Introduction
Chronic myelogenous leukemia (CML), one of the three
most common forms of leukemia, is a hematopoietic stem cell disease
which is mostly caused by abnormal expression of the oncoprotein
BCR-ABL (1,2). At present, chemotherapy is the main
clinical treatment strategy for CML. However, serious drug
side-effects and multi-drug resistance caused by long-term
chemotherapy reduce the efficacy of treatment (3,4).
Hematopoietic stem cell transplantation is regarded as the most
effective treatment for CML, but it is limited by numerous factors,
including the availability of a donor, transplant rejection and the
high expense (5). For these
reasons, it is necessary to develop novel methods for CML
therapy.
Polyamines, including spermidine, spermine and their
diamine precursor putrescine, are polycationic alkylamines that are
ubiquitously present in all mammalian cells. Polyamines are
essential for eukaryotic cell growth, as these molecules
participate in multi-physical processes, including gene
transcription, regulation of protein function and cell membrane
stability (6,7). Notably, tumor cells contain a higher
concentration of polyamines than normal cells. The high level of
polyamines promotes the proliferation, invasion and migration of
tumor cells. Conversely, growth inhibition and apoptosis can be
induced in tumor cells by reducing the polyamine concentration
(8). Therefore, polyamine
metabolism has been identified as an important novel target for
antitumor therapy (9,10). Polyamine analogs are organic
chemical compounds that are analogous to polyamines in molecular
structure. Certain polyamine analogs can impose an inhibitory
effect on tumor cells by disturbing polyamine metabolism.
Tetrabutyl propanediamine (TBP) is a newly developed putricine
analog developed by the present group. In our previous studies, TBP
has been demonstrated to inhibit the growth and migration of human
hepatocellular carcinoma HepG2 and osteosarcoma MG-63 cells by
inducing apoptosis (11,12). In the present study, the effects of
TBP on K562 cells and the underlying mechanism of these effects
were further observed. The aim of the present study was to explore
the potential value of TBP for the clinical therapy of human
CML.
Materials and methods
Drugs and chemicals
The polyamine analog tetrabutyl propanediamine (TBP)
was synthesized by Dr. Wang Kai (Wuhan Engineering University,
Wuhan, China). Doxorubicin was produced by Zheijiang Hisun
Pharmaceutical Co., Ltd. (Taizhou, China). RPMI-1640, Triton X-100,
ribonuclease, propidium iodide (PI) and
3-(4,5-Dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Calf serum and
the Annexin V kit were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA). Agarose was purchased from Biowest LLC
(Kansas, MO, USA).
Cell culture
The K562 cells (China Center for Type Culture
Collection, Wuhan, China) were grown in RPMI-1640 medium
supplemented with 10% calf serum, 100 μg/ml streptomycin (North
China Pharmaceutical Co., Ltd., Shijiazhuang, China) and 100 U/ml
penicillin (North China Pharmaceutical Co., Ltd.) in a humidified
5% CO2 and 95% air incubator at 37°C.
Assay of cellular proliferation
The K562 cells were seeded into 96-well culture
plates [2,000 cells/well in 150 μl standard RPMI-1640 with the
different concentrations (0, 12.5, 25,50 and 100 μmol/l) of TBP] in
triplicate. After 24, 48 and 72 h of exposure to TBP, 50 μl MTT
solution (250 μg/ml in RPMI-1640 medium) was added into each well
and the cells were incubated at 37°C for 4 h. The plates were
centrifuged at 380 × g for 5 min, the supernatant medium was
removed and then 200 μl of dimethylsulfoxide was added to each
well. After 20 min, the absorbance (A) of each well at 490 nm was
recorded. The cell survival rate was calculated according to the
following formula: Cell survival rate (%) = Adrug /
Acontrol × 100.
Cell cycle assay by flow cytometry
The K562 cells were treated with various
concentrations of TBP for 24 h and harvested by centrifuging at 380
× g for 5 min. The cells were washed twice in ice-cold
phosphate-buffered saline (PBS), suspended in PBS (containing 75%
ethanol and 0.5 mmol/l EDTA) at 4°C for 30 min. The cells were then
centrifuged to remove the supernatant and washed with PBS,
resuspended in 500 μl PBS (containing 0.1% Triton X-100, 50 μg/ml
ribonuclease and 0.1 mg/ml PI) at 4°C for 30 min in the dark and
then assayed by flow cytometry.
Detection of apoptosis by DNA
fragmentation analysis
The K562 cells were treated with 50 and 100 μmol/l
TBP for 24 h and harvested by centrifuging at 380 × g for 5 min.
