Introduction
Lung cancer is one of the primary causes of
cancer-associated mortality in numerous countries (1,2), with
non-small cell lung cancer (NSCLC) being the cause of 80–85% of all
diagnosed lung cancers (3). At the
time of diagnosis, numerous patients with NSCLC already exhibit
advanced stage IIIB or IV disease, which makes these patients
candidates for systemic chemotherapy (4). Cisplatin (diamminedichloroplatinum;
DDP) is a platinum-coordinated complex that has been extensively
used for first-line chemotherapy to treat human NSCLC (5). However, drug resistance often develops
in NSCLC following continuous infusion or multiple administration
of DDP, leading to treatment failure characterized by tumor growth
or tumor relapse (6,7). Therefore, it is extremely important to
decrease the resistance to chemotherapeutic drugs in order to treat
NSCLC.
Genistein (GEN) is a major isoflavone in soy and red
clover that has potential beneficial effects and numerous
biological actions, which have become a focus for research. An
increasing number of studies have revealed that dietary
isoflavones, including GEN, may protect against several cancer
types, including lung cancer (8–10),
without exerting toxic effects on normal cells. In addition,
several studies have demonstrated that GEN inhibits the
proliferation of cancer cells by cooperatively or synergistically
enhancing the effect of anticancer drugs (8,11,12).
Schabath et al (13) found
that 10 μM GEN enhanced trichostatin A-induced apoptosis in human
lung carcinoma A549 cells, but not in normal human lung
fibroblasts. It has been reported that GEN may sensitize estrogen
receptor-positive breast cancer cells to tamoxifen treatment
(14). Mohammad et al
revealed that in in vitro and in vivo pancreatic
carcinoma cells, GEN enhanced DDP-induced growth inhibition and
apoptosis (15). These studies
indicate that GEN may cause other drug-resistant carcinoma cells to
become sensitized to common chemotherapy drugs and may also
decrease the toxicity of these common agents, as a lower dose of
the chemotherapy drug is required.
Currently, the effect of combined GEN and DDP
treatment on NSCLC cell proliferation and growth in vitro
and in vivo has not been explored in any systemic study. The
present study, therefore, aimed to evaluate the feasibility of
using DDP in combination with GEN to inhibit NSCLC cell growth,
proliferation and apoptosis, and to investigate the underlying
molecular mechanisms involved in GEN-induced apoptosis, thereby
providing additive or synergistic benefits against NSCLC.
Materials and methods
Reagents
All chemicals used were reagent grade or higher. DDP
and GEN were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
nonidet P-40 lysis buffer, chemiluminescent peroxidase substrate,
propidium iodide (PI), 4′,6-diamidino-2-phenylindole (DAPI),
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT),
pyrogallol and H2O2 were obtained from Merck
(Whitehouse Station, NJ, USA). Dimethyl sulfoxide (DMSO) was
purchased from Merck (Darmstadt, Germany). The stock solutions of
PI, DAPI and MTT were prepared by dissolving 1 mg of each compound
in 1 ml phosphate-buffered saline (PBS). The solutions were
protected from light, stored at 4°C and used within one month.
Cell culture and cell viability
assay
The A549 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL, Grand Island,
NY, USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; GIBCO-BRL) at 37°C in a 5% CO2 atmosphere and at
95% humidity.
The A549 cells were grown in monolayers and were
harvested and dispensed in 96-well culture plates in 100 μl DMEM at
a concentration of 5×103 cells per well. After 24 h, the
assigned concentrations of DDP, GEN or a combination of the two
were added to the cells. Wells containing medium alone were used as
the negative controls, while the wells containing cells but without
any treatment were used as the positive controls. The cells were
then incubated for an additional 4 h. At the end of the treatment,
200 μl DMSO was added to each well following the removal of the
supernatant. The cell viability was determined by measuring the
absorbance of the wells at a wavelength of 490 nm using the Thermo
Multiskan MK3 microplate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). This assay was performed in triplicate. The
inhibition rate was calculated according to the following formula:
Inhibition rate (%) = [1 - (average absorbance of experimental
group / average absorbance of blank control group)] × 100.
