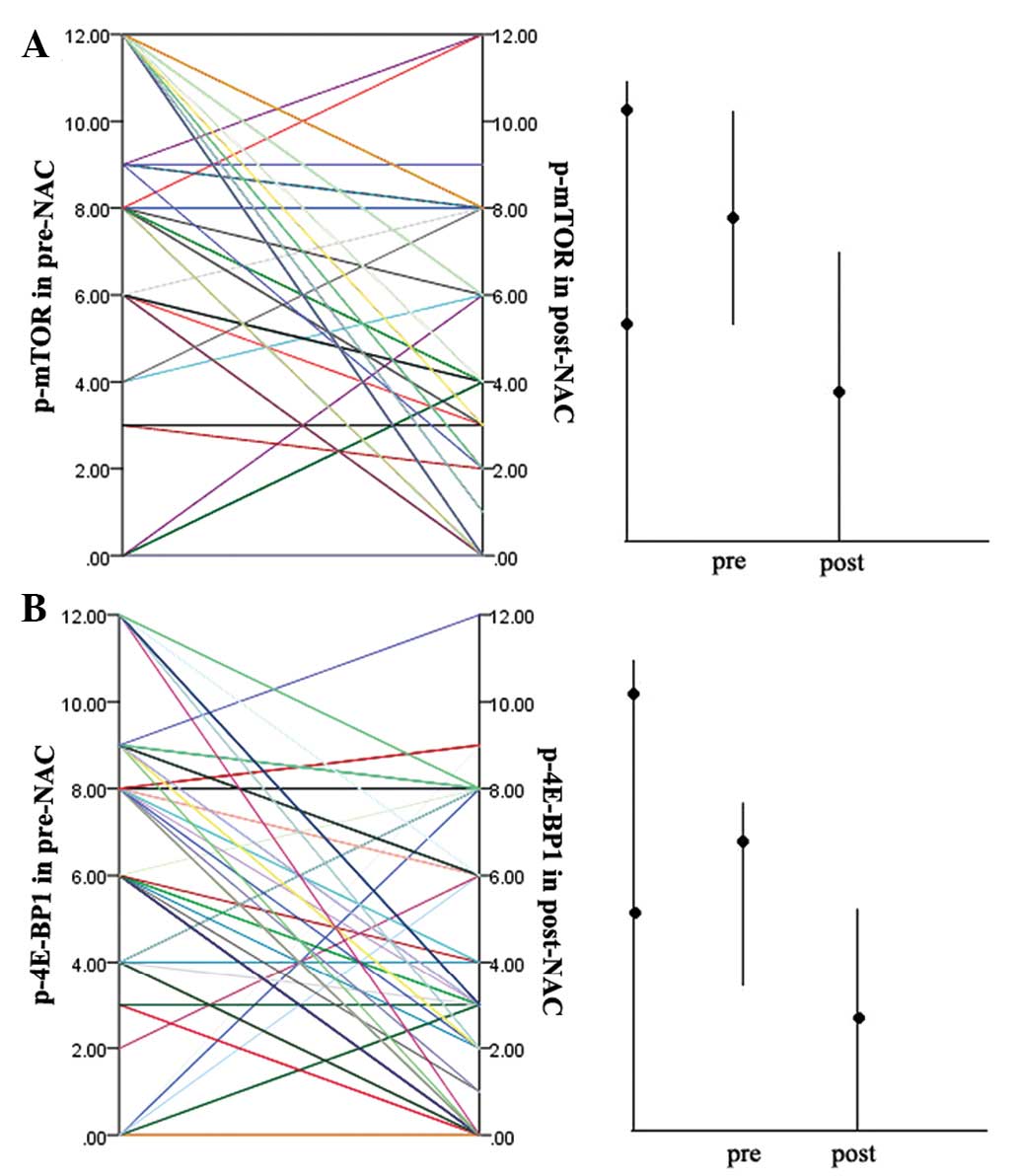
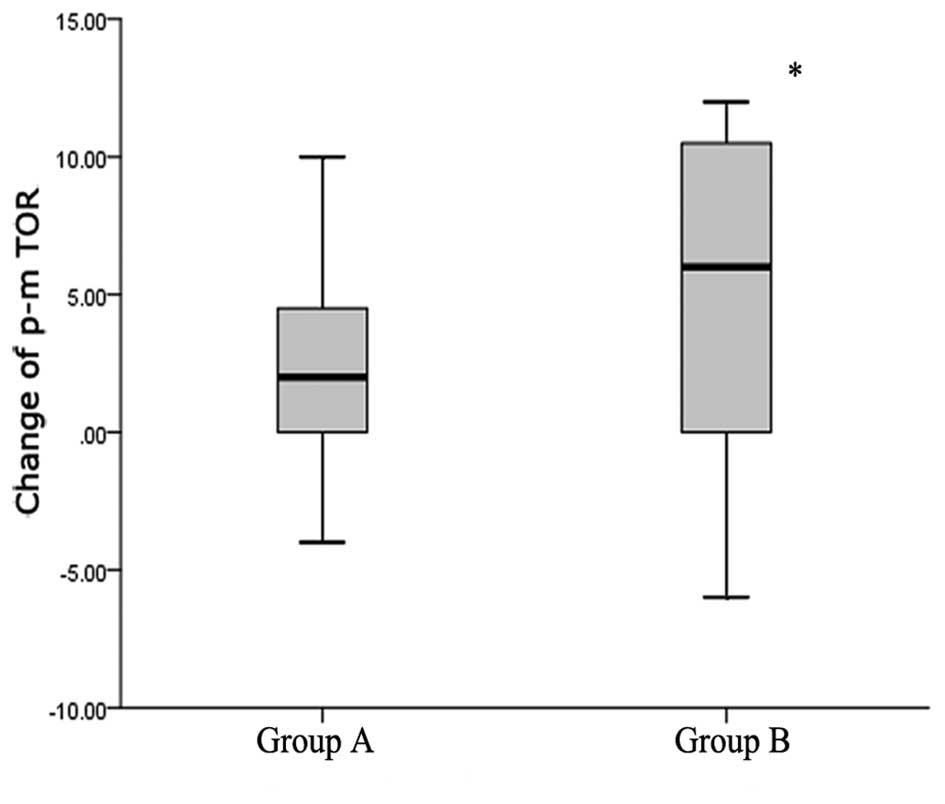
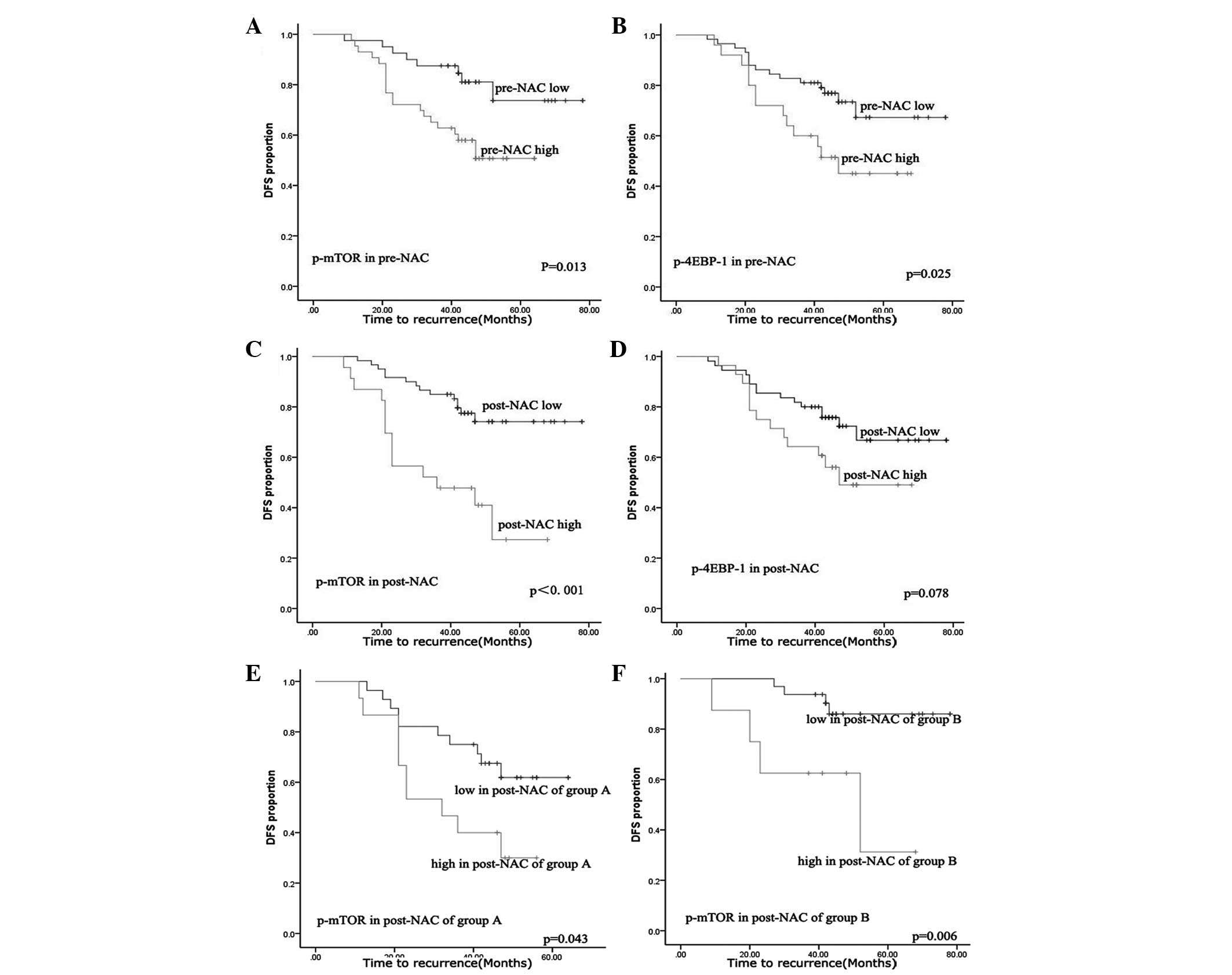
|
1
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nature Rev Mol Cell Biol.