The cells were suspended in cell lysate buffer (50 mmol/l Tris-HCl
pH 8.0, 20 mmol/l EDTA and 0.5% Triton X-100) and incubated on ice
for 20 min. The lysate was centrifuged at 13,800 × g for 5 min to
remove the nuclei. The supernatant was then harvested and extracted
using an equal volume of phenol/chloroform. The DNA fragments in
the extracted supernatant were precipitated by adding two volumes
of 100% ethanol, 1/10 volume of 3 mol/l sodium acetate (pH 5.2) and
centrifuging at 13,800 × g for 10 min. The pellets were suspended
in 0.1X saline-sodium citrate buffer. Following treatment with
deoxyribonuclease-free ribonuclease (10 mg/ml) for 30 min at 37°C,
an equal volume of 2 mol/l NaCl was added and the solution was
extracted again by phenol/chloroform and precipitated by two
volumes of ethanol. The DNA pellets were then suspended in 30 μl
double distilled water, separated by 1.5% agarose gel and
visualized under UV illumination.
Detection of apoptosis by flow cytometry
following Annexin V/PI staining
The K562 cells were cultured in RPMI-1640 medium
containing 0, 12.5, 25, 50 or 100 μmol/l TBP for 24 h and were
harvested by centrifugation at 380 × g for 5 min. The cells were
treated according to the manufacturer’s instructions, suspended in
500 μl PBS and then analyzed by flow cytometry.
Determination of the activity of
acetylpolyamine oxidase (APAO) and spermine oxidase (SMO)
The activity of APAO and SMO was detected by a
modified chemiluminescent analysis (13). Briefly, the K562 cells were
harvested, resuspended in 300 μl cell lysis buffer (glycine 83
mmol/l, Triton X-100 0.25%, pH 8.0) and stored at −80°C for 24 h to
prepare the cell lysate. The enzyme activity in the cell lysate was
then assayed in a 300-μl reaction, which consisted of 83 mmol/l
glycine buffer (pH 8.0), 15 μmol/l luminol, 20 μg horseradish
peroxidase, 0.2 mmol/l bromoethylamine (catalase inhibitor), 15
μmol/l deprenyl (copper containing amine inhibitor) and 0.15 mmol/l
clorgyline (mitochondrial oxidase inhibitor), with 250 μmol/l
N1-acetylspermine (for APAO activity) or 250 μmol/l spermine (for
SMO activity) as substrates. All reagents, with the exception of
the substrates, were combined and incubated for 2 min at 37°C, the
tube was then transferred to the luminometer and the substrates
were added. The resulting chemiluminescence was recoded over 20
sec. The enzyme activities were expressed as relative light units
(RLU; RLU/μg protein/min).
Statistical analysis
Data were presented as the mean ± standard deviation
and were analyzed using a t-test performed using SPSS 17.0 software
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
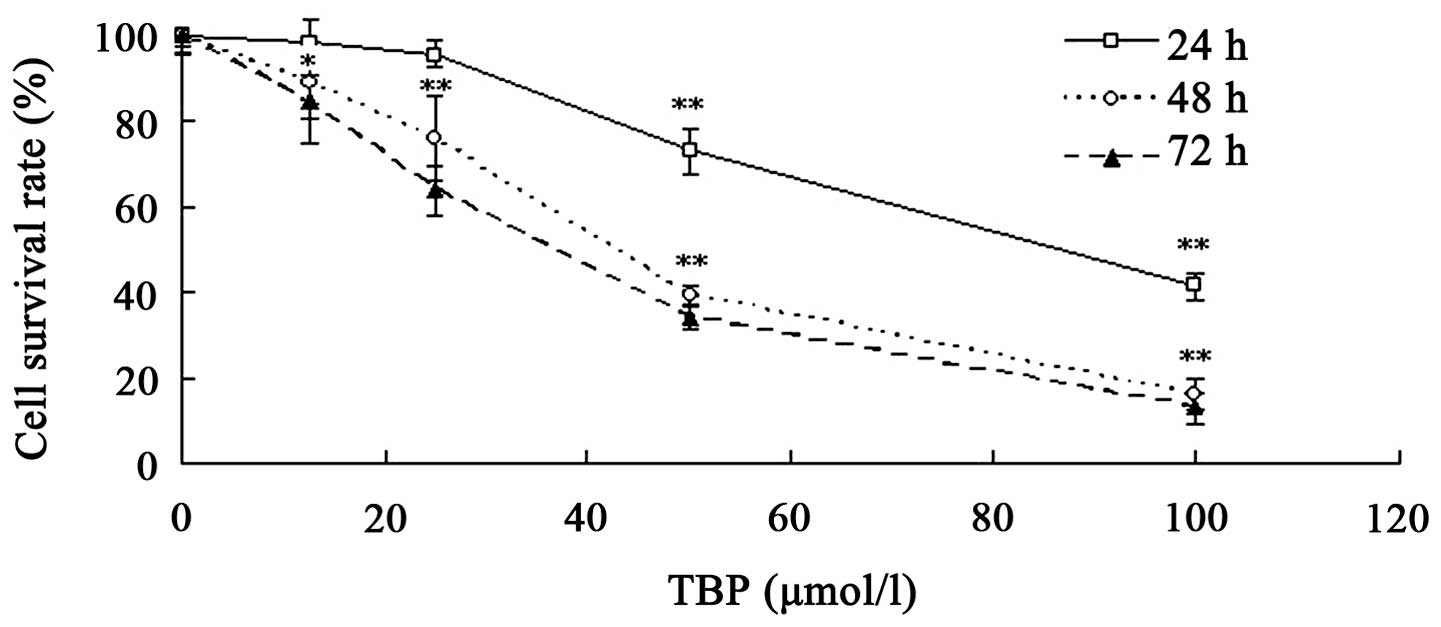
TBP inhibits the growth of K562
cells
TBP has been demonstrated to inhibit the growth of
solid tumor cell lines, including HepG2 and MG63 cells (11,12).