Detection of apoptosis
The A549 cells were cultured in six-well plates in
DMEM supplemented with 10% FBS medium and were treated with DDP,
GEN or DDP and GEN in combination for 48 h. The cover slips were
washed three times with PBS and single cell suspensions were fixed
in 1% PBS. The cells were stained with 100 μg/ml acridine orange
(Sigma-Aldrich) and 100 μg/ml ethidium bromide (Sigma-Aldrich) for
1 min. The cells were then observed under a fluorescence microscope
(BX43, Olympus Corporation, Tokyo, Japan). In total, ≥200 cells
were counted and the percentage of apoptotic cells was determined.
Triplicates were performed in all experiments and the experiments
were performed on five occasions.
Caspase activity
The activity of caspase-3, -8 and -10 was measured
using caspase colorimetric protease assay kits (Millipore,
Billerica, MA, USA) according to the manufacturer’s instructions.
In brief, the A549 cells were treated with DDP and GEN alone or in
combination for 24 h. Following treatment, the cells were washed
twice with ice-cold PBS and harvested by centrifugation at 1,000 ×
g for 10 min. The cell pellets were then lysed in 150 μl buffer.
Protein concentrations in the lysate were determined using the
Lowry method (16). An 80-μl
aliquot of the lysate was incubated with 10 μl of substrate for
each caspase at 37°C for 2 h. The samples were analyzed at 405 nm
in a microplate reader (Thermo Fisher Scientific Inc.). The
relative caspase activity of the control group was set as 100.
Tumor xenograft assay
Female BALB/c nude mice, 4–5 weeks old, were
obtained from the Experimental Animal Center of the Jilin
University (Changchun, Jilin, China). All the animal experiments
were performed in accordance with institutional guidelines,
following a protocol approved by the Ethics Committees of the
Disease Model Research Center (The First Hospital of Jilin
University, Changchun, Jilin, China). Female BALB mice, 6–7 weeks
old, were maintained under specific pathogen-free conditions and
provided with food and water ad libitum. All the animals
were fed with a normal pellet diet for one week prior to the
experiments.
The exponentially growing A549 cells were harvested
and a tumorigenic dose of 2.5×106 cells was injected
intraperitoneally into the 4–5 week-old mice. When the tumors
reached 100–200 mm3 in size, the mice were divided
randomly into four groups, with 10 mice per group. The control
group received 1% polysorbate resuspended in deionized water. In
the DDP group, DDP was administered at a dose of 9 mg/kg as an
intraperitoneal bolus injection, while in the GEN group, GEN was
administered at a dose of 800 μg/kg each day orally for five days.
In the combination group, DDP (5 mg/kg) was administered with GEN
on day one, followed by four days of GEN (500 μg/kg) treatment. The
tumor weight was measured subsequent to the mice being sacrificed
by cervical dislocation, and the tumor volume was measured prior to
the treatment injections being administered and on days seven, 14
and 21 of treatment.