10:307–318. 2009.
|
|
2
|
Zhou X, Tan M, Stone Hawthorne V, et al:
Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway
by ErbB2 overexpression predicts tumor progression in breast
cancers. Clinical Cancer Res. 10:6779–6788. 2004.
|
|
3
|
Meric-Bernstam F and Gonzalez-Angulo AM:
Targeting the mTOR signaling network for cancer therapy. J Clin
Oncol. 27:2278–2287. 2009.
|
|
4
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006.
|
|
5
|
Margariti N, Fox SB, Bottini A and
Generali D: ‘Overcoming breast cancer drug resistance with mTOR
inhibitors’. Could it be a myth or a real possibility in the
short-term future? Breast Cancer Res Treat. 128:599–606. 2011.
|
|
6
|
Gingras AC, Raught B and Sonenberg N: eIF4
initiation factors: effectors of mRNA recruitment to ribosomes and
regulators of translation. Annu Rev Biochem. 68:913–963. 1999.
|
|
7
|
Hara K, Yonezawa K, Kozlowski MT, et al:
Regulation of eIF-4EBP1 phosphorylation by mTOR. J Biol Chem.
272:26457–26463. 1997.
|
|
8
|
von Manteuffel SR, Dennis PB, Pullen N, et
al: The insulin-induced signalling pathway leading to S6 and
initiation factor 4E binding protein 1 phosphorylation bifurcates
at a rapamycin-sensitive point immediately upstream of p70s6k. Mol
Cell Biol. 17:5426–5436. 1997.
|
|
9
|
Brunn GJ, Hudson CC, Sekulić A, et al:
Phosphorylation of the translational repressor PHAS-I by the
mammalian target of rapamycin. Science. 277:99–101. 1997.
|
|
10
|
Heikkinen T, Korpela T, Fagerholm R, et
al: Eukaryotic translation initiation factor 4E (eIF4E) expression
is associated with breast cancer tumor phenotype and predicts
survival after anthracycline chemotherapy treatment. Breast Cancer
Ress Treat. 141:79–88. 2013.
|
|
11
|
Coleman LJ, Peter MB, Teall TJ, et al:
Combined analysis of eIF4E and 4E-binding protein expression
predicts breast cancer survival and estimates eIF4E activity. Br J
Cancer. 100:1393–1399. 2009.
|
|
12
|
Jefferies HB, Fumagalli S, Dennis PB, et
al: Rapamycin suppresses 5′TOP mRNA translation through inhibition
of p70s6k. EMBO J. 16:3693–3704. 1997.
|
|
13
|
Kim EK, Kim HA, Koh JS, et al:
Phosphorylated S6K1 is a possible marker for endocrine therapy
resistance in hormone receptor-positive breast cancer. Breast
Cancer Res Treat. 126:93–99. 2011.
|
|
14
|
Fuksa L, Micuda S, Grim J, Ryska A and
Hornychova H: Predictive biomarkers in breast cancer: their value
in neoadjuvant chemotherapy. Cancer Invest. 30:663–678. 2012.
|
|
15
|
Mieog JS, van der Hage JA and van de Velde
CJ: Neoadjuvant chemotherapy for operable breast cancer. Br J Surg.