The present study further investigated the effect of TBP on the
proliferation of K562 cells using MTT assay. Various concentrations
of TBP (range, 12.5–100 μmol/l) were used to treat K562 cells for
24, 48 or 72 h. The results revealed that TBP exerted a significant
growth inhibition effect on K562 cells in a dose- and
time-dependent manner. As shown in Fig.
1, the survival rates were 73.1, 39.4 and 34.1% when the cells
were treated with 50 μmol/l TBP for 24, 48 and 72 h,
respectively.
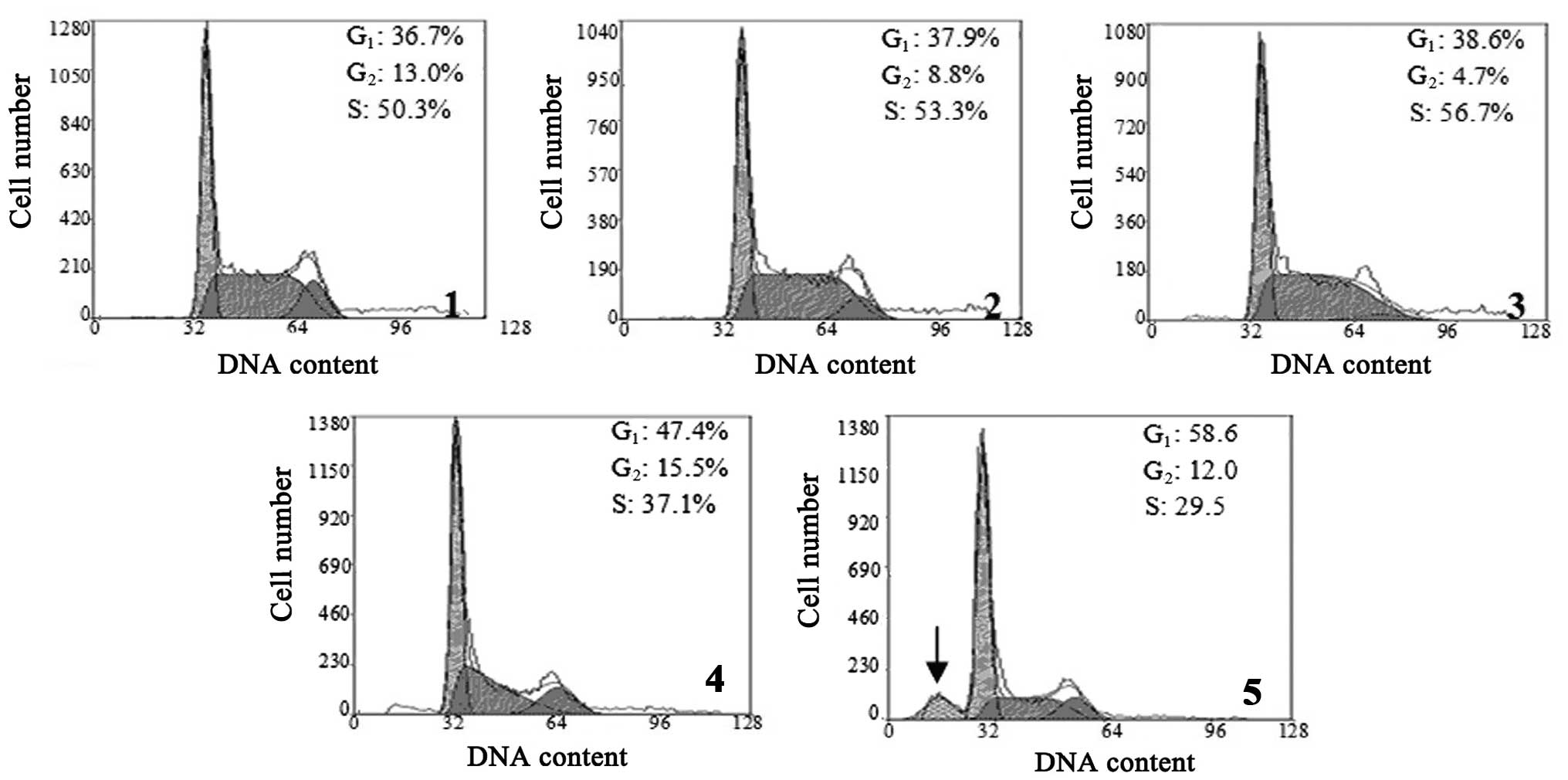
TBP interferes with the cell cycle of
K562 cells
The effect of TBP on the cell cycle of K562 cells
was analyzed by flow cytometry. The results revealed that treatment
with higher concentrations of TBP (50 and 100 μmol/l) resulted in a
significantly increased cell ratio in the
G0/G1 phase, but a decreased cell ratio in S
phase. When the K562 cells were treated with 100 μmol/l TBP for 24
h, the sub-G1 apoptotic peak was visible. These results
indicated that high concentrations of TBP can induce
G0/G1-phase arrest and induce apoptosis in
K562 cells (Fig. 2).