Western blotting
The cultured cells were washed twice with PBS (pH,
7.2) and the cells were then lysed with Triton X-100
9Sigma-Aldrich) in Hepes buffer (Sigma-Aldrich), which consisted of
150 mm NaCl, 50 mm Hepes, 1.5 mm MgCl2, 1% Triton X-100,
0.1% SDS, protease inhibitor cocktail, 100 mm NaF and 100 mm
Na3VO4, for 30 min. The cell lysates were
clarified using centrifugation at 10,000 × g for 15 min and the
protein concentrations were determined using the Bradford reagent
(Sigma-Aldrich). The protein samples were separated on an 8–15%
SDS-polyacrylamide gel and transferred onto nitrocellulose
membranes (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Subsequent to blocking in Tris-buffered saline containing 5%
skimmed milk and 0.1% Tween 20 for 2 h at room temperature, the
protein samples were then immunoblotted with primary mouse
monoclonal anti-human PI3K (1:3,000), anti-human phosphorylated(p)
PI3K(p-PI3K; 1:2,000), anti-human AKT (1:3,000), anti-human pAKT
(1:3,000) and anti-human β-actin (1:5,000) antibodies (Abs), which
were all purchased from Cell Signaling Technology, Inc., (Beverly,
MA, USA) for 2 h at room temperature. Next, the samples were
incubated with horseradish peroxidase-conjugated polyclonal goat
anti-mouse immunoglobulin G Abs (1:5,000, Amersham Biosciences,
Uppsala, Sweden) for 2 h at room temperature. All the immunoblots
were visualized using an enhanced chemiluminescence kit (Pierce
Protein Biology, Rockford, IL, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical comparisons between more than two groups
were performed using one-way analysis of variance followed by
Tukey’s post-hoc test. Statistical analyses were undertaken using
the SPSS® statistical package, version 19.0 (SPSS Inc.,
Chicago, IL, USA) for Windows® and GraphPad Prism
version 5.01 (GraphPad Software, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of DDP and GEN alone or in
combination on A549 cell proliferation
To evaluate the effect of DDP and GEN alone or in
combination on the cell viability of NSCLC cells in vitro, a
MTT assay was performed for 48 h whilst the A549 cells were being
treated with DPP and GEN alone or in combination. It was found that
