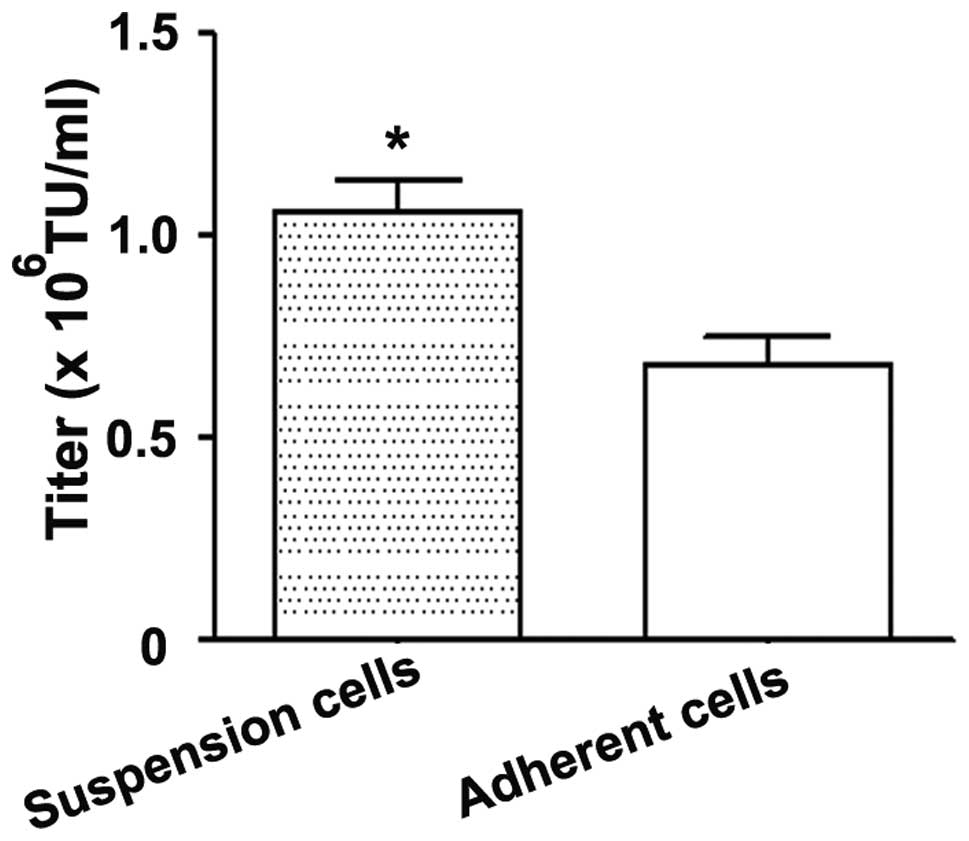
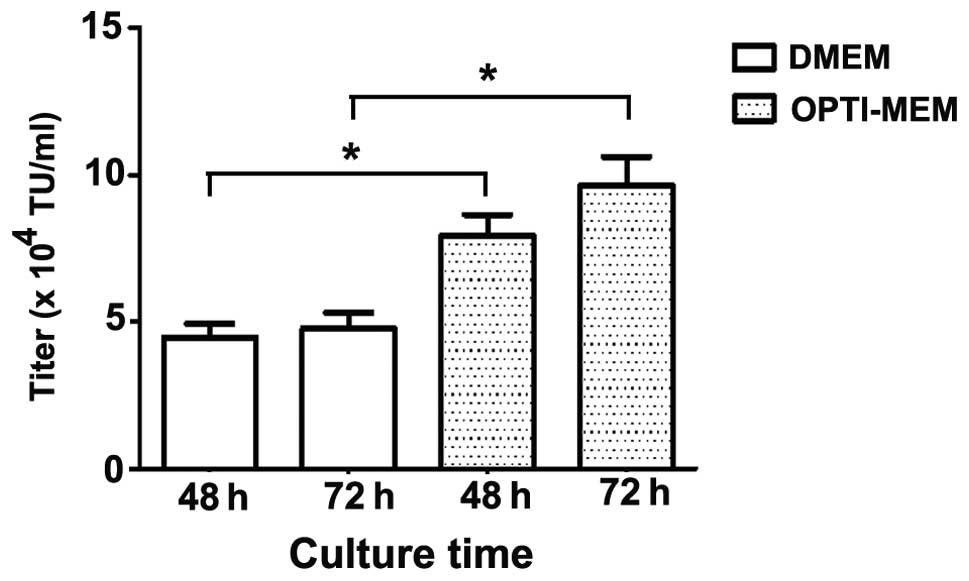