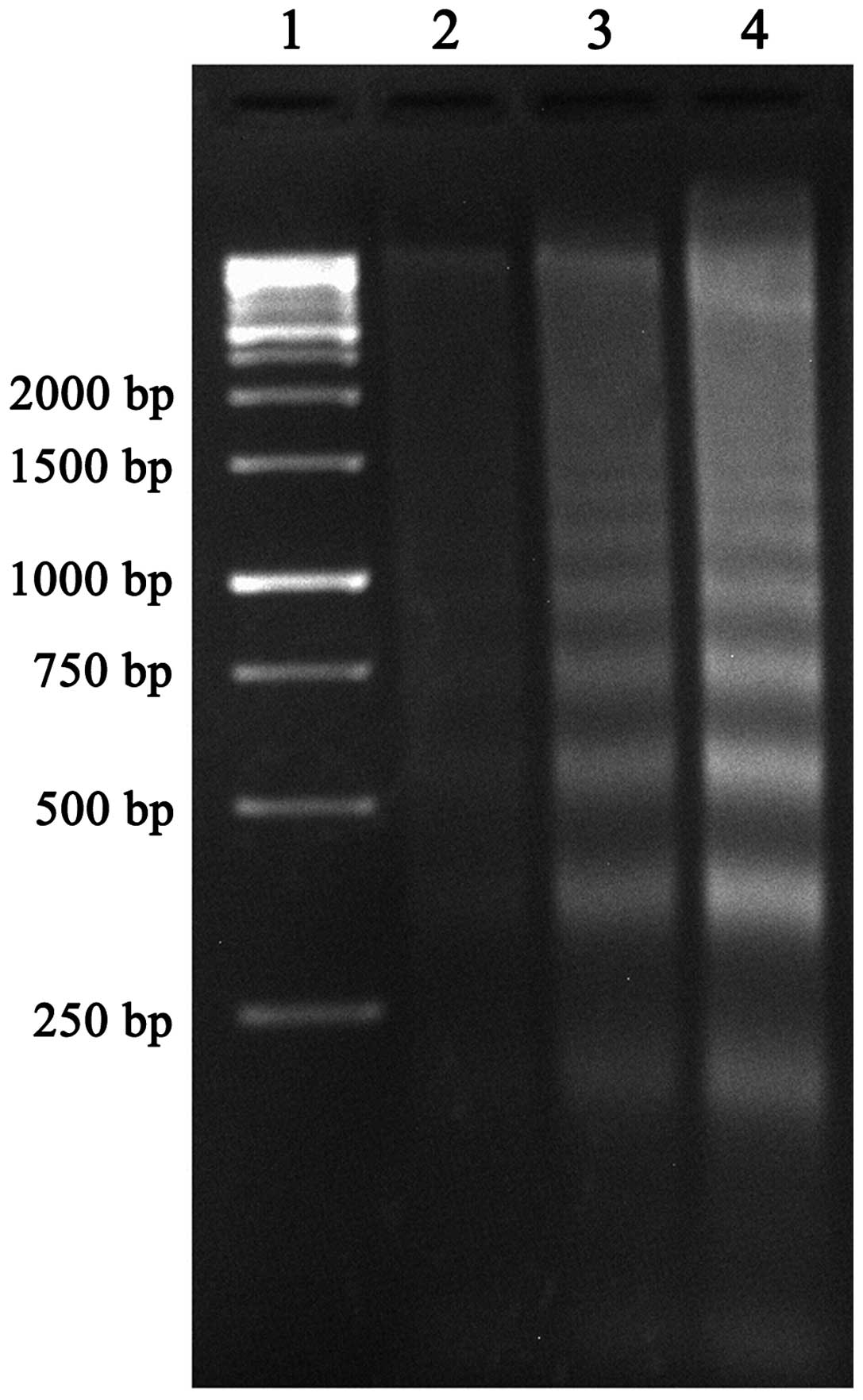
TBP induces apoptosis of K562 cells
DNA fragmentation analysis and Annexin V/PI double
staining were further used to identify TBP-induced apoptosis in the
K562 cells.
As shown in Fig. 3,
the clear DNA ladders that are a typical characteristic for
apoptosis were observed in agarose gel electrophoresis subsequent
to treating the K562 cells with TBP (50 and 100 μmol/l) for 24 h.
The strength of the DNA ladders is dose-dependent.