the inhibitory rates of DDP and GEN alone or in combination were
higher compared with the control group (P<0.01). There was no
significance different between the DDP and GEN groups (P>0.05).
However, the inhibitory rate in the group treated with GEN and DDP
in combination was higher compared with either agent alone
(P<0.05; Fig. 1).
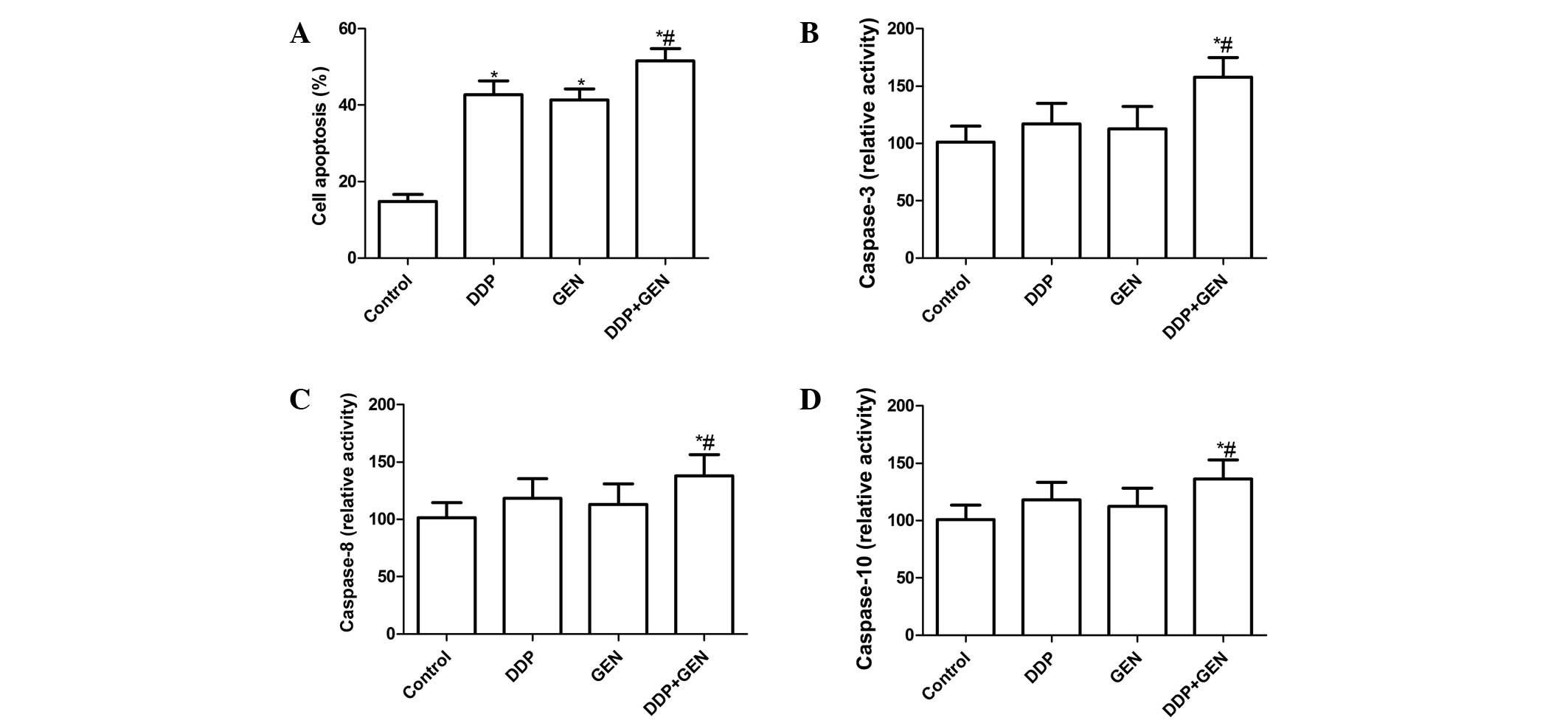
Effects of DDP and GEN alone or in
combination on A549 cell apoptosis
To investigate whether DDP and GEN alone or in
combination could induce apoptosis, apoptosis was analyzed
following treatment with DDP and GEN. It was found that the A549
cells treated with DDP or GEN had significant levels of cell
apoptosis compared with the untreated cells (Fig. 2A). Treatment with a combination of
DDP and GEN led to a marked increase in the level of apoptotic
cells compared with either agent alone (P<0.01; Fig. 2A).
To explore the possible mechanism for the induction
of cell apoptosis in cells treated with a combination of DDP and
GEN, the activity of caspase-3, -8 and -10 was determined by ELISA.
The results revealed that treatment with a combination of DDP and
GEN could significantly increase the activity of caspase-3, -8 and
-10 compared with treatment using DPP or GEN alone (P<0.01)
(Fig. 2B, C and D).
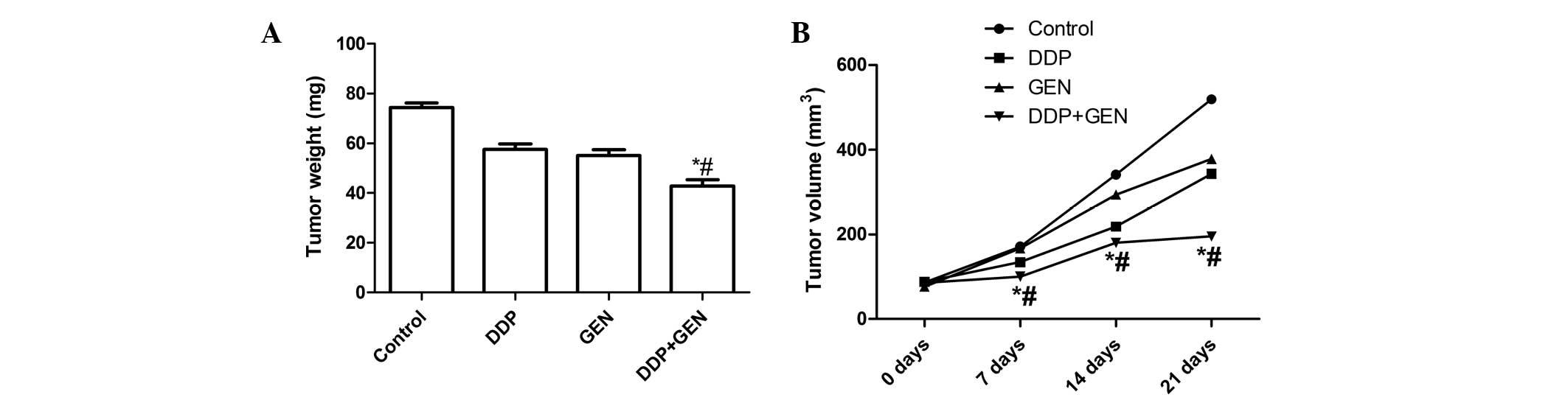
Treatment with DDP and GEN in combination
causes significant inhibition of tumor growth
The in vivo therapeutic efficacy of DDP and
GEN was assessed in female BALB/c nude A549 tumor-bearing mice. The
mice were sacrificed and the tumor tissue was removed 21 days after
treatment. The tumor weight was then measured and it was found that
the tumor weight in the mice treated with a combination of DDP and
GEN was lower compared with the tumor weight in the untreated group
and the groups treated with either agent alone (P<0.01)
(Fig. 3A). In addition, it was also
found that the tumor volume following treatment with DDP and GEN in
combination was significantly lower compared with the untreated
group and the groups treated with either agent alone (P<0.01;
Fig. 3B). These results indicate
that treatment with DDP in combination with GEN markedly suppresses
the tumorigenicity of A549 cells in mice.