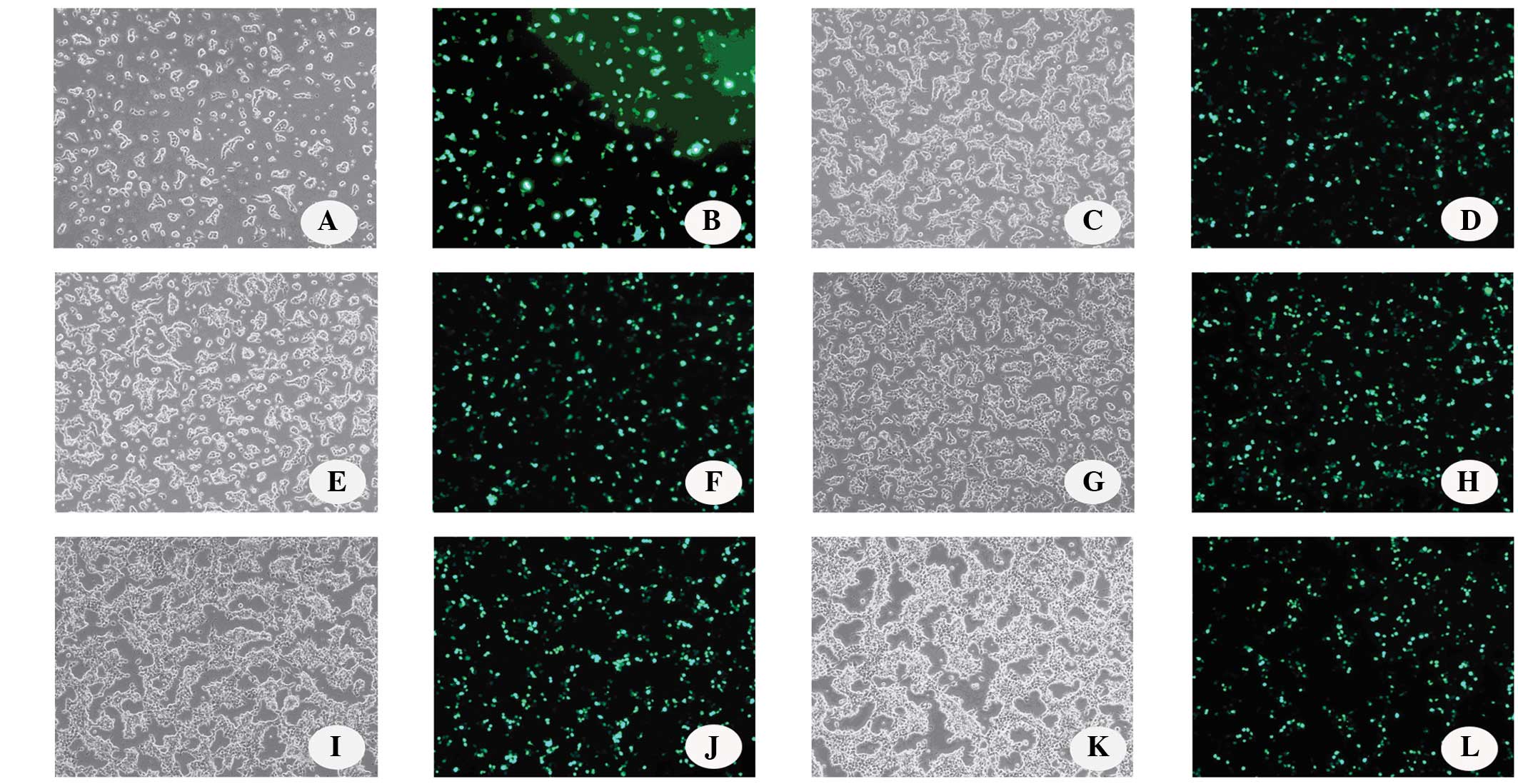
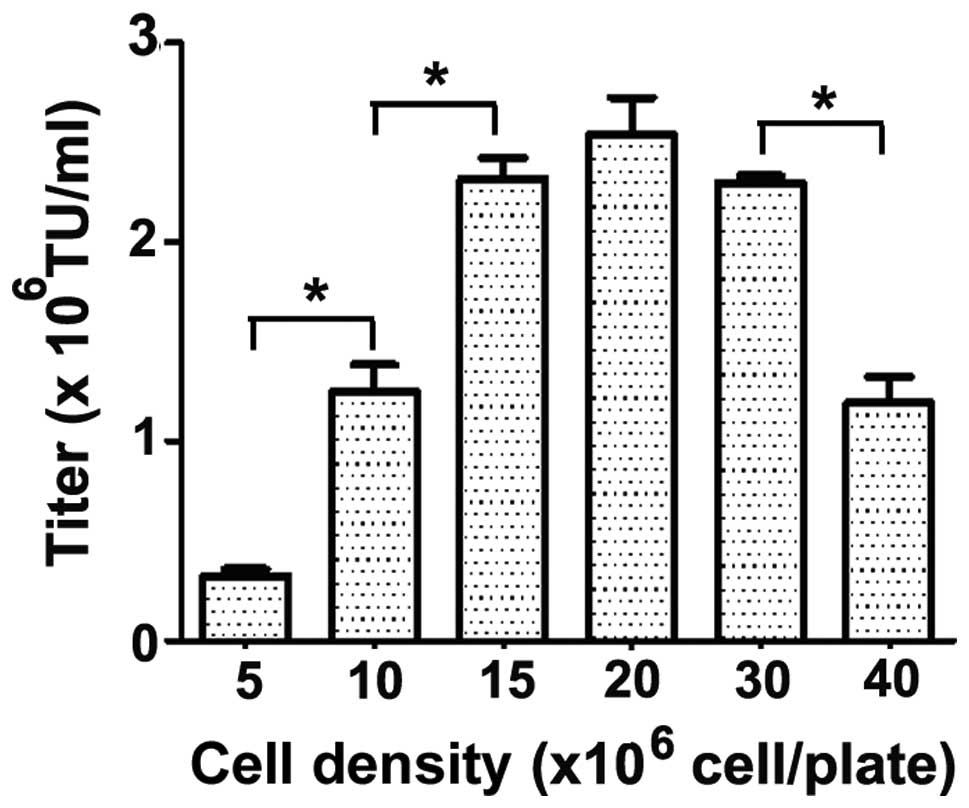
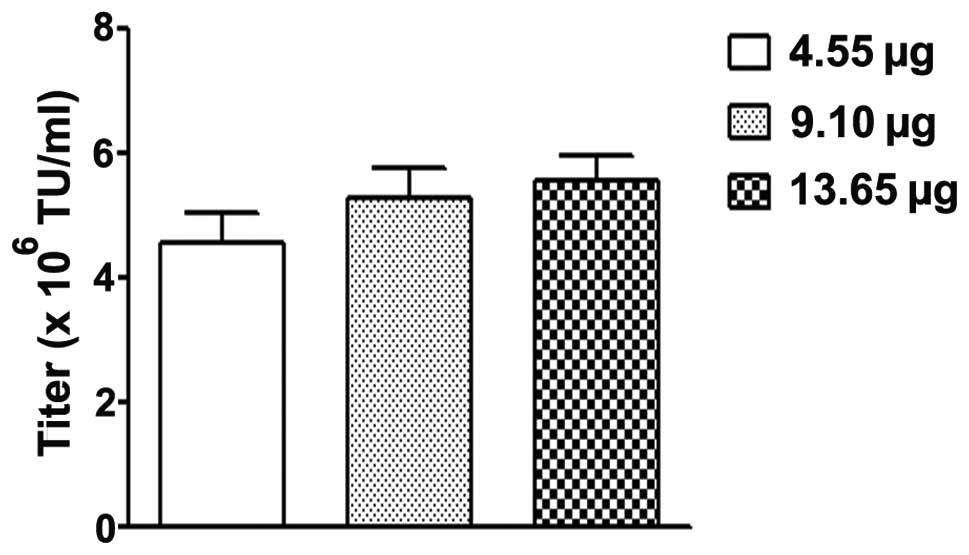