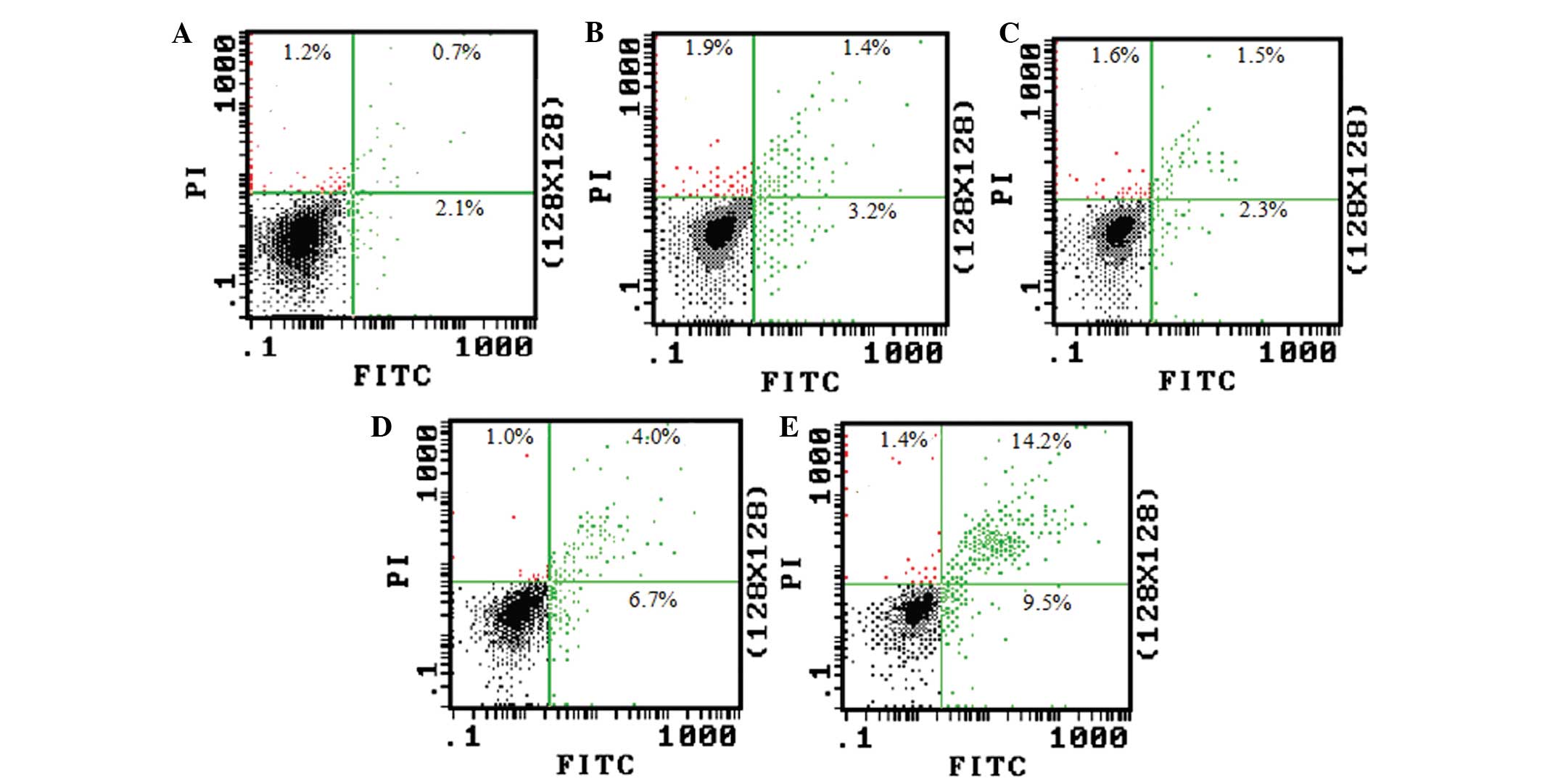
The Annexin V/PI double staining and flow cytometry
also revealed that the ratio of Annexin V/PI double-positive and
Annexin V single-positive cells dramatically increased when the
cells were treated with 50 and 100 μmol/l TBP (Fig. 4). This result is consistent with the
DNA fragmentation assay.