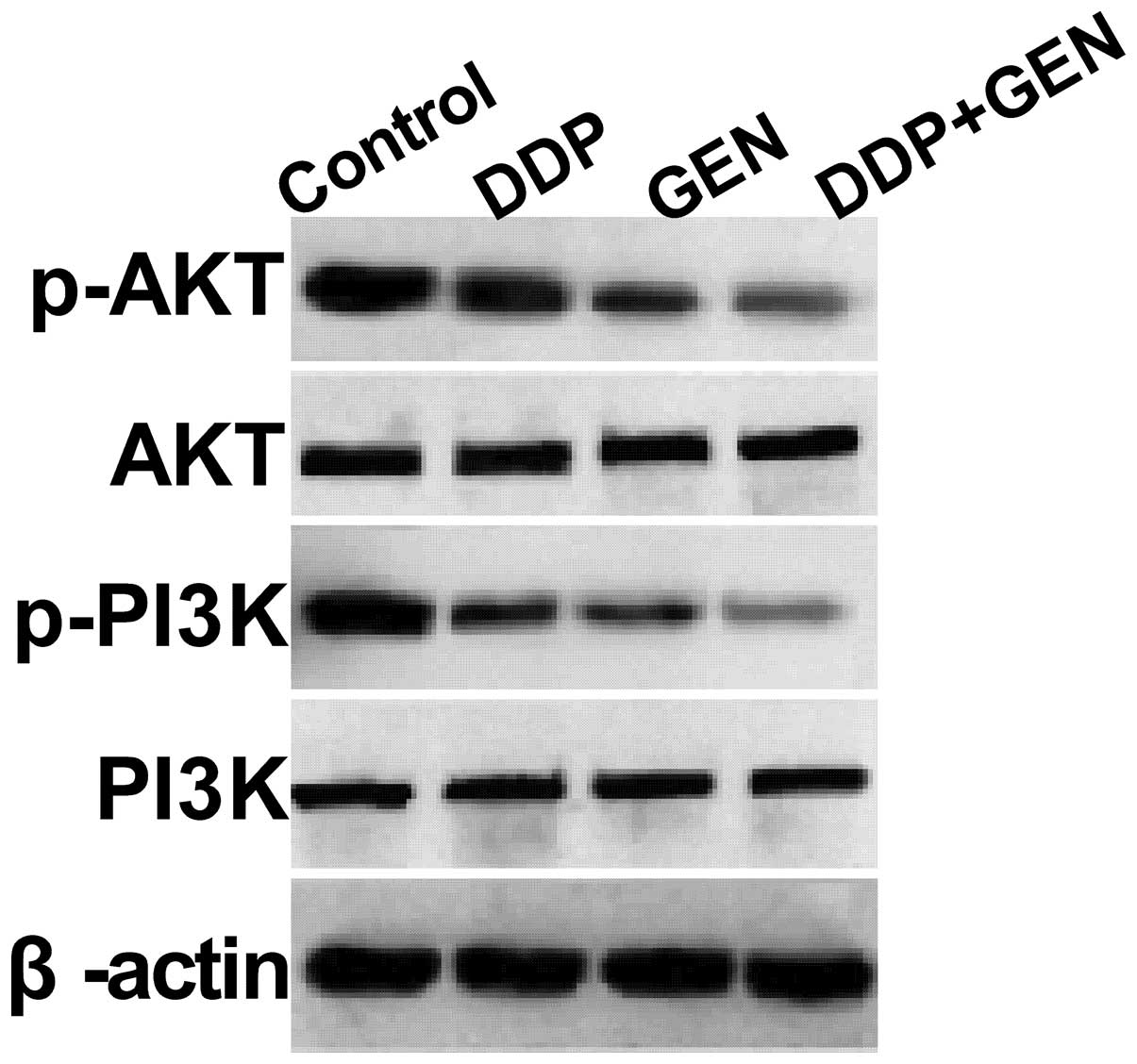
GEN in combination with DDP suppresses
the PI3K/AKT pathway in A549 cells
To clarify the molecular mechanisms involved in the
effect of GEN in combination with DDP on A549 cell proliferation,
the present study focused on the effects of GEN and DDP alone and
in combination on the activation of PI3K/AKT pathway, which
participates in the main intracellular signaling required for cell
proliferation and survival. GEN and DDP alone or in combination
were found to inhibit the tyrosine phosphorylation of AKT and PI3K
(Fig. 4). In addition, treatment
with a combination of GEN and DDP resulted in a greater reduction
in p-AKT and PI3K compared with treatment with either agent alone.
These results indicate that treatment with DDP in combination with
GEN inhibits tumor cell growth, to a certain extent, by suppressing
the PI3K/AKT pathway.
Discussion
Despite the substantial effort made to increase the
survival rate of patients with NSCLC, the majority of patients
exhibit advanced-stage, unresectable disease at the time of
diagnosis and the lesions possess inherent resistance to
chemotherapy and radiotherapy. Therefore, a satisfactory survival
rate has not been reached. Administered as either a single drug or
as a combination therapy with other agents, DDP is one of the
chemotherapeutic agents used in the clinic to treat lung carcinoma
(17). A previous study has
suggested an association between DDP-based doublet regimens and a
slightly improved survival rate compared with non-platinum-based
doublet regimens (18). However,
the outcome of DDP therapy for NSCLC appears to have reached a