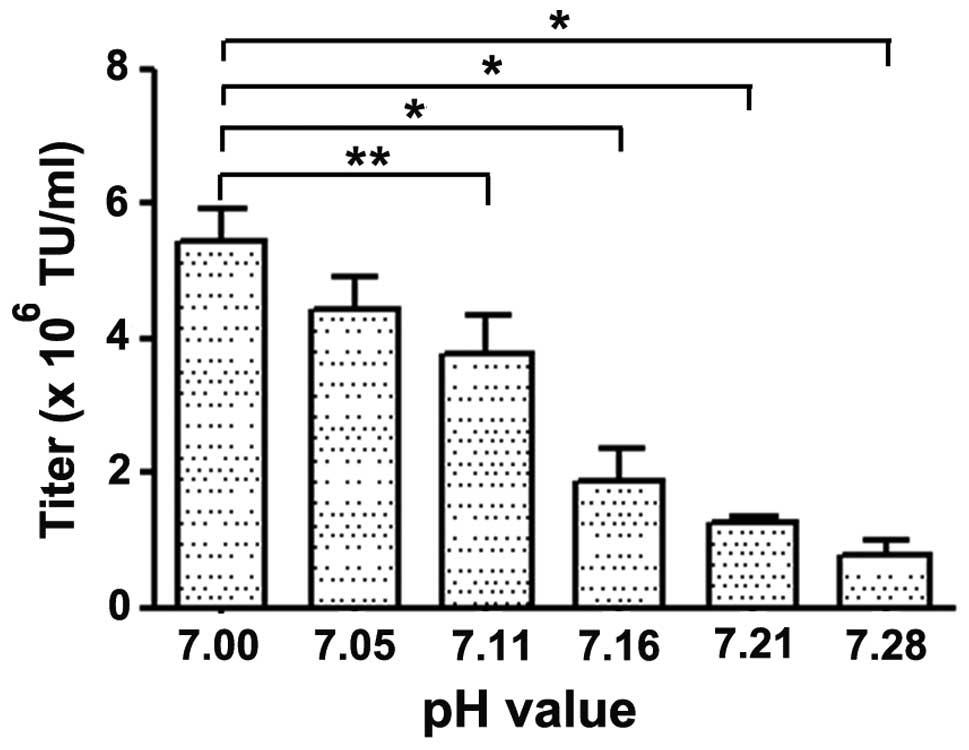
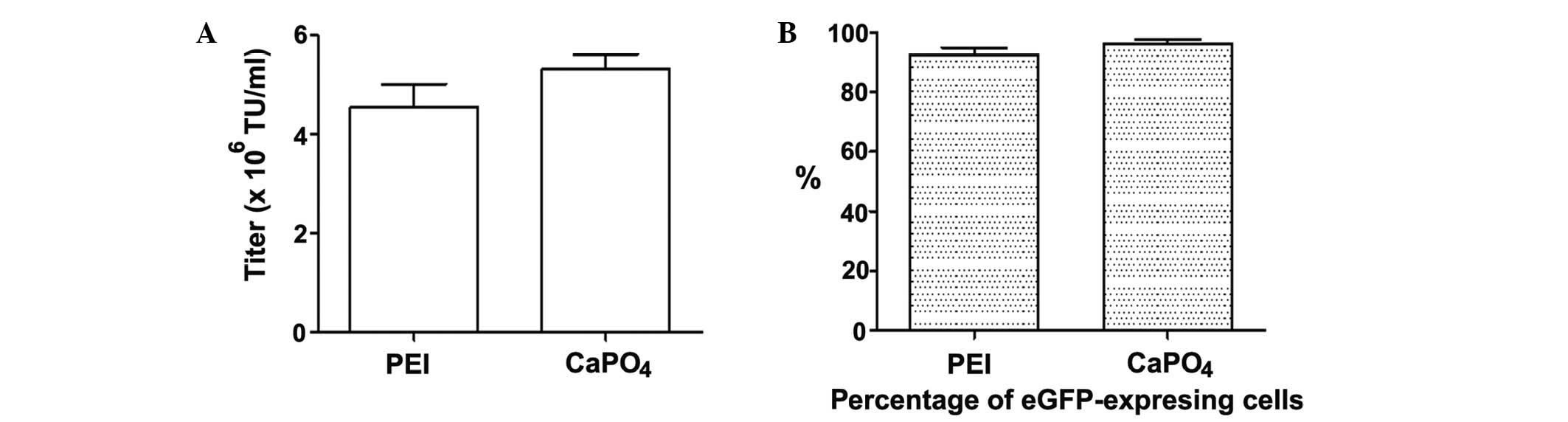
|
1
|
Quinonez R and Sutton RE: Lentiviral
vectors for gene delivery into cells. DNA Cell Biol. 21:937–951.