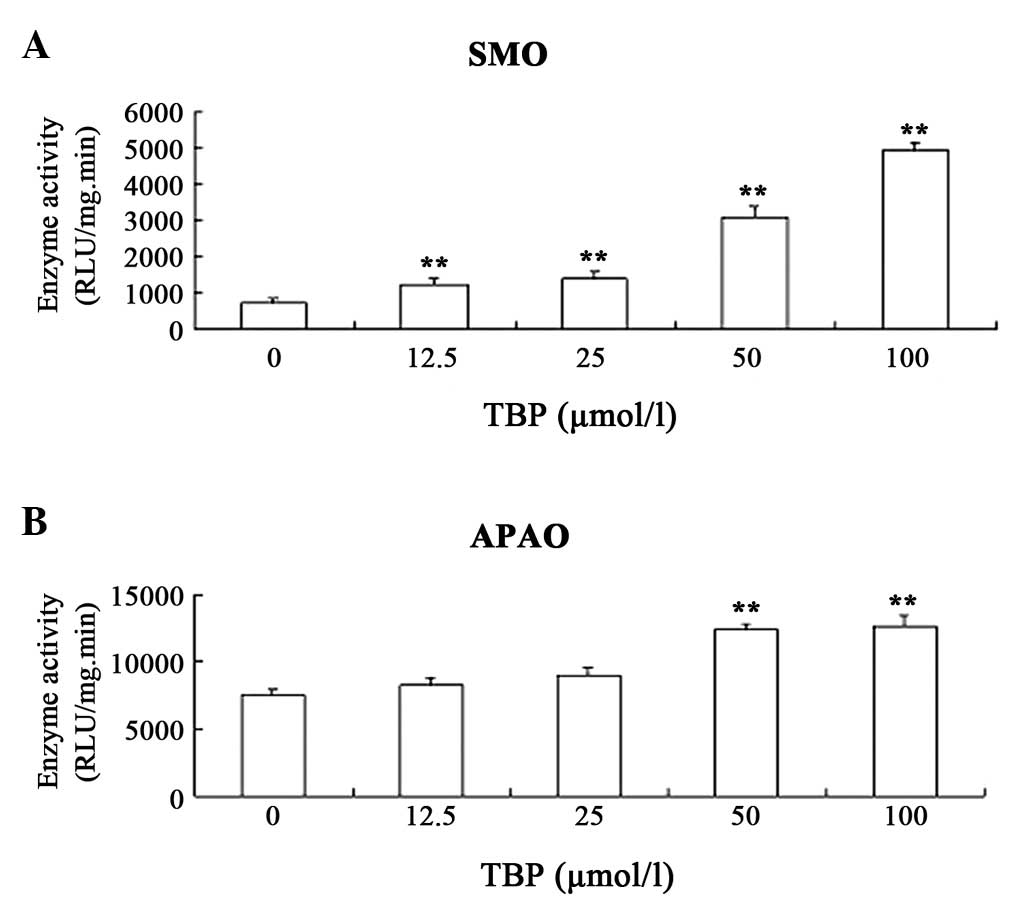
TBP upregulates the activity of SMO and
APAO in K562 cells
The aforementioned results revealed that TBP
inhibits K562 cell proliferation by inducing apoptosis. In order to
explore the mechanism underlying these effects, the effect of TBP
on the activity of SMO and APAO in K562 cells was investigated. SMO
and APAO are important enzymes involved in polyamine catabolism.
The enzymes catalyze degradation of polyamines and, at the same
time, produce hydrogen peroxide (H2O2). SMO
and APAO have each been proved to be important factors in inducing
cell apoptosis (14). The results
in the present study indicated that TBP upregulates the enzymatic
activity of SMO and APAO in K562 cells. Notably, TBP could
stimulate SMO activity much more strongly compared with APAO
activity. Exposure of K562 cells to 50 μmol/l TBP for 24 h resulted
in a ~4.5-fold induction of SMO activity and only a ~70% increase
in APAO activity (Fig. 5).
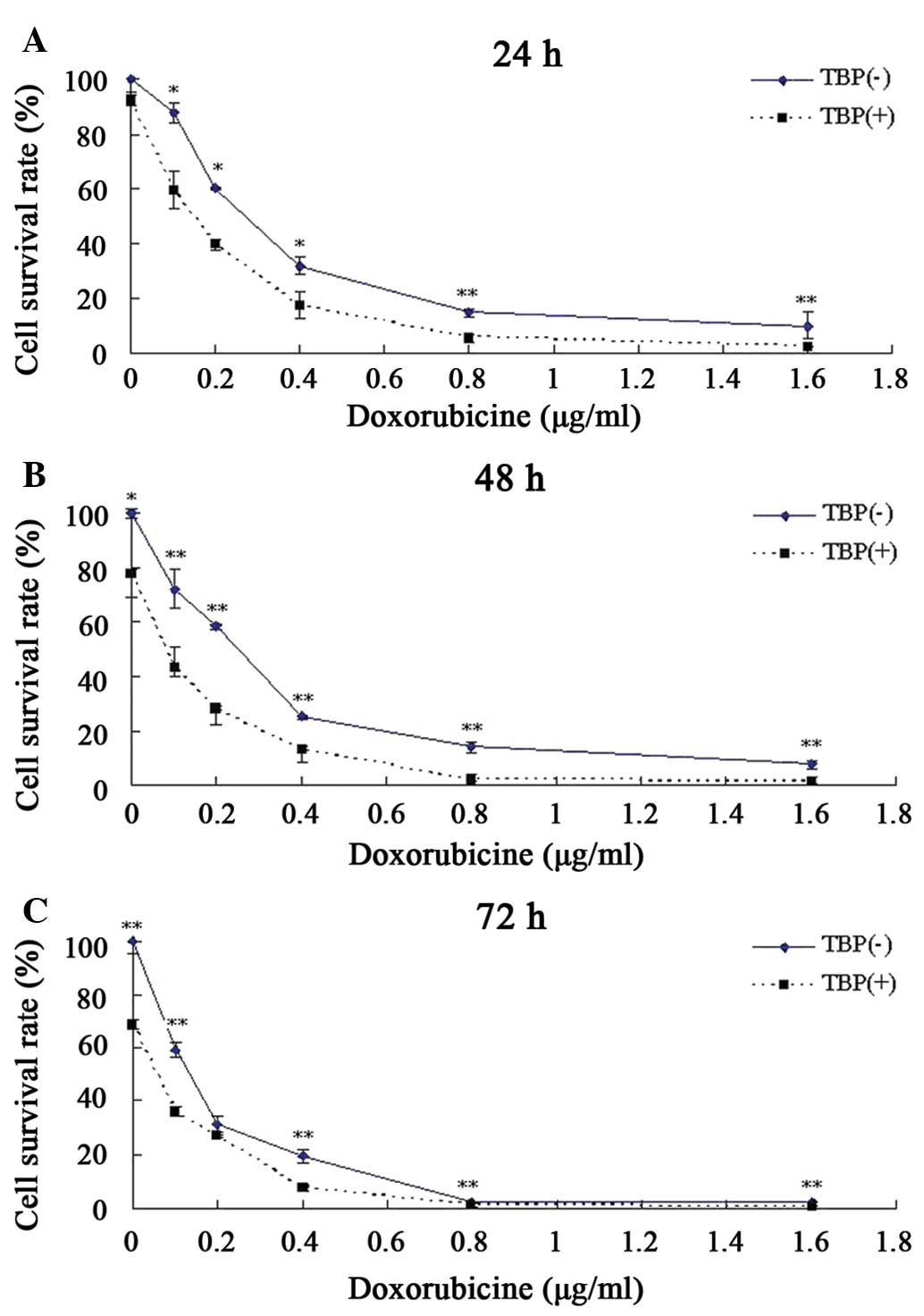
TBP improves the toxic response of K562
cells to doxorubicin
Doxorubicin is one of the first-line drugs in the
clinical treatment of chronic myeloid leukemia, but long-term use
of this drug can lead to drug resistance and reduce its therapeutic
effect (15,16). One clinical strategy to reduce the
drug resistance of tumor cells and enhance the antitumor effect is
combination therapy using two or more drugs. The present study
attempted to explore whether TBP can increase the sensitivity of
K562 to doxorubicin when these two drugs are co-administered.
According to the aforementioned data, although no evident growth
inhibition was observed when K562 cells were treated with 25 μmol/l
TBP, this same concentration of TBP could induce a ~2-fold increase
in SMO activity. Therefore, this TBP concentration was used
together with doxorubicin to co-treat K562 cells, and the results
revealed that TBP significantly enhanced the toxic effect of
doxorubicin on K562 cells. Compared with the K562 cells treated by
doxorubicin only, TBP (25 μmol/l) co-treatment resulted in a 74%