plateau. Therefore, the resistance to DDP must be decreased for the
treatment of NSCLC.
In the present study, DDP in combination with GEN
was selected to reduce the doses of DDP required for the treatment
of NSCLC. The present results revealed that the combination of low
concentrations of DDP and GEN resulted in significantly increased
growth inhibition (P<0.01) and an increased level of apoptosis
in the A549 cells compared with either agent alone. These findings
suggested that GEN is able to increase the anti-neoplastic activity
of DDP and decrease the inherent resistance of NSCLC to DDP.
GEN is a small, biologically active flavonoid that
is found in high amounts in soy products and in other plants and
vegetables. GEN is able to inhibit tumor cells in vitro and
in vivo without evident toxicity to normal cells (19–21),
making GEN a promising agent for use in conjunction with toxic
chemotherapy agents, including DDP. It has been reported that GEN
enhances DDP-induced cell growth inhibition and apoptosis in
pancreatic and ovarian carcinoma cells (15,22). A
previous study has also revealed that pretreatment with GEN
inactivated NF-κB and may have contributed to an increase in
DDP-induced growth inhibition and apoptosis in various cancers,
without exhibiting systemic toxicity (23). The results of the present study
revealed that GEN enhances DDP-induced cell growth inhibition and
apoptosis in NSCLC cells in vitro and in vivo, which
is in agreement with previous results (15,22,23).
These findings and the present results demonstrate that GEN
decreases the inherent resistance to DDP and increases the
inhibition of tumor growth.