2002. View Article : Google Scholar
|
|
2
|
Vigna E and Naldini L: Lentiviral vectors:
excellent tools for experimental gene transfer and promising
candidates for gene therapy. J Gene Med. 2:308–316. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Segura MM, Garnier A, Durocher Y, Ansorge
S and Kamen A: New protocol for lentiviral vector mass production.
Methods Mol Biol. 614:39–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin KJ, Wall EA, Zavzavadjian JR, et al:
A single lentiviral vector platform for microRNA-based conditional
RNA interference and coordinated transgene expression. Proc Natl
Acad Sci USA. 103:13759–13764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman JE, Huentelman MJ, Kasparov S, et
al: Efficient large-scale production and concentration of
HIV-1-based lentiviral vectors for use in vivo. Physiol Genomics.
12:221–228. 2003.
|
|
6
|
Kosaka Y, Kobayashi N, Fukazawa T, et al:
Lentivirus-based gene delivery in mouse embryonic stem cells. Artif
Organs. 28:271–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salmon P and Trono D: Production and
titration of lentiviral vectors. Curr Protoc Neurosci.
37:4.21.1–4.21.24. 2006.
|
|
8
|
Lee JH and Welsh MJ: Enhancement of
calcium phosphate-mediated transfection by inclusion of adenovirus
in coprecipitates. Gene Ther. 6:676–682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mochizuki H, Schwartz JP, Tanaka K, Brady
RO and Reiser J: High-titer human immunodeficiency virus type
1-based vector systems for gene delivery into nondividing cells. J
Virol. 72:8873–8883. 1998.PubMed/NCBI
|
|
10
|
Cronin J, Zhang XY and Reiser J: Altering
the tropism of lentiviral vectors through pseudotyping. Curr Gene
Ther. 5:387–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segura MM, Kamen A and Garnier A:
Downstream processing of oncoretroviral and lentiviral gene therapy
vectors. Biotechnol Adv. 24:321–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Segura MM, Garnier A, Durocher Y, Coelho H
and Kamen A: Production of lentiviral vectors by large-scale
transient transfection of suspension cultures and affinity
chromatography purification. Biotechnol Bioeng. 98:789–799. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reed SE, Staley EM, Mayginnes JP, Pintel
DJ and Tullis GE: Transfection of mammalian cells using linear
polyethylenimine is a simple and effective means of producing
recombinant adeno-associated virus vectors. J Virol Methods.
138:85–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Durocher Y, Pham PL, St-Laurent G, et al:
Scalable serum-free production of recombinant adeno-associated
virus type 2 by transfection of 293 suspension cells. J Virol
Methods. 144:32–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geraerts M, Michiels M, Baekelandt V,
Debyser Z and Gijsbers R: Upscaling of lentiviral vector production
by tangential flow filtration. J Gene Med. 7:1299–1310. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boussif O, Lezoualc’h F, Zanta MA, et al:
A versatile vector for gene and oligonucleotide transfer into cells
in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA.
92:7297–7301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pham PL, Kamen A and Durocher Y:
Large-scale transfection of mammalian cells for the fast production
of recombinant protein. Mol Biotechnol. 34:225–237. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derouazi M, Girard P, Van Tilborgh F, et
al: Serum-free large-scale transient transfection of CHO cells.
Biotechnol Bioeng. 87:537–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Durocher Y, Perret S and Kamen A:
High-level and high-throughput recombinant protein production by
transient transfection of suspension-growing human 293-EBNA1 cells.
Nucleic Acids Res. 30:E92002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao DS, Li L, Garson K and Vanderhyden BC:
OPCML gene transferred by recombinant lentiviruses in vitro and its
inhibition to ovarian cancer cells. Zhonghua Fu Chan Ke Za Zhi.
41:333–338. 2006.(In Chinese). PubMed/NCBI
|
|
21
|
Jounai N, Okuda K, Kojima Y, et al:
Contribution of the rev gene to the immunogenicity of DNA vaccines
targeting the envelope glycoprotein of HIV. J Gene Med. 5:609–617.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuroda H, Kutner RH, Bazan NG and Reiser
J: Simplified lentivirus vector production in protein-free media
using polyethylenimine-mediated transfection. J Virol Methods.
157:113–121. 2009. View Article : Google Scholar
|
|
23
|
Kahl CA, Marsh J, Fyffe J, Sanders DA and
Cornetta K: Human immunodeficiency virus type 1-derived lentivirus
vectors pseudotyped with envelope glycoproteins derived from Ross
River virus and Semliki Forest virus. J Virol. 78:1421–1430. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kafri T, van Praag H, Ouyang L, Gage FH
and Verma IM: A packaging cell line for lentivirus vectors. J
Virol. 73:576–584. 1999.
|
|
25
|
Ni Y, Sun S, Oparaocha I, et al:
Generation of a packaging cell line for prolonged large-scale
production of high-titer HIV-1-based lentiviral vector. J Gene Med.
7:818–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sinn PL, Sauter SL and McCray PB Jr: Gene
therapy progress and prospects: development of improved lentiviral
and retroviral vectors - design, biosafety, and production. Gene
Ther. 12:1089–1098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitta B, Rimann M and Fussenegger M:
Detailed design and comparative analysis of protocols for optimized
production of high-performance HIV-1-derived lentiviral particles.
Metab Eng. 7:426–436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sena-Esteves M, Tebbets JC, Steffens S,
Crombleholme T and Flake AW: Optimized large-scale production of
high titer lentivirus vector pseudotypes. J Virol Methods.
122:131–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas M, Ge Q, Lu JJ, Chen J and Klibanov
AM: Cross-linked small polyethylenimines: while still nontoxic,
deliver DNA efficiently to mammalian cells in vitro and in vivo.
Pharm Res. 22:373–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Hia HC, Goh PE and Yap MG:
High-density transient gene expression in suspension-adapted 293
EBNA1 cells. Biotechnol Bioeng. 99:108–116. 2008. View Article : Google Scholar
|
|
31
|
Pham PL, Perret S, Doan HC, et al:
Large-scale transient transfection of serum-free suspension-growing
HEK293 EBNA1 cells: peptone additives improve cell growth and
transfection efficiency. Biotechnol Bioeng. 84:332–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pham PL, Perret S, Cass B, et al:
Transient gene expression in HEK293 cells: peptone addition
posttransfection improves recombinant protein synthesis. Biotechnol
Bioeng. 90:332–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toledo JR, Prieto Y, Oramas N and Sánchez
O: Polyethylenimine-based transfection method as a simple and
effective way to produce recombinant lentiviral vectors. Appl
Biochem Biotechnol. 157:538–544. 2009. View Article : Google Scholar
|
|
34
|
Geraerts M, Willems S, Baekelandt V,
Debyser Z and Gijsbers R: Comparison of lentiviral vector titration
methods. BMC Biotechnol. 6:342006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reiser J: Production and concentration of
pseudotyped HIV-1-based gene transfer vectors. Gene Ther.
7:910–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakoda T, Kasahara N, Kedes L and Ohyanagi
M: Calcium phosphate coprecipitation greatly enhances transduction
of cardiac myocytes and vascular smooth muscle cells by lentivirus
vectors. Exp Clin Cardiol. 12:133–138. 2007.
|
|
37
|
Trobridge G and Russell DW: Cell cycle
requirements for transduction by foamy virus vectors compared to
those of oncovirus and lentivirus vectors. J Virol. 78:2327–2335.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O’Rourke JP, Newbound GC, Kohn DB, Olsen
JC and Bunnell BA: Comparison of gene transfer efficiencies and
gene expression levels achieved with equine infectious anemia
virus- and human immunodeficiency virus type 1-derived lentivirus
vectors. J Virol. 76:1510–1515. 2002. View Article : Google Scholar
|
|
39
|
Kutner RH, Puthli S, Marino MP and Reiser
J: Simplified production and concentration of HIV-1-based
lentiviral vectors using HYPERFlask vessels and anion exchange
membrane chromatography. BMC Biotechnol. 9:102009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koldej R, Cmielewski P, Stocker A, Parsons
DW and Anson DS: Optimisation of a multipartite human
immunodeficiency virus based vector system; control of virus
infectivity and large-scale production. J Gene Med. 7:1390–1399.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McGarrity GJ, Hoyah G, Winemiller A, et
al: Patient monitoring and follow-up in lentiviral clinical trials.
J Gene Med. 15:78–82. 2013. View Article : Google Scholar : PubMed/NCBI
|