decrease in the required doses of doxorubicin for a 50% growth
inhibition after 48 h (Fig. 6).
Discussion
As a novel polyamine analog, TBP has been proved to
inhibit the growth and migration of human hepatocellular carcinoma
HepG2 cells and osteosarcoma MG-63 cells by inducing apoptosis
(11,12). The present study further observed
the effects of TBP on K562 cells and the underlying mechanism of
these effects.
The present data revealed that TBP treatment
significantly inhibited K562 cell proliferation and induced
G0/G1-phase arrest in a time- and
dose-dependent manner. In order to explore whether drug-induced
apoptosis was the molecular mechanism underlying TBP-mediated
growth inhibition, DNA fragmentation analysis and Annexin V/PI
double staining were performed. The results revealed that, in the
K562 cells treated with TBP, a DNA fragment ladder with the
fragment lengths being multiples of 200 bp was observed on the
agarose electrophoresis, which is a typical characteristic for
apoptosis. Annexin V/PI and flow cytometry also revealed a
significantly increased ratio of apoptotic cells following TBP
treatment. These results demonstrated that TBP could inhibit the
growth of K562 cells by inducing apoptosis.
Reactive oxygen species, including
H2O2, are generally known as powerful
inducers of apoptosis. Numerous studies have also demonstrated that
polyamine depletion can trigger apoptosis in tumor cells. SMO and
APAO are important enzymes involved in polyamine catabolism. These
enzymes synergistically catalyze the degradation of
spermine/spermidine and produce H2O2 as a
side product (13,14). In order to evaluate the effect of
TBP on SMO and APAO expression, the chemiluminescent assay was used
to determine the enzymatic activity of SMO and APAO. The data
revealed that the enzymatic activity of SMO and APAO in K562 cells
were upregulated by TBP treatment. However, TBP imposed a more
powerful induction on SMO compared with APAO. A low concentration
of TBP (25 μmol/l) resulted in a ~2-fold induction in SMO enzymatic
activity, but no evident induction for APAO activity was observed.