Caspases are members of a cysteine protease family
that consists of integral components of the apoptotic pathway,
including caspase-3. Caspase-3 is a cell death protease that is
activated by a variety of apoptotic stimuli. Once activated,
caspase-3 acts on numerous cellular targets that induce the
morphological appearance of apoptotic cells when they are cleaved
or activated (24,25). A large number of studies have
concluded that numerous chemotherapy drugs exert apoptotic effects
through the activation of caspases (24–26).
In the present study, the results revealed that treatment with a
combination of DDP and GEN significantly increased the activity of
caspases-3, -8 and -10 compared with treatment with DDP or GEN
alone (P<0.01), which indicates that caspases are vital protease
mediators of apoptosis initiated by combined treatment with DDP and
GEN.
The PI3K/AKT pathway is known to play a significant
role in regulating the chemoresistance of cancer cells (27–29).
In addition, Li and Sarkar also demonstrated that GEN inhibits
NF-κB activation, which is partly mediated through the AKT
signaling pathway (30). A study by
Park and Seol also revealed that the combination of GEN and TRAIL
enhanced TRAIL-induced apoptosis in A549 cells by regulating the
PI3K/AKT pathway (31). In the
present study, the results revealed that the use of DDP in
combination with GEN as a treatment for A549 cells resulted in a
marked reduction of phosphorylated PI3K and AKT relative to the
untreated cells, without altering the total protein levels of PI3K
or AKT. Previous studies and the present results have revealed that
the combination of GEN and DDP enhanced DDP-induced apoptosis in
A549 cells by regulating the PI3K/AKT pathway, at least in
part.
In conclusion, the present study reveals that DPP in
combination with GEN could enhance the anti-proliferative and
pro-apoptotic effects on NSCLC cell via effects on the
anti-apoptotic PI3K/AKT signaling pathways, at least in part. Thus,
it may be worthwhile to consider further evaluating the combination
treatment for NSCLC in clinical trials.
Acknowledgements
This study was supported by the Science and
Technology Research and Innovation Team funded by Jilin Province
(grant no. JL20130531).
References
|
1
|
Méndez M, Custodio A and Provencio M: New
molecular targeted therapies for advanced non-small-cell lung
cancer. J Thorac Dis. 3:30–56. 2011.
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009.
|
|
3
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: insight into multitargeted small-molecule
growth factor receptor inhibitors. Biomed Res Int.
2013:9647432013.
|
|
4
|
Raez LE and Lilenbaum R: Chemotherapy for
advanced non-small-cell lung cancer. Clin Adv Hematol Oncol.
2:173–178. 2004.
|
|
5
|
Rinaldi M, Belvedere O, Cauchi C, et al:
Maintenance chemotherapy in non-small cell lung cancer. Ann Oncol.
17(Suppl 2): ii67–ii70. 2006.
|
|
6
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011.
|
|
7
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006.
|
|
8
|
Gadgeel SM, Ali S, Philip PA, Wozniak A
and Sarkar FH: Genistein enhances the effect of epidermal growth
factor receptor tyrosine kinase inhibitors and inhibits nuclear
factor kappa B in nonsmall cell lung cancer cell lines. Cancer.
115:2165–2176. 2009.
|
|
9
|
Li Q and Chen H: Epigenetic modifications
of metastasis suppressor genes in colon cancer metastasis.
Epigenetics. 6:849–852. 2011.
|
|
10
|
Nagata Y, Sonoda T, Mori M, et al: Dietary
isoflavones may protect against prostate cancer in Japanese men. J
Nutr. 137:1974–1979. 2007.
|
|
11
|
Ali S, Varghese L, Pereira L, et al:
Sensitization of squamous cell carcinoma to cisplatin induced
killing by natural agents. Cancer Lett. 278:201–209. 2009.
|
|
12
|
Lattrich C, Lubig J, Springwald A, et al:
Additive effects of trastuzumab and genistein on human breast
cancer cells. Anticancer Drugs. 22:253–261. 2011.
|
|
13
|
Schabath MB, Hernandez LM, Wu X, Pillow PC
and Spitz MR: Dietary phytoestrogens and lung cancer risk. JAMA.