94:1189–1200. 2007.
|
|
16
|
Kuerer HM, Newman LA, Smith TL, et al:
Clinical course of breast cancer patients with complete pathologic
primary tumor and axillary lymph node response to doxorubicin-based
neoadjuvant chemotherapy. J Clin Oncol. 17:460–469. 1999.
|
|
17
|
Ogston KN, Miller ID, Payne S, et al: A
new histological grading system to assess response of breast
cancers to primary chemotherapy: prognostic significance and
survival. Breast. 12:320–327. 2003.
|
|
18
|
Thomas E, Holmes FA, Smith TL, et al: The
use of alternate, non-cross-resistant adjuvant chemotherapy on the
basis of pathologic response to a neoadjuvant doxorubicin-based
regimen in women with operable breast cancer: long-term results
from a prospective randomized trial. J Clin Oncol. 22:2294–2302.
2004.
|
|
19
|
Heys SD, Hutcheon AW, Sarkar TK, et al:
Neoadjuvant docetaxel in breast cancer: 3-year survival results
from the Aberdeen trial. Clin Breast Cancer. 3(Suppl 2): S69–S74.
2002.
|
|
20
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007.
|
|
21
|
Dillon RL, White DE and Muller WJ: The
phosphatidyl inositol 3-kinase signaling network: implications for
human breast cancer. Oncogene. 26:1338–1345. 2007.
|
|
22
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012.
|
|
23
|
Banerji S, Cibulskis K, Rangel-Escareno C,
et al: Sequence analysis of mutations and translocations across
breast cancer subtypes. Nature. 486:405–409. 2012.
|
|
24
|
Saal LH, Holm K, Maurer M, et al: PIK3CA
mutations correlate with hormone receptors, node metastasis, and
ERBB2, and are mutually exclusive with PTEN loss in human breast
carcinoma. Cancer Res. 65:2554–2559. 2005.
|
|
25
|
Stemke-Hale K, Gonzalez-Angulo AM, Lluch
A, et al: An integrative genomic and proteomic analysis of PIK3CA,
PTEN, and AKT mutations in breast cancer. Cancer Res. 68:6084–6091.
2008.
|
|
26
|
Mondesire WH, Jian W, Zhang H, et al:
Targeting mammalian target of rapamycin synergistically enhances
chemotherapy-induced cytotoxicity in breast cancer cells. Clinical
Cancer Res. 10:7031–7042. 2004.
|
|
27
|
Jerusalem G, Fasolo A, Dieras V, et al:
Phase I trial of oral mTOR inhibitor everolimus in combination with
trastuzumab and vinorelbine in pre-treated patients with
HER2-overexpressing metastatic breast cancer. Breast Cancer Res
Treat. 125:447–455. 2011.
|
|
28
|
Andre F, Campone M, O’Regan R, et al:
Phase I study of everolimus plus weekly paclitaxel and trastuzumab
in patients with metastatic breast cancer pretreated with
trastuzumab. J Clin Oncol. 28:5110–5115. 2010.
|
|
29
|
Huober J, Hanusch C, Fasching PA, et al:
Neoadjuvant chemotherapy of paclitaxel with or without Rad001:
Results of the non-responder part of the GEPARUINTO study. In:
Presented at 33rd Annual San Antonio Breast Cancer Symposium; San
Antonio, TX. 2011
|
|
30
|
Yardley DA: Combining mTOR inhibitors with
chemotherapy and other targeted therapies in advanced breast
cancer: Rationale, clinical experience, and future directions.
Breast Cancer (Auckl). 7:7–22. 2013.
|
|
31
|
Yang F and Li J: WHO classification of
tumors of the breast. Zhonghua Wai Ke Za Zhi. 52:1–3. 2014.(In
Chinese).
|
|
32
|
Chen J and Fang Y: A novel pathway
regulating the mammalian target of rapamycin (mTOR) signaling.
Biochemical Pharmacol. 64:1071–1077. 2002.
|
|
33
|
Mita MM, Mita A and Rowinsky EK: The
molecular target of rapamycin (mTOR) as a therapeutic target
against cancer. Cancer Biol Ther. 2(4 Suppl 1): S169–S177.
2003.
|
|
34
|
Hiller DJ, Chu Q, Meschonat C, et al:
Predictive value of eIF4E reduction after neoadjuvant therapy in
breast cancer. J Surg Res. 156:265–269. 2009.
|
|
35
|
Klos KS, Wyszomierski SL, Sun M, et al:
ErbB2 increases vascular endothelial growth factor protein
synthesis via activation of mammalian target of rapamycin/p70S6K
leading to increased angiogenesis and spontaneous metastasis of
human breast cancer cells. Cancer Res. 66:2028–2037. 2006.
|
|
36
|
Hanrahan EO, Hennessy BT and Valero V:
Neoadjuvant systemic therapy for breast cancer: an overview and
review of recent clinical trials. Expert Opin Pharmacother.
6:1477–1491. 2005.
|