A high concentration of TBP (100 μmol/l) can lead to a ~7-fold
induction in SMO activity, but only ~1.7-fold induction for APAO
activity. This result was consistent with the former results
reported by Wang et al, who demonstrated that SMO was
upregulated in response to exposure to the polyamine analog
(17). These data suggest that the
key mechanism underlying TBP induced-apoptosis in K562 cells may be
associated with polyamine depletion and H2O2
accumulation caused by the powerful induction of SMO activity.
The present study also discovered that TBP at a low
concentration (25 μmol/l) can significantly increase the
sensitivity of the K562 cells to the antitumor drug doxorubicine
and produce a synergistic antitumor effect, which means that in CML
treatment, TBP could be used to increase the therapeutic efficacy
and simultaneously reduce the side-effects and multi-drug
resistance of doxorubicine by reducing the drug dosage.
In conclusion, the findings in the present study
indicate that TBP can inhibit the growth and induce apoptosis in
K562 cells by upregulating the key enzymes in polyamine catabolism
and has a potential value for clinical therapy of human CML. Future
studies are required, to clarify the effect of TBP in combination
with other drugs on K562 cells and the effect of TBP on other
leukemia cells.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81372265).
References
|
1
|
Wang Y, Chen J, Wang L, et al:
Fangchinoline induces G0/G1 arrest by modulating the expression of
CDKN1A and CCND2 in K562 human chronic myelogenous leukemia cells.
Exp Ther Med. 5:1105–1112. 2013.PubMed/NCBI
|
|
2
|
Lee YL, Chen CW, Liu FH, et al:
Aclacinomycin A sensitizes K562 chronic myeloid leukemia cells to
imatinib through p38MAPK-mediated erythroid differentiation. PLoS
One. 8:e619392013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng H, Yuan X, Shi R, et al: PHII-7
inhibits cell growth and induces apoptosis in leukemia cell line
K562 as well as its MDR-counterpart K562/A02 through producing
reactive oxygen species. Eur J Pharmacol. 2013.(In press).
View Article : Google Scholar
|
|
4
|
Shi R, Li W, Zhang X, et al: A novel
indirubin derivative PHII-7 potentiates adriamycin cytotoxicity via
inhibiting P-glycoprotein expression in human breast cancer
MCF-7/ADR cells. Eur J Pharmacol. 669:38–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brusic A, Hainz U, Wadleigh M, et al:
Detecting T-cell reactivity to whole cell vaccines: Proof of
concept analysis of T-cell response to K562 cell antigens in CML
patients. Oncol Immunol. 1:1095–1103. 2012.
|
|
6
|
Broshtilova V, Lozanov V and Miteva L:
Polyamine metabolism changes in psoriasis. Indian J Dermatol.
8:306–309. 2013. View Article : Google Scholar
|
|
7
|
Soda K, Kano Y, Chiba F, et al: Increased
polyamine intake inhibits age-associated alteration in global DNA
methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS
One. 8:e643572013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soda K: The mechanisms by which polyamines
accelerate tumor spread. J Exp Clin Cancer Res. 30:95–103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gamble LD, Hogarty MD, Liu X, et al:
Polyamine pathway inhibition as a novel therapeutic approach to
treating neuroblastoma. Front Oncol. 11:162–172. 2012.
|
|
10
|
Scuoppo C, Miething C, Lindqvist L, et al:
A tumour suppressor network relying on the polyamine-hypusine axis.
Nature. 487:244–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang JL, Han Y, Wang YL, et al: Polyamine
Analogues Tetrabutyl Propanediamine Inhibits Proliferation,
Invasion and Migration of Human Liver Cell Line HepG2 Cells in
vitro. Chin J Lab Diagn. 16:1350–1354. 2012.
|
|
12
|
Zhang HJ, Wang K, Wang YL, et al: Effects
of polyamine analogues tetrabutyl propanediamine on proliferation,
apoptosis and migration of human MG63 myeloma cells. Chinese
Pharmacological Bulletin. 28:974–977. 2012.
|
|
13
|
Wang Y, Hacher A, Casero RA, et al: The
induction of human spermine oxidase PAOh1/SMO is regulated at the
level of increased new mRNA, mRNA stabilization, and newly synthe
sized protein. Biochem J. 386:543–547. 2005. View Article : Google Scholar :
|
|
14
|
Wang Y, Murray-Stewart T, Casero RA Jr, et
al: Properties of purified recombinant human polyamine oxidase,
PAOh1/SMO. Biochem Biophys Res Commun. 304:605–611. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tyleckova J, Hrabakova R, Mairychova K, et
al: Cancer cell response to anthracyclines effects: mysteries of
the hidden proteins associated with these drugs. Int J Mol Sci.
13:15536–15564. 2012. View Article : Google Scholar
|
|
16
|
Xin H, Kong Y, Jiang X, et al:
Multi-drug-resistant cells enriched from chronic myeloid leukemia
cells by doxorubicin possess tumor-initiating-cell properties. J
Pharmacol Sci. 122:299–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Devereux W, Casero RA Jr, et al:
Cloning and characterization of a human polyamine oxidase that is
inducible by polyamine analogue exposure. Cancer Res. 61:5370–5373.
2001.PubMed/NCBI
|