294:1493–1504. 2005.
|
|
14
|
Mai Z, Blackburn GL and Zhou JR: Genistein
sensitizes inhibitory effect of tamoxifen on the growth of estrogen
receptor-positive and HER2-overexpressing human breast cancer
cells. Mol Carcinog. 46:534–542. 2007.
|
|
15
|
Mohammad RM, Banerjee S, Li Y, et al:
Cisplatin-induced antitumor activity is potentiated by the soy
isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer.
106:1260–1268. 2006.
|
|
16
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.
|
|
17
|
Petty RD, Nicolson MC, Skaria S, et al:
Aberdeen Pancreatic Cancer Focus Group: A phase II study of
mitomycin C, cisplatin and protracted infusional 5-fluorouracil in
advanced pancreatic carcinoma: efficacy and low toxicity. Ann
Oncol. 14:1100–1105. 2003.
|
|
18
|
Rajeswaran A, Trojan A, Burnand B and
Giannelli M: Efficacy and side effects of cisplatin- and
carboplatin-based doublet chemotherapeutic regimens versus
non-platinum-based doublet chemotherapeutic regimens as first line
treatment of metastatic non-small cell lung carcinoma: a systematic
review of randomized controlled trials. Lung Cancer. 59:1–11.
2008.
|
|
19
|
Choi YH, Zhang L, Lee WH and Park KY:
Genistein-induced G2/M arrest is associated with the inhibition of
cyclin B1 and the induction of p21 in human breast carcinoma cells.
Int J Oncol. 13:391–396. 1998.
|
|
20
|
Choi YH, Lee WH, Park KY and Zhang L:
p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin
B1 and G2/M arrest by the isoflavone genistein in human prostate
carcinoma cells. Jpn J Cancer Res. 91:164–173. 2000.
|
|
21
|
Sarkar FH, Adsule S, Padhye S, Kulkarni S
and Li Y: The role of genistein and synthetic derivatives of
isoflavone in cancer prevention and therapy. Mini Rev Med Chem.
6:401–407. 2006.
|
|
22
|
Thasni KA, Rojini G, Rakesh SN, et al:
Genistein induces apoptosis in ovarian cancer cells via different
molecular pathways depending on Breast Cancer Susceptibility gene-1
(BRCA1) status. Eur J Pharmacol. 588:158–164. 2008.
|
|
23
|
Li Y, Ahmed F, Ali S, et al: Inactivation
of nuclear factor kappaB by soy isoflavone genistein contributes to
increased apoptosis induced by chemotherapeutic agents in human
cancer cells. Cancer Res. 65:6934–6942. 2005.
|
|
24
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008.
|
|
25
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326(Pt 1): 1–16. 1997.
|
|
26
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
|
|
27
|
Gagnon V, Van Themsche C, Turner S,
Leblanc V and Asselin E: AKT and XIAP regulate the sensitivity of
human uterine cancer cells to cisplatin, doxorubicin and taxol.
Apoptosis. 13:259–271. 2008.
|
|
28
|
McDonald GT, Sullivan R, Paré GC and
Graham CH: Inhibition of phosphatidylinositol 3-kinase promotes
tumor cell resistance to chemotherapeutic agents via a mechanism
involving delay in cell cycle progression. Exp Cell Res.
316:3197–3206. 2010.
|
|
29
|
Chekenya M, Krakstad C, Svendsen A, et al:
The progenitor cell marker NG2/MPG promotes chemoresistance by
activation of integrin-dependent PI3K/AKT signaling. Oncogene.
27:5182–5194. 2008.
|
|
30
|
Li Y and Sarkar FH: Inhibition of nuclear
factor kappaB activation in PC3 cells by genistein is mediated via
AKT signaling pathway. Clin Cancer Res. 8:2369–2377. 2002.
|
|
31
|
Park SY and Seol DW: Regulation of AKT by
EGF-R inhibitors, a possible mechanism of EGF-R inhibitor-enhanced
TRAIL-induced apoptosis. Biochem Biophys Res Commun. 295:515–518.
2002